Introduction
Lung cancer is the type of cancer with the highest
morbidity and mortality in the world; its 5-year survival rate is
less than 16% (1). Clinical
studies have revealed that 80% of lung cancer cases are non-small
cell lung cancer (NSCLC) and that the majority of NSCLC patients
are not diagnosed until an advanced stage. Chemotherapy is the main
strategy for the treatment of NSCLC. However, the sensitivity to
chemotherapy of NSCLC patients is extremely poor, and this is one
of the main factors affecting patient survival rate. Therefore, it
is important to study the mechanisms of poor sensitivity to
chemotherapy to improve the survival rate of patients with lung
cancer.
Forkhead box P3 (Foxp3) is a member of the
forkhead/winged-helix transcription factor family and is the key
regulatory gene and specific molecular marker for the development
and function of CD4+CD25+ regulatory T cells
(Tregs) (2,3). The function of Tregs in the tumour
microenvironment is to inhibit the local immune response, which
promotes tumour progression (4,5). In
patients with various types of cancer, increased levels of Tregs
and Foxp3 expression in peripheral blood and tumour specimens have
been associated with poor prognoses (6–9).
Foxp3 has previously been reported to be expressed not only in
Tregs but also in the tumour cells of patients with pancreatic
cancer, melanoma and other types of tumour (10–14).
Some of these studies have shown that the expression of Foxp3
within tumour cells is associated with tumour progression and a
poor prognosis, suggesting that Foxp3 expression within tumour
cells mimics the function of Tregs (12–14).
In addition, certain studies have suggested that Tregs are
resistant to conventional chemotherapy, thus improving tumour
immune evasion (15,16). However, the correlation between the
expression of Foxp3 within tumour cells and sensitivity to
chemotherapy remains unclear.
It was hypothesised that Foxp3 expression within
lung cancer cells is linked to the resistance to chemotherapeutic
agents and the progression of lung cancer. In the current study,
after detecting the expression of Foxp3 in mouse Lewis lung cancer
(LLC) cells, which are NSCLC cells, the role of Foxp3 in the
sensitivity to chemotherapy and the expression of multidrug
resistance protein 1 (mdr1) mRNA and its protein product,
P-glycoprotein (P-gp), was evaluated after transiently transfecting
pcDNA3.1-Foxp3 recombinant plasmids into LLC cells.
Materials and methods
Reagents and antibodies
The rat anti-mouse Foxp3 (FJK-16s) monoclonal
antibody and the goat anti-rat IgG/HRP antibody were purchased from
eBioscience (San Diego, CA, USA). The FuGENE transfection reagent
and mitomycin C (MMC) were purchased from Roche (Mannheim,
Germany). The rabbit anti-mouse P-gp polyclonal antibody was
purchased from Boster Biological Technology Ltd. (Wuhan, China).
The goat anti-rabbit FITC labelled IgG was purchased from Zhongshan
Goldenbridge Biotechnology (Beijing, China). The Ready-to-Use
Immunohistochemistry Hypersensitivity UltraSensitive™ S-P kit was
purchased from Maixin-Bio (Fujian, China). Adriamycin (ADM) was
purchased from Hisun Chemical Co., Ltd. (Zhejiang, China).
Cells and culture conditions
The mouse LLC cell line was obtained from the
Department of Immunology in the Norman Bethune College of Medicine
at Jilin University (Changchun, China) and cultured at 37°C with 5%
CO2 in complete DMEM (Gibco-BRL, Carlsbad, CA, USA)
containing 10% FBS (Gibco-BRL).
Immunocytochemical staining
Cells were grown overnight on coverslips in 6-well
plates to allow for cell attachment. The cells were then washed 3
times with PBS prior to the addition of ice-cold 80% ethanol at 4°C
for 10 min. The cells were subsequently incubated with 5 ml of
perforated liquid (0.1% saponin) for 20 min at room temperature
(RT). The cells were incubated with rat anti-mouse Foxp3 antibody
(dilution 1:100) at 4°C overnight, followed by biotin-labelled goat
anti-rat IgG and streptomycin anti-biotin peroxidase for 10 min at
RT. Diaminobenzidine (DAB) was used as a chromogen. For the
negative control, PBS was used instead of rat anti-mouse Foxp3
antibody.
Transfection of an expression plasmid
encoding for mouse Foxp3
The mouse pcDNA3.1-Foxp3 plasmid was previously
constructed in our laboratory. LLC cells were transfected with the
mouse pcDNA3.1-Foxp3 plasmid or the pcDNA3.1 empty plasmid using
FuGENE (Roche) according to the manufacturer’s instructions. The
expression of Foxp3 was detected by measuring the mRNA and protein
levels after 48 h.
RT-PCR analysis
Total cellular RNA was extracted with RNAiso™ Plus
(Takara Bio, Inc., Shiga, Japan) according to the manufacturer’s
instructions. For reverse transcription, cDNA was generated using 1
μg of total RNA, 2 μl of oligo(dT) primers (Takara Bio, Inc.), 1 μl
of reverse transcriptase (M-MLV, Takara Bio, Inc.) and 1 μl of
dNTPs (10 mM) in a total volume of 20 μl. For the PCR reactions,
the primer sequences were as follows: Foxp3 sense,
5′-CAGCTGCCTACAGTGCCCCTAG-3′ and antisense,
5′-CATTTGCCAGCAGTGGGTAG-3′ (17);
mdr1 sense, 5′-GGCATTGCCTACCTGTTGG-3′ and antisense,
5′-GCTTTCTGTGGACACTTCTG-3′ (18);
and β-actin sense, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and antisense,
5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (19). For Foxp3, the reaction conditions
were as follows: 94°C for 30 sec, 60°C for 30 sec and 72°C for 60
sec for 35 cycles. For mdr1, the reaction conditions were as
follows: 94°C for 30 sec, 55°C for 30 sec and 72°C for 90 sec for
35 cycles. For β-actin, the reaction conditions were as follows:
94°C for 30 sec, 52°C for 30 sec and 72°C for 60 sec for 25 cycles.
All reactions were followed by an elongation step of 10 min at
72°C. RT-PCR products were analysed by 1% agarose gel
electrophoresis and stained by ethidium bromide. The ratio of Foxp3
or mdr1 to β-actin was calculated as the relative level of mRNA
expression.
Western blot analysis
Nuclear proteins from LLC cells were extracted as
described previously (20).
Western blot analysis was performed as follows: 30–50 μg of total
protein was separated by 10% SDS-polyacrylamide gel electrophoresis
and then transferred onto PVDF membranes. The membranes were
blocked with 5% non-fat dry milk in phosphate-buffered saline plus
0.03% Tween-20 (PBST) at RT for 2 h. Immunoblotting was performed
using the rat anti-mouse Foxp3 monoclonal antibody (dilution,
1:200) in non-fat milk Tris-buffer at 4°C overnight. The membrane
was then incubated with the goat anti-rat IgG/HRP (dilution,
1:2,000) for 2 h at RT. The rabbit anti-mouse β-actin antibody was
used as an internal control. Protein expression was detected using
BeyoECL Plus (Beyotime Biotech., Jiangsu, China).
Cell proliferation assay
To assess the chemosensitivity of LLC cells to
various chemoreagents, the inhibitory rate of cell proliferation
was determined by the MTT assay. pcDNA3.1-Foxp3-LLC, pcDNA3.1-LLC
and LLC cells were plated in 96-well plates (1×104
cells/well) and cultured with 10% FBS at 37°C and 5% CO2
overnight to allow cell attachment. The cells were then incubated
with fresh DMEM containing various concentrations (0, 5, 10, 20 or
40 μg/ml) of ADM or MMC. Following 48 h of treatment, 20 μl/well of
MTT was added to the cells to reach a concentration of 0.5 mg/ml.
Following 4 h of reaction time, the supernatant was discarded and
200 μl of DMSO (Gibco-BRL) was added. The optical density (OD) at
570 nm of each well was measured with the enzyme immunoassay
instrument (Bio-Rad 2550, Bio-Rad, Hercules, CA, USA). Triplicate
wells were used in each group. The IC50 was defined as
the concentration of drug eliciting 50% cell death. Inhibitory rate
(%) = [(OD of control group - OD of treated group)/OD of control
group] ×100.
Flow cytometry for the expression of
P-gp
Cells (1,000,000) were collected and washed with PBS
and then fixed with 4% paraformaldehyde (1 ml/tube) for 20 min at
4°C. The cells were centrifuged at 1,500 rpm for 5 min and then
incubated with 100 μl of rabbit anti-mouse P-gp polyclonal antibody
(dilution, 1:100) for 40 min at 4°C. Following the incubation, the
cells were washed with PBS and incubated with 100 μl of
FITC-conjugated goat anti-rabbit IgG (dilution, 1:100) for 30 min
at 4°C in the dark. Following the incubation, the cells were washed
with PBS and fixed in 4% paraformaldehyde (0.5 ml/tube) prior to
detection by flow cytometry.
Statistical analysis
Statistical analysis was conducted using SPSS 12.0
software. All results are presented as the mean ± SD. The Student’s
t-test was used for statistical analysis. P<0.05 was considered
the threshold for statistical significance.
Results
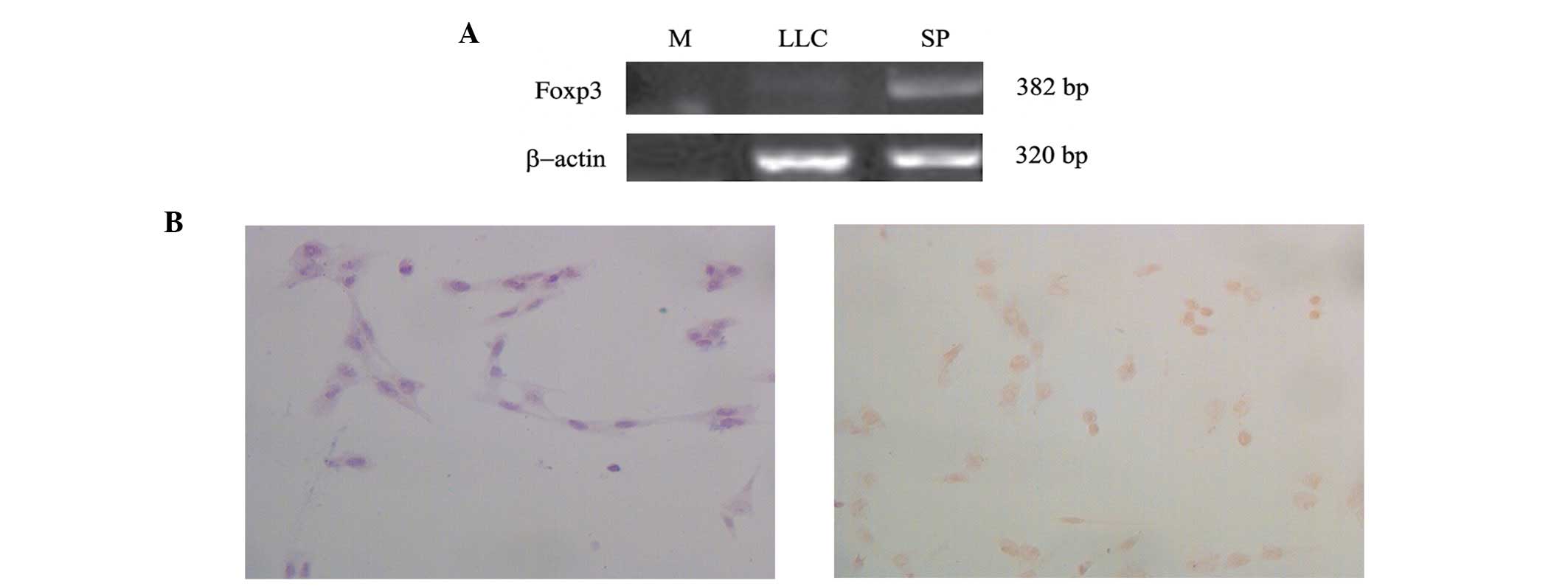
Foxp3 expression in LLC cells
The expression of Foxp3 mRNA was examined in mouse
LLC cell lines by RT-PCR. The results revealed that Foxp3 was
expressed in LLC cells (Fig. 1A).
The protein expression of Foxp3 was also confirmed by
immunocytochemistry. Foxp3 protein was localised in the nucleus of
LLC cells, as shown in Fig.
1B.
Establishment of Foxp3-overexpressing LLC
cells
pcDNA3.1-Foxp3 recombinant or pcDNA3.1 empty
plasmids were transiently transfected into LLC cells. The
overexpression of Foxp3 in pcDNA3.1-Foxp3-LLC was confirmed by
RT-PCR and western blot analysis. The results revealed that the
expression of Foxp3 mRNA in the pcDNA3.1-Foxp3-LLC group was
significantly increased compared with that of the LLC and
pcDNA3.1-LLC groups (P<0.01; Fig.
2A and B). Western blot analysis also confirmed that the
protein level of Foxp3 was significantly increased in the
pcDNA3.1-Foxp3-LLC group compared with that of the LLC and
pcDNA3.1-LLC groups (P<0.01; Fig.
2C and D).
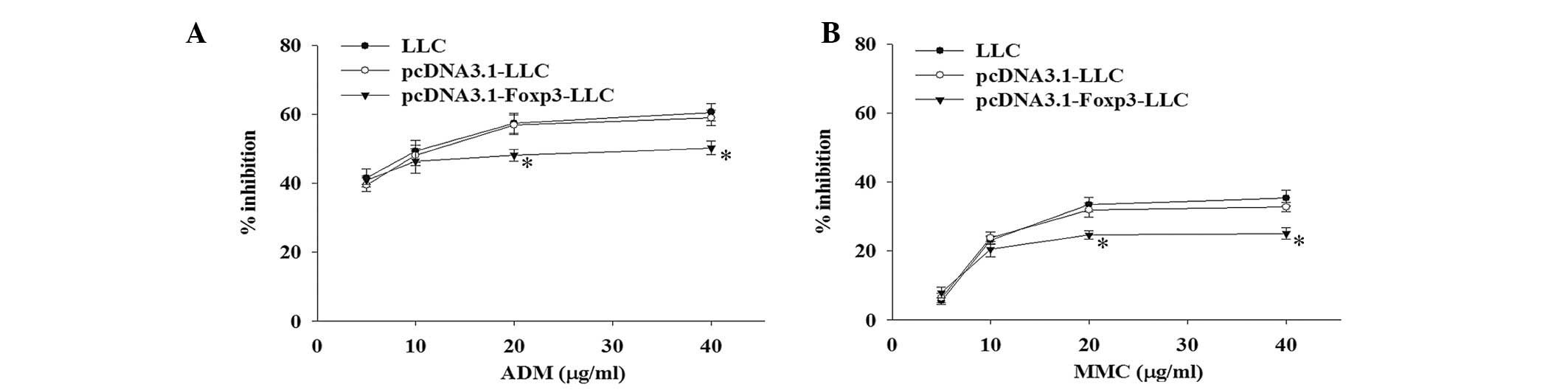
Sensitivity to ADM and MMC chemotherapy
in Foxp3-overexpressing LLC cells
To determine the effects of Foxp3 overexpression in
LLC on sensitivity to chemotherapeutic agents, pcDNA3.1-Foxp3-LLC,
pcDNA3.1-LLC and LLC cells were treated with various concentrations
of ADM and MMC. The inhibitory rate of cell proliferation was
measured by the MTT assay at 48 h. As shown in Fig. 3A, the inhibitory rate of cell
proliferation in the pcDNA3.1-Foxp3-LLC group was significantly
lower than that of the pcDNA3.1-LLC and LLC groups following
treatment with 20 and 40 μg/ml of ADM (P<0.05). The
IC50 of ADM for the pcDNA3.1-Foxp3-LLC group (32.78
μg/ml) was higher than that of the pcDNA3.1-LLC group (12.97 μg/ml)
and the LLC group (11.27 μg/ml). Similarly, after treatment with
MMC at the concentrations of 20 and 40 μg/ml, the
pcDNA3.1-Foxp3-LLC group also exhibited a significantly lower
growth inhibitory rate compared with the pcDNA3.1-LLC and LLC
groups (P<0.05; Fig. 3B). The
IC50 of MMC for the pcDNA3.1-Foxp3-LLC group (162.1
μg/ml) was markedly higher than that of the pcDNA3.1-LLC group
(63.7 μg/ml) and the LLC group (50.8 μg/ml).
Expression of mdr1 mRNA and P-gp protein
in Foxp3 overexpressing LLC cells
To investigate the mechanism of the Foxp3-induced
reduction in sensitivity to chemotherapy in LLC cells, RT-PCR was
first used to examine whether Foxp3 expression was associated with
mdr1 gene expression. The results revealed that the expression of
mdr1 mRNA was significantly increased in the pcDNA3.1-Foxp3-LLC
group but not in the pcDNA3.1-LLC and LLC groups (P<0.05;
Fig. 4A and B). To further confirm
the upregulation of mdr1 by Foxp3, the expression of P-gp, the
protein product of the mdr1 gene, was examined by flow cytometry.
Similarly, the expression of P-gp was also significantly increased
in the pcDNA3.1-Foxp3-LLC group compared with the pcDNA3.1-LLC and
LLC groups (P<0.05; Fig. 4C and
D).
Discussion
Chemotherapy is the main strategy used in the
clinical treatment of patients with NSCLC. However, patients with
NSCLC are often resistant to the chemotherapeutic agents. This
sensitivity to chemotherapy is the most significant factor
affecting the survival rate of patients with NSCLC.
Foxp3 has been identified as the master regulator of
the development and function of Tregs (2,3).
Tregs have been reported to be associated with tumourigenesis and
poor prognosis in various types of cancer (6–9).
Previous studies have demonstrated the expression of Foxp3 not only
in Tregs but also in tumour cells from patients with pancreatic and
breast cancer and other types of tumour (10–14).
Some of these studies have shown that the expression of Foxp3
within tumour cells is associated with tumour progression and poor
prognosis. Hinz et al indicated that Foxp3 was expressed in
the nucleus of pancreatic carcinoma cells and that it shared
similar growth-suppressive effects with Tregs (10). Merlo et al indicated that
Foxp3 expression in primary breast carcinoma tumour cells was
associated with a risk of poor overall survival rate and that this
risk was correlated with the increased intensity of Foxp3
immunostaining (12). These
studies suggest that Foxp3 expression within tumour cells mimics
the function of Tregs. The present study also demonstrates the
expression of Foxp3 in LLC cells at the genetic and protein levels.
Similar to Tregs, Foxp3 protein is located in the nucleus of LLC
cells.
In addition, certain studies have revealed that
Tregs may be resistant to conventional chemotherapy, thus improving
tumour immune evasion (15,16).
Szczepanski et al reported that acute myelogenous leukaemia
patients with lower circulating Treg frequencies have an improved
response to the induction of chemotherapy. During the
post-induction period however, the Treg frequency and suppressive
activity remain elevated, even in complete remission, suggesting
that Tregs are resistant to conventional chemotherapy (15). In addition, it has been reported
that advanced stage gastrointestinal cancer patients present with
increased levels of Tregs 3 weeks after chemotherapy, which
correlated with a poor prognosis (16). However, the correlation between
Foxp3 within tumour cells and sensitivity to chemotherapy remains
unclear.
ADM and MMC are conventional chemotherapeutic agents
that are used for the treatment of NSCLC. The current study used
mouse LLC cells to investigate whether Foxp3 is involved in the
resistance to chemotherapy. The results demonstrated that the
inhibitory rate of cell proliferation in Foxp3-overexpressing cells
was significantly reduced following treatment with ADM and MMC,
suggesting that Foxp3-overexpressing LLC cells are resistant to
cell death by ADM and MMC. In general, patients exhibit lower
chemosensitivity to chemotherapeutic agents with higher
IC50 values. In the current study, the IC50
values of ADM and MMC for Foxp3-overexpressing LLC cells were
higher than those in the empty plasmid-transfected and
untransfected LLC cells, indicating that Foxp3-overexpressing LLC
cells are less sensitive to ADM and MMC. The results of this study
suggest that Foxp3 expression in LLC cells reduces the sensitivity
of LLC cells to ADM and MMC, resulting in reduced tumour cell death
by ADM and MMC.
Multidrug resistance, particularly when mediated by
P-gp, is the main cause of reduced chemosensitivity, which is a
major factor in the failure of chemotherapy (21,22).
P-gp, encoded by the mdr1 gene, is a 170-kDa transmembrane
glycoprotein and is an energy-dependent drug pump of ATP-binding
cassette (ABC) transporters. P-gp pumps intracellular drugs outside
the cell by ATP-dependent conformational changes, thus reducing the
concentration of drugs within tumour cells (23,24).
In tumour patients, P-gp effluxes natural hydrophobic anticancer
drugs, including alkaloids (vinblastine, vincristine), antitumour
antibiotics (ADM and actinomycin D), paclitaxel and the alkylating
agent, MMC (25–27). We found that ADM and MMC are
substrates of P-gp and therefore the expression levels of mdr1 mRNA
and P-gp protein were measured. The results demonstrated that mdr1
mRNA and P-gp protein were upregulated in Foxp3-overexpressing LLC
cells, suggesting that Foxp3 upregulates the expression of P-gp,
resulting in a lower sensitivity of the LLC cells to ADM and
MMC.
This study demonstrates that Foxp3 may reduce the
sensitivity of LLC cells to ADM and MMC by upregulating the
expression of P-gp. Foxp3 may be the significant factor responsible
for the insensitivity to chemotherapy in LLC cells. These results
may provide a new mechanism of resistance to chemotherapy for
NSCLC.
Acknowledgements
This study was supported by grants from the
Department of Immunology in the Norman Bethune College of Medicine
at Jilin University.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008.
|
|
2
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fontenot JD, Rasmussen JP, Williams LM,
Dooley JL, Farr AG and Rudensky AY: Regulatory T cell lineage
specification by the forkhead transcription factor Foxp3. Immunity.
22:329–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woo EY, Yeh H, Chu CS, Schlienger K,
Carroll RG, Riley JL, Kaiser LR and June CH: Cutting edge:
regulatory T cells from lung patients directly inhibit autologous T
cell proliferation. J Immunol. 168:4272–4276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schneider T, Kimpfler S, Warth A, Schnabel
PA, Dienemann H, Schadendorf D, Hoffmann H and Umansky V:
Foxp3+ regulatory T cells and killer cells distinctly
infiltrate primary tumors and draining lymph nodes in pulmonary
adenocarcinoma. J Thorac Oncol. 6:432–438. 2011.
|
|
6
|
Shimizu K, Nakata M, Hirami Y, Yukawa T,
Maeda A and Tanemoto K: Tumor-infiltrating Foxp3+
regulatory T cells are correlated with cyclooxygenase-2 expression
and are associated with recurrence in resected non-small cell lung
cancer. J Thorac Oncol. 5:585–590. 2010.
|
|
7
|
Tokuno K, Hazama S, Yoshino S, Yoshida S
and Oka M: Increased prevalence of regulatory T-cells in the
peripheral blood of patients with gastrointestinal cancer.
Anticancer Res. 29:1527–1532. 2009.PubMed/NCBI
|
|
8
|
Deng L, Zhang H, Luan Y, Zhang J, Xing Q,
Dong S, Wu X, Liu M and Wang S: Accumulation of foxp3+ T
regulatory cells in draining lymph nodes correlates with disease
progression and immune suppression in colorectal cancer patients.
Clin Cancer Res. 16:4105–4112. 2010.
|
|
9
|
Petersen RP, Campa MJ, Sperlazza J, et al:
Tumor infiltrating Foxp3+ regulatory T-cells are
associated with recurrence in pathologic stage I NSCLC patients.
Cancer. 107:2866–2872. 2006.PubMed/NCBI
|
|
10
|
Hinz S, Pagerols-Raluy L, Oberg HH, et al:
Foxp3 expression in pancreatic carcinoma cells as a novel mechanism
of immune evasion in cancer. Cancer Res. 67:8344–8350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuo T, Liu R, Zhang H, Chang X and Liu Y,
Wang L, Zheng P and Liu Y: FOXP3 is a novel transcriptional
repressor for the breast cancer oncogene SKP2. J Clin Invest.
117:3765–3773. 2007.PubMed/NCBI
|
|
12
|
Merlo A, Casalini P, Carcangiu ML,
Malventano C, Triulzi T, Mènard S, Tagliabue E and Balsari A: FOXP3
expression and overall survival in breast cancer. J Clin Oncol.
27:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LH, Su L and Wang JT: Correlation
between elevated FOXP3 expression and increased lymph node
metastasis of gastric cancer. Chin Med J (Engl). 123:3545–3549.
2010.PubMed/NCBI
|
|
14
|
Wang G, Liu G, Liu Y, Li X and Su Z: FOXP3
expression in esophageal cancer cells is associated with poor
prognosis in esophageal cancer. Hepatogastroenterology. 59:Mar
2–2012.(Epub ahead of print). View
Article : Google Scholar
|
|
15
|
Szczepanski MJ, Szajnik M, Czystowska M,
et al: Increased frequency and suppression by regulatory T cells in
patients with acute myelogenous leukemia. Clin Cancer Res.
15:3325–3332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu H, Mao Y, Dai Y, Wang Q and Zhang X:
CD4+CD25+ regulatory T cells in patients with
advanced gastrointestinal cancer treated with chemotherapy.
Onkologie. 32:246–252. 2009.
|
|
17
|
Ruan Q, Kameswaran V, Tone Y, Li L, Liou
HC, Greene MI, Tone M and Chen YH: Development of Foxp3+
regulatory T cells is driven by A c-Rel enhanceosome. Immunity.
31:932–940. 2009.PubMed/NCBI
|
|
18
|
Melaine N, Liénard MO, Dorval I, Le
Goascogne C, Lejeune H and Jégou B: Multidrug resistance genes and
p-glycoprotein in the testis of the rat, mouse, Guinea pig, and
human. Biol Reprod. 67:1699–1707. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simeone L, Straubinger M, Khan MA,
Nalleweg N, Cheusova T and Hashemolhosseini S: Identification of
Erbin interlinking MuSK and ErbB2 and its impact on acetylcholine
receptor aggregation at the neuromuscular junction. J Neurosci.
30:6620–6634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shim GS, Manandhar S, Shin DH, Kim TH and
Kwak MK: Acquisition of doxorubicin resistance in ovarian carcinoma
cells accompanies activation of the NRF2 pathway. Free Radic Biol
Med. 47:1619–1631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Widmer N, Colombo S, Buclin T and
Decosterd LA: Functional consequence of MDR1 expression on imatinib
intracellular concentrations. Blood. 102:11422003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aller SG, Yu J, Ward A, et al: Structure
of P-glycoprotein reveals a molecular basis for poly-specific drug
binding. Science. 323:1718–1722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loo TW and Clarke DM: Location of the
rhodamine-binding site in the human multidrug resistance
P-glycoprotein. J Biol Chem. 277:44332–44338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ambudkar SV, Kim IW and Sauna ZE: The
power of the pump: mechanisms of action of P-glycoprotein (ABCB1).
Eur J Pharm Sci. 27:392–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szachowicz-Petelska B, Figaszewski Z and
Lewandowski W: Mechanisms of transport across cell membranes of
complexes contained in antitumour drugs. Int J Pharm. 222:169–182.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pires MM, Emmert D, Hrycyna CA and
Chmielewski J: Inhibition of P-glycoprotein-mediated paclitaxel
resistance by reversibly linked quinine homodimers. Mol Pharmacol.
75:92–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Enokida H, Gotanda T, Oku S, et al:
Reversal of P-glycoprotein mediated paclitaxel resistance by new
synthetic isoprenoids in human bladder cancer cell line. Jpn J
Cancer Res. 93:1037–1046. 2002. View Article : Google Scholar : PubMed/NCBI
|