Introduction
Osteosarcoma is an aggressive malignant neoplasm
with poor prognosis. The use of local excision of the tumor tissue
with limb salvage surgery combined with neoadjuvant chemotherapy
has improved the survival rate markedly (1,2).
However, the local excision is not always complete, which triggers
recurrence at the primary site, leading to treatment failure
(3,4). Local perfusion thermochemotherapy has
long been proposed as an anticancer treatment, specifically for the
prevention of local recurrence, for use in combination with surgery
and chemotherapy (5–7). It has been shown that, at high
temperatures, chemotherapeutic drugs kill the osteosarcoma tumor
tissue that has not been removed by surgery, thereby reducing
recurrence at the primary site (8,9).
Our laboratory has focused on identifying strategies
for improving the thermochemotherapeutic effect of etoposide on
osteosarcoma. We hypothesized that the combination of paclitaxel
and etoposide in the presence of hyperthermia is likely to have an
improved killing effect on osteosarcoma cells. The purpose of this
study was to evaluate the cytotoxic effects of a combination of
paclitaxel and etoposide on an osteosarcoma cell line in the
presence of hyperthermia and investigate the effects of the
combination on the Fas-associated death receptor pathway.
Materials and methods
Materials
The OS732 osteosarcoma cell line was bought from
Beijing Jishuitan Hospital and the RPMI-1640 powder was acquired
from Gibco-BRL (Carlsbad, CA, USA). Trypsin, MTT and RNase A were
acquired from Huamei Biological Co. (Beijing, China). The following
materials were also used: paclitaxel (Beijing Concord
Pharmaceutical, Beijing, China), etoposide (Hisun Pharmaceutical
Co., Zhejiang, China), an enzyme meter (Bio-Rad, Hercules, CA,
USA), a FACScan flow cytometer (Becton-Dickinson, Franklin Lakes,
NJ, USA), an LH50A inverted phase contrast microscope (Olympus,
Tokyo, Japan) and a fluorescence microscope (Nikon, Tokyo,
Japan).
Cell culture and research methods
The OS732 osteosarcoma cell line was added to
RMPI-1640 solution with 10% fetal bovine serum and cultured in an
incubator at 37°C in a humidified 5% CO2 atmosphere. The
cells that had entered the logarithmic growth phase were selected
and heated using a numerical display constant temperature (±0.1°C)
water bath, set at various temperatures (37, 40 and 43°C) for 1 h.
We selected concentrations of 1, 10, 50 and 100 μg/ml for the
paclitaxel group, 1, 5, 10 and 100 μg/ml for the etoposide group
and 10 μg/ml paclitaxel with 5 μg/ml etoposide for the combination
group. PBS was used for the blank control group. These
concentrations were selected on the basis of EC50 doses
that we established in a preliminary trial. All trials were
repeated four times. The study was approved by the ethics committee
of China Medical University.
Measurement of the survival rates of
tumor cells by the MTT method
The treated cells (5×105/ml) were seeded
in a 96-well plate with a 200 μl reaction volume per well, using 4
parallel wells per group. After culturing for 24 h, freshly
prepared 5 mg/ml MTT was added to each well and incubation was
continued at 37°C for 4 h. The supernatant was then discarded and
150 μl DMSO was added. The absorbance (A) was measured at 540 nm.
The survival rate of the tumor cells (%) = Aexperimental
group/Acontrol group × 100.
Observation of the morphology of
apoptotic cells
The morphology, number and adherence of the tumor
cells were directly observed using an inverted phase contrast
microscope. A cover slide was placed in a 6-well plate, seeded with
OS732 cells, fixed for 10 min and stained with 0.5 ml Hoechst 33258
staining solution for 5 min. Images were then captured using a
fluorescence microscope.
Measurement of the proportion of
apoptotic cells by flow cytometry (FCM)
The digested cells were collected, washed with PBS,
centrifuged and then treated with 70% cold ethanol to fix
overnight. The cells were then centrifuged to remove ethanol and
washed twice with PBS. The cells were stained in the dark with 100
μl PI staining solution at 4°C for 1 h. The strength of the
fluorescence was measured using a FACScan flow cytometer. The
wavelength of the activating light was 488 nm, and the apoptotic
rates were measured using CellQuest analysis software.
Immunocytochemistry to detect Fas
expression in OS732 cells
Digested cells (2×105/ml) were placed in
a 6-well culture plate with a pre-treated cover slide in each well.
After culturing for 24 h, the supernatants were discarded, the drug
or drug combination was added and culturing was continued for 24 h.
A blank control group was also established. The cover slides were
removed, fixed with acetone at 4°C for 10 min and stained by the SP
method. A brown-yellow cytoplasm indicated a Fas-positive cell, and
the expression levels of Fas were determined from the average gray
levels obtained using a micro-picture analysis system.
Statistic method
Experimental data are the mean ± SD. Comparisons
among groups were made by ANOVA, and for any two groups, a t-test
was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant result. All results were
analyzed using Windows SPSS 15.0 software.
Results
Changes in the survival rates of the
tumor cells for the three thermochemotherapeutic drug
treatments
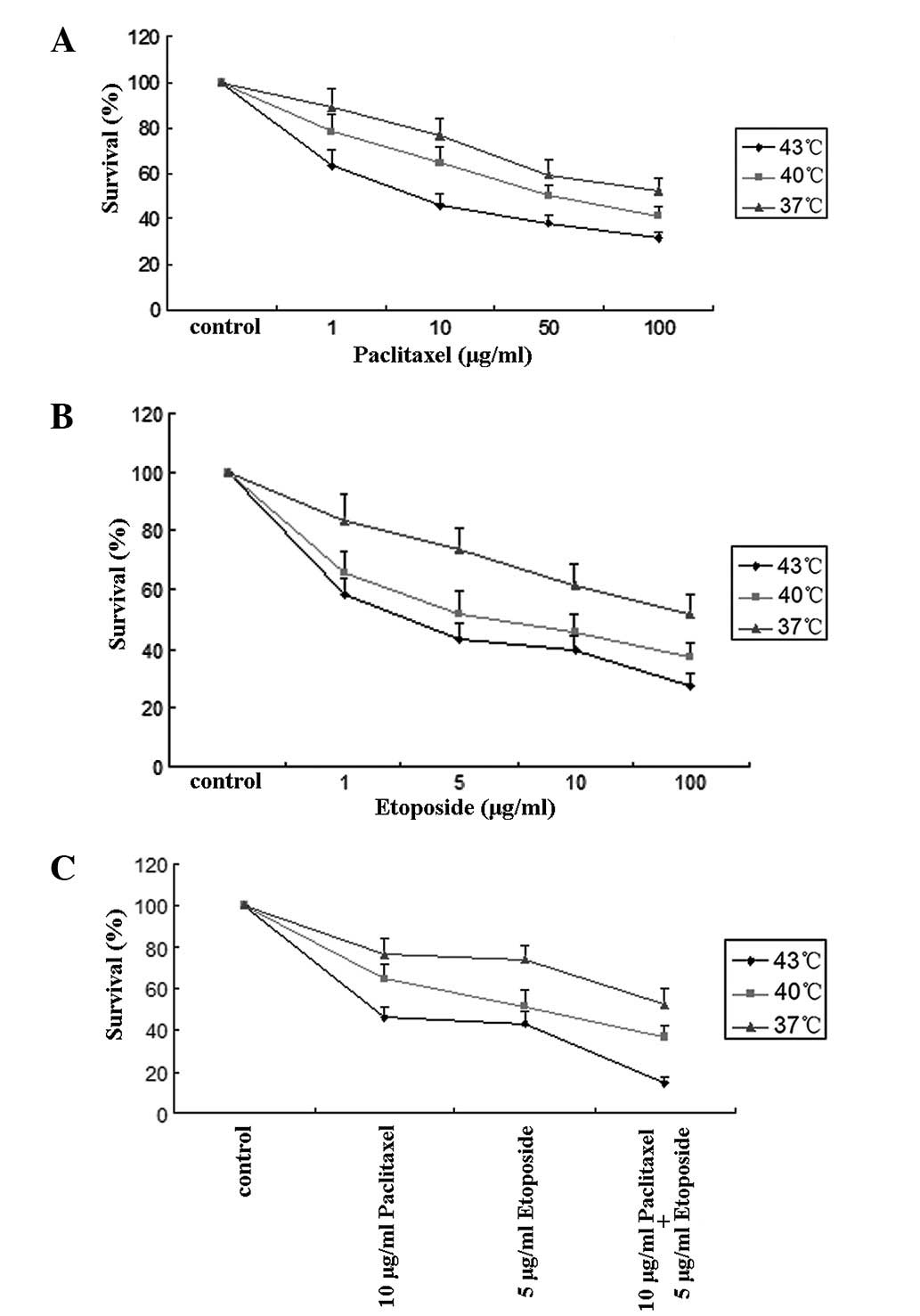
Following the treatment of the OS732 cells at
various temperatures for 1 h with paclitaxel at concentrations of
1, 10, 50 and 100 μg/ml, the cell growth was inhibited in a
dose-dependent manner and there were significant differences
between the groups (P<0.05; Fig.
1A). Similarly, when etoposide was used at concentrations of 1,
5, 10 and 100 μg/ml, the cell growth also varied between the groups
(P<0.05; Fig. 1B). For the
combination of 10 μg/ml paclitaxel and 5 μg/ml etoposide, the
survival rate was significantly lower (P<0.01; Fig. 1C) than for either 10 μg/ml
paclitaxel or 5 μg/ml etoposide alone, showing that the combination
of paclitaxel and etoposide has a stronger inhibitory effect than
either single agent. More significantly, we found that the cell
growth was inhibited in a temperature-dependent manner since the
survival rates of the OS732 cells were lowest at 43°C and highest
at 37°C.
Morphological changes of apoptosis in
OS732 cells
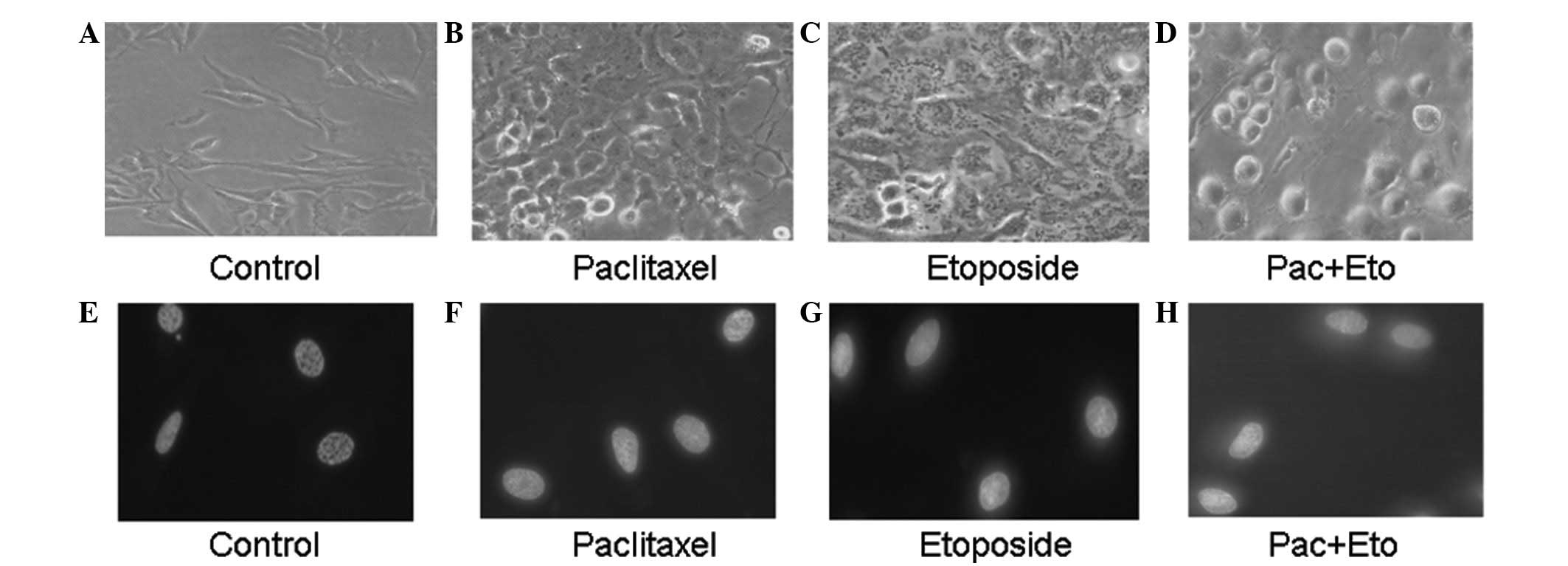
Under an inverted phase contrast microscope, the
normal OS732 cells were observed to be attached to the dish; the
cells were rhomboid and angular and were growing adhered to the
dish (Fig. 2A). Following the
application of either paclitaxel (10 μg/ml) or etoposide (5 μg/ml),
certain parts of the cells became small and round (Fig. 2B and C) whereas following the
combined application, chromatin and cytoplasm condensation occurred
and numerous cells exfoliated and were suspended in the culture
medium (Fig. 2D). Using a
fluorescence microscope, lightly-stained control OS732 cells were
observed (Fig. 2E). Following the
application of either paclitaxel (10 μg/ml) or etoposide (5 μg/ml),
only parts of the cells demonstrated condensed and flared
fluorescence (Fig. 2F and G)
whereas, following the combined application, condensed and flared
fluorescence was clearly observed, revealing the presence of
numerous apoptotic cells (Fig.
2H).
Comparison of the apoptotic rates in
OS732 cells for various medication methods by FCM
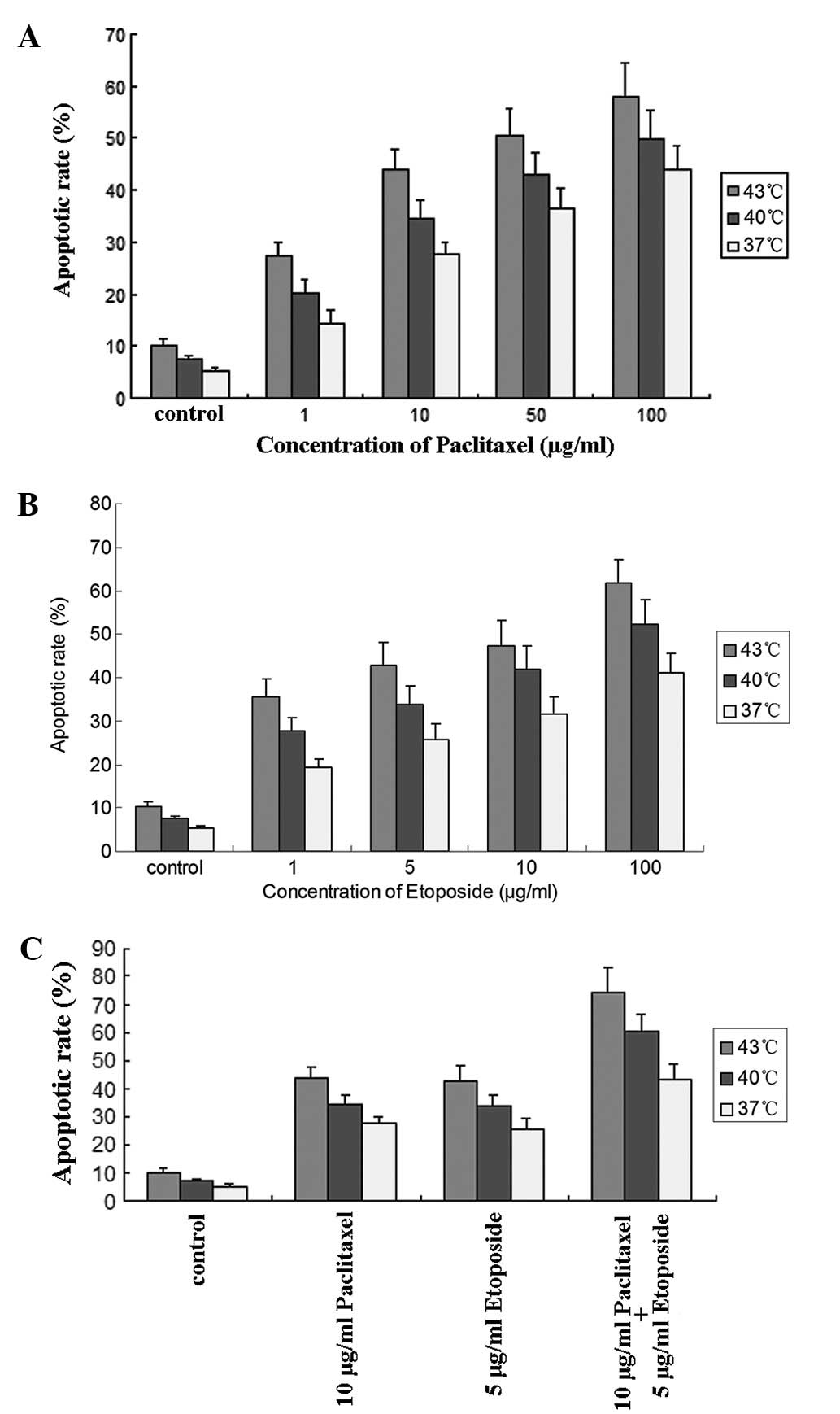
Following the treatment of the cells at various
temperatures for 1 h with paclitaxel at concentrations of 1, 10, 50
and 100 μg/ml, the apoptotic rate increased in a dose-dependent
manner and there were significant differences between the groups
(P<0.05; Fig. 3A). For
etoposide concentrations of 1, 5, 10 and 100 μg/ml, the apoptotic
rate also increased in a dose-dependent manner, and there were
significant differences between groups (P<0.01; Fig. 3B). For the combination of 10 μg/ml
paclitaxel and 5 μg/ml etoposide, the apoptotic rate was
significantly higher (P<0.01; Fig.
3C) than for 10 μg/ml paclitaxel or 5 μg/ml etoposide alone,
indicating that the combined use of paclitaxel and etoposide has a
stronger apoptosis-inducing effect than the use of either
individually.
Fas expression in OS732 cells by
immunocytochemistry
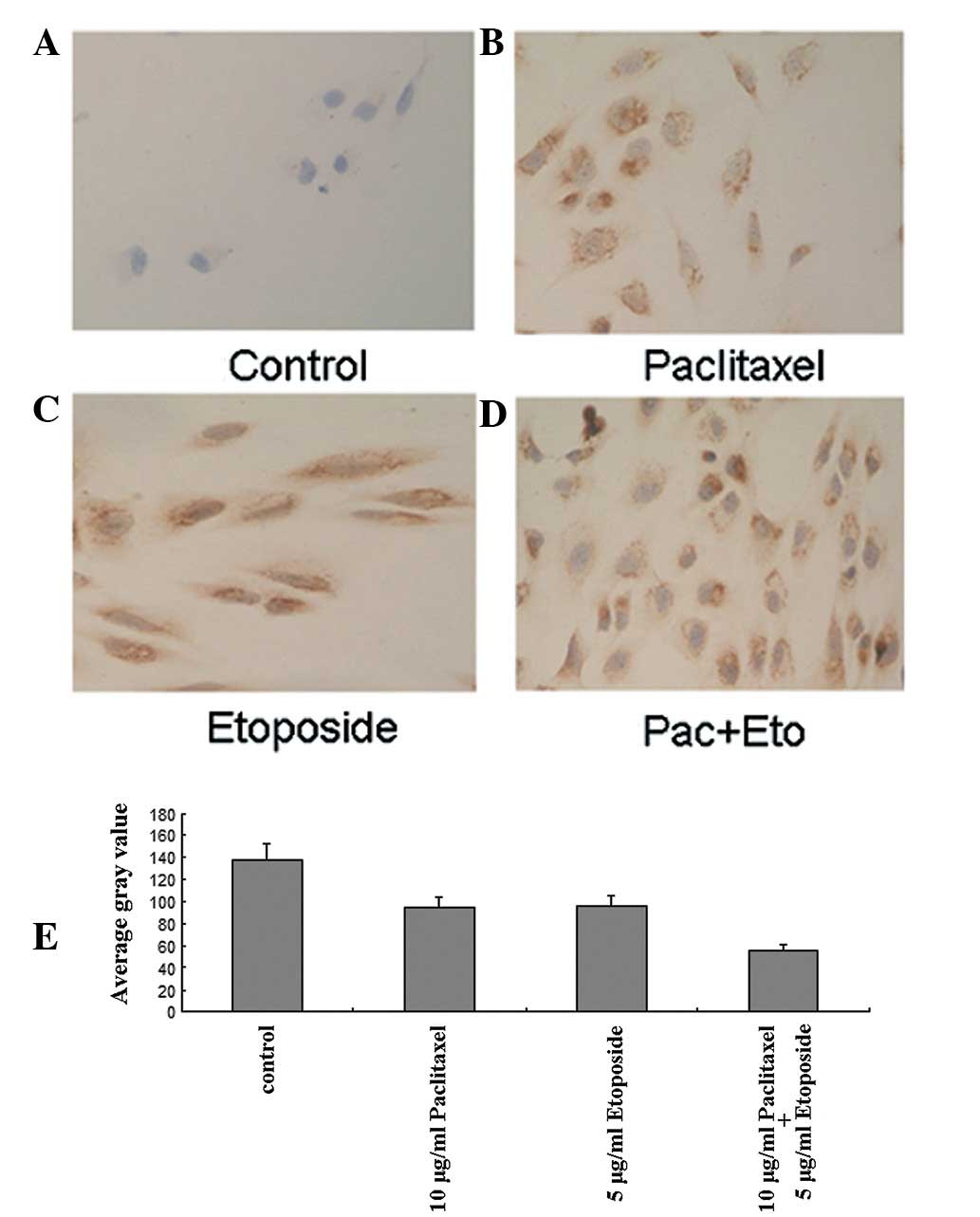
We observed only a small number of brown particles
in the cytoplasms of the control OS732 cells (Fig. 4A). Deeper staining of the
cytoplasms was observed following treatment with 10 μg/ml
paclitaxel (Fig. 4B) or 5 μg/ml
etoposide (Fig. 4C). For cells
treated with a combination of 10 μg/ml paclitaxel and 5 μg/ml
etoposide, the cytoplasms were stained much more strongly; staining
was observed in the entire field of vision (Fig. 4D). We further measured the Fas
levels quantitatively using a MetaMorph automatic image analyzer to
compare the average gray values, which are inversely proportional
to the levels of Fas expression (Fig.
4E)
Discussion
Thermochemotherapy is a comprehensive method that
has been developed based on the hyperthermal therapy of malignant
tumors. The isolated perfused chemotherapy of osteosarcoma
hyperthermally may not only effectively control the primary local
tumor, but also greatly increase the rate of successful limb
salvage (6,8). Although the efficacy of thermotherapy
with etoposide has been extensively demonstrated in a number of
tumor tissues (10–12), there are only limited studies
available in the field of osteosarcoma. It is well known that the
optimal therapeutic temperature clinically is 42–43°C for 1 h
(10) as this maximizes the tumor
damage while preserving the surrounding normal tissue. We also
found that 43°C is the ideal temperature for a combination of
paclitaxel and etoposide to kill the OS732 cell lines in
thermochemotherapy.
In the current study, we found that following the
use of etoposide or paclitaxel at various temperatures for 1 h, the
proliferation of tumor cells was inhibited in a dose-dependent
manner, indicating that each drug had a characteristic
thermotherapy-enhanced effect (Fig. 1A
and B). From observation of the morphological changes in the
apoptotic cells by inverted phase contrast microscopy (Fig. 2B and C) and by fluorescence
microscopy (Fig. 2F and G), we
deduced that paclitaxel and etoposide induced apoptosis in the
osteosarcoma cells. Assessment of the proportion of apoptotic cells
by FCM (Fig. 3A and B) revealed
that the killing effect on OS732 cells was due to the induction of
apoptosis.
Currently, there is a consensus on the enhancing
effect of thermotherapy on etoposide (10–12),
and positive views on the enhancing effect of thermotherapy on
paclitaxel have been reported (13–15).
However, there are also contrary opinions; Mohamed et al
reported that the cytotoxicity of docetaxel is enhanced by
hyperthermia whereas that of paclitaxel is not (16).
We found that following the joint application of low
doses of paclitaxel and etoposide to OS732 cells at 43°C for 1 h,
the cell survival rates decreased sharply compared with those
treated individually with paclitaxel or etoposide (Fig. 1C), demonstrating that the combined
use of paclitaxel and etoposide has a stronger inhibitory effect.
We also found apoptotic changes in the combination group when
observing the cells under an inverted phase contrast microscope
(Fig. 2D) as well as with a
fluorescence microscope (Fig. 2H).
The apoptotic rates in the combination group were clearly higher
(P<0.01) than in the individual treatment groups, indicating
that the inhibitory effect on the tumor cells was predominantly
accomplished by the induction of apoptosis (Fig. 3C), in agreement with studies in
other tumors (17).
There are two accepted theories concerning the
antitumor mechanism of thermochemotherapy. Firstly, thermotherapy
may alter the membranes of the tumor cells and thereby enable the
drugs to enter the tumor cells more easily (18). Secondly, thermotherapy may promote
the ability of the drugs to induce apoptosis in the tumor cells. A
number of chemotherapeutic agents induce cellular apoptosis by
various mechanisms which may be promoted by thermotherapy. Our
study indicates that, under hyperthermal conditions, paclitaxol and
etoposide each individually increased Fas expression levels
compared with the control group; however, the combination of
paclitaxel and etoposide greatly increased the Fas expression
levels in the OS732 cells compared with the individual treatments
(Fig. 4).
Previous studies have revealed that Fas-FADD
signaling is critical in the induction of apotosis in tumor cells
by etoposide (19–21). Our results are essentially
consistent with those of previous studies. As for the antitumor
mechanism of paclitaxel, it is primarily regarded to be the
induction of cell accumulation in the G2/M phase of the cell cycle
(22). Certain studies have
revealed that Fas-FADD signaling is also influential in the
induction of apoptosis in tumor cells by paclitaxel (23,24).
It has also been reported that paclitaxel triggers cell death in
H460 cells via an unidentified caspase-independent mechanism
(25). Our results reveal that
paclitaxel increases the expression levels of Fas in OS732 cells;
however, we should not neglect the possibility that hyperthermia is
also involved in the upregulation of Fas expression since previous
studies have indicated that hyperthermia affects Fas levels
(26,27). We found that, in the presence of
hyperthermia, small doses of paclitaxel and etoposide
synergistically contributed to the upregulation of Fas, which we
reveal is the probable mechanism by which paclitaxel acts in the
thermochemotherapy of osteosarcoma. Since local thermal etoposide
infusion chemotherapy has been widely used clinically, we expect
that the upregulation of Fas is likely to help to improve the
sensitivity of the osteosarcoma cells to thermochemotherapy with
etoposide and maximize the cytotoxic effects on the primary tumor
so as to prevent recurrence following surgery.
In conclusion, our results demonstrate that
paclitaxel is capable of sensitizing the OS732 cell line to
etoposide in the presence of hyperthermia by upregulation of Fas
expression. Paclitaxel is commonly used in cancer treatment
(28,29). However, we consider that the use of
paclitaxel in the thermochemotherapy of osteosarcoma would be a
more suitable therapeutic method. Since etoposide and paclitaxel
are antitumor drugs with specific therapeutic effects, toxicity and
resistance may readily occur following their large-dose and
long-term use, whereas the combined application of small doses of
etoposide and paclitaxel in the presence of hyperthermia is likely
to enhance the apoptosis-inducing effect, resulting in improved
drug sensitivity in osteosarcoma patients and minimizing the
cytotoxicity caused by clinical chemotherapy.
In this study, the combined application of 10 μg/ml
paclitaxel and 5 μg/ml etoposide to OS732 cells in the presence of
hyperthermia greatly inhibited the OS732 cells by inducing
apoptosis more strongly than the application of 10 μg/ml paclitaxel
or 5 μg/ml etoposide alone. Paclitaxel enhances the
thermochemotherapy of osteosarcoma cell lines and this is primarily
accomplished by the upregulation of Fas expression and the
induction of apoptosis.
Acknowledgements
This article was checked by Dr Shavali Shaik of Beth
Israel Deaconess Medical Center, Harvard Medical School.
References
|
1
|
Bacci G, Balladelli A, Palmerini E, et al:
Neoadjuvant chemotherapy for osteosarcoma of the extremities in
preadolescent patients: the Rizzoli Institute experience. J Pediatr
Hematol Oncol. 30:908–912. 2008.
|
|
2
|
Bacci G, Rocca M, Salone M, et al: High
grade osteosarcoma of the extremities with lung metastases at
presentation: treatment with neoadjuvant chemotherapy and
simultaneous resection of primary andmetastatic lesions. J Surg
Oncol. 98:415–420. 2008.
|
|
3
|
Franke M, Hardes J, Helmke K, et al:
Solitary skeletal osteosarcoma recurrence. Findings from the
Cooperative Osteosarcoma Study Group. Pediatr Blood Cancer.
56:771–776. 2011.
|
|
4
|
Andreou D, Bielack SS, Carrle D, et al:
The influence of tumor- and treatment-related factors on the
development of local recurrence in osteosarcoma after adequate
surgery. An analysis of 1355 patients treated on neoadjuvant
Cooperative Osteosarcoma Study Group protocols. Ann Oncol.
22:1228–1235. 2011.
|
|
5
|
Routt SM, Zhu J, Zaleski JM and Dynlacht
JR: Potentiation of metalloenediyne cytotoxicity by hyperthermia.
Int J Hyperthermia. 27:435–444. 2011.
|
|
6
|
Streckfus CF, Brown RE and Bull JM:
Proteomics, morphoproteomics, saliva and breast cancer: an emerging
approach to guide the delivery of individualised thermal therapy,
thermochemotherapy and monitor therapy response. Int J
Hyperthermia. 26:649–661. 2010.
|
|
7
|
Trieb K, Blahovec H and Kubista B: Effects
of hyperthermia on heat shock protein expression, alkaline
phosphatase activity and proliferation in human osteosarcoma cells.
Cell Biochem Funct. 25:669–672. 2007.
|
|
8
|
Fan QY, Ma BA, Zhou Y, et al: Bone tumors
of the extremities or pelvis treated by microwave-induced
hyperthermia. Clin Orthop Relat Res. 406:165–175. 2003.
|
|
9
|
Shido Y, Nishida Y, Suzuki Y, et al:
Targeted hyperthermia using magnetite cationic liposomes and an
alternating magnetic field in a mouse osteosarcoma model. J Bone
Joint Surg Br. 92:580–585. 2010.
|
|
10
|
Vujaskovic Z, Kim DW, Jones E, et al: A
phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel,
and hyperthermia in locally advanced breast cancer. Int J
Hyperthermia. 26:514–521. 2010.
|
|
11
|
Tang Y and McGoron AJ: Combined effects of
laser-ICG photothermotherapy and doxorubicin chemotherapy on
ovarian cancer cells. J Photochem Photobiol B. 97:138–144.
2009.
|
|
12
|
Fiorillo A, DeRosa G, Giugliano F, et al:
Efficacy of pegylated lyposomal anthracyclines and of
intra-arterial carboplatin and doxorubicin combined with local
hyperthermia in a case of malignant endovascular papillary
angioendothelioma. Curr Drug Deliv. 6:58–61. 2009.
|
|
13
|
Liu B, Yang M, Li X, et al: Enhanced
efficiency of thermally targeted taxanes delivery in a human
xenograft model of gastric cancer. J Pharm Sci. 97:3170–3181.
2008.
|
|
14
|
Michalakis J, Georgatos SD, de Bree E, et
al: Short-term exposure of cancer cells to micromolar doses of
paclitaxel, with or without hyperthermia, induces long-term
inhibition of cell proliferation and cell death in vitro. Ann Surg
Oncol. 14:1220–1228. 2007.PubMed/NCBI
|
|
15
|
Zoul Z, Filip S, Melichar B, et al: Weekly
paclitaxel combined with local hyperthermia in the therapy of
breast cancer locally recurrent after mastectomy - a pilot
experience. Onkologie. 27:385–388. 2004.
|
|
16
|
Mohamed F, Marchettini P, Stuart OA, et
al: Thermal enhancement of new chemotherapeutic agents at moderate
hyperthermia. Ann Surg Oncol. 10:463–468. 2003.
|
|
17
|
de Bree E, Theodoropoulos PA, Rosing H, et
al: Treatment of ovarian cancer using intraperitoneal chemotherapy
with taxanes: from laboratory bench to bedside (Review). Cancer
Treat Rev. 32:471–482. 2006.
|
|
18
|
de Bree E, Rosing H, Michalakis J, et al:
Intraperitoneal chemotherapy with taxanes for ovarian cancer with
peritoneal dissemination (Review). Eur J Surg Oncol. 32:666–670.
2006.
|
|
19
|
Miyata S, Takemura G, Kosai K, et al:
Anti-Fas gene therapy prevents doxorubicin-induced acute
cardiotoxicity through mechanisms independent of apoptosis. Am J
Pathol. 176:687–698. 2010.
|
|
20
|
Kim HS, Lee YS and Kim DK: Doxorubicin
exerts cytotoxic effects through cell cycle arrest and Fas-mediated
cell death. Pharmacology. 84:300–309. 2009.
|
|
21
|
Li S, Zhou Y, Dong Y and Ip C: Doxorubicin
and selenium cooperatively induce Fas signaling in the absence of
Fas/Fas ligand interaction. Anticancer Res. 27:3075–3082. 2007.
|
|
22
|
Drago-Ferrante R, Santulli A, Di Fiore R,
et al: Low doses of paclitaxel potently induce apoptosis in human
retinoblastoma Y79 cells by up-regulating E2F1. Int J Oncol.
33:677–687. 2008.PubMed/NCBI
|
|
23
|
Pires NM, Eefting D, de Vries MR, et al:
Sirolimus and paclitaxel provoke different vascular pathological
responses after local delivery in a murine model for restenosis on
underlying atherosclerotic arteries. Heart. 93:922–927. 2007.
|
|
24
|
Stumm S, Meyer A, Lindner M, et al:
Paclitaxel treatment of breast cancer cell lines modulates Fas/Fas
ligand expression and induces apoptosis which can be inhibited
through the CD40 receptor. Oncology. 66:101–111. 2004.
|
|
25
|
Huisman C, Ferreira CG, Bröker LE, et al:
Paclitaxel triggers cell death primarily via caspase-independent
routes in the non-small cell lung cancer cell line NCI-H460. Clin
Cancer Res. 8:596–606. 2002.
|
|
26
|
Wang X, Gao XH, Li X, et al: Local
hyperthermia induces apoptosis of keratinocytes in both normal skin
and condyloma acuminata via different pathways. Apoptosis.
14:721–728. 2009.
|
|
27
|
Yu DY, Matsuya Y, Zhao QL, et al:
Enhancement of hyperthermia-induced apoptosis by a new synthesized
class of furan-fused tetracyclic compounds. Apoptosis.
12:1523–1532. 2007.
|
|
28
|
Ohguri T, Imada H, Narisada H, et al:
Systemic chemotherapy using paclitaxel and carboplatin plus
regional hyperthermia and hyperbaric oxygen treatment for non-small
cell lung cancer with multiple pulmonary metastases: preliminary
results. Int J Hyperthermia. 25:160–167. 2009.
|
|
29
|
Hulshof MC, Van Haaren PM, Van Lanschot
JJ, et al: Preoperative chemoradiation combined with regional
hyperthermia for patients with resectable esophageal cancer. Int J
Hyperthermia. 25:79–85. 2009.
|