Introduction
Liver fibrosis is a common consequence of chronic
liver injury that is induced by a variety of etiological factors
that lead to liver cirrhosis. This progressive pathological process
is characterized by the accumulation of extracellular matrix (ECM)
proteins (1–3). The activation and proliferation of
hepatic stellate cells (HSCs) has been shown to promote the
excessive production and secretion of ECM and therefore to play a
central role in liver fibrogenesis (4). Moreover, the activated HSCs are
eliminated mainly through apoptosis, since it is difficult for them
to return to quiescence (5,6),
making them an appealing target for the treatment of liver
fibrosis.
Increased attention has been paid to the
antifibrotic activity of natural herbs, in particular Salvia
miltiorrhiza, a traditional Chinese herbal medicine that has a
variety of pharmacological effects, including anti-inflammatory,
oxygen free radical-removing and antioxidant activities (7,8). In
order to explore the antifibrotic mechanism of Salvia
miltiorrhiza, the monomer IH764-3, a major potent component
extracted from Salvia miltiorrhiza, was investigated. In our
previous study, we demonstrated that the monomer IH764-3 inhibited
proliferation and induced apoptosis in HSCs stimulated by
H2O2 in vitro (9–11).
Moreover, it increased the ratio of matrix metalloproteinase
(MMP)-13 to tissue inhibitor of MMP (TIMP)-1 in HSCs by
downregulating the expression of focal adhesion kinase (FAK) and
extracellular signal-regulated kinase (ERK) in vitro
(12). However, there is no direct
evidence that the monomer IH764-3 has a potential role in the
treatment of liver fibrosis.
The purpose of the present study was to investigate
the impact of the monomer IH764-3 on the proliferation and
apoptosis of HSCs in a rat model of liver fibrosis induced by bile
duct ligation (BDL) in vivo.
Materials and methods
Reagents
The Salvia miltiorrhiza monomer IH764-3 was
donated by Professor Yang Chunzheng from the Hematology Institute
of the Chinese Academy of Medical Sciences. The mouse anti-α-smooth
muscle actin (α-SMA) monoclonal antibody, rabbit anti-FAK and p-FAK
(Tyr397) polyclonal antibodies and rabbit anti-ERK and mouse
anti-p-ERK monoclonal antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The streptavidin
peroxidase (SP) immunohistochemical kit was acquired from Zhongshan
Biotechnology Corporation (Beijing, China). The terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
detection kit was purchased from Boster Biotechnology, Inc. (Wuhan,
China). The reverse transcription-polymerase chain reaction
(RT-PCR) amplification system was purchased from Promega
Biotechnology, Inc. (Madison, WI, USA).
Animal models
A total of 40 adult male Sprague-Dawley rats
weighing 350–450 g were obtained from the Experimental Animal
Center of Hebei Medical University (Certificate No. 04037). The
rats were randomly divided into three groups: a control group
(n=10), a model group (n=15) and a IH764-3 group (n=15). All rats
were housed in plastic cages with free access to clean-grade food
and water and received human care. The experiments were performed
in compliance with the national ethical guidelines for the care and
use of laboratory animals.
A rat model of hepatic fibrosis was established by
common BDL as previously described (13). All rats undergoing laparotomy were
injected intraperitoneally with 100 ml/kg ketamine hydrochloride
and chloral hydrate to induce deep anesthesia. In the model and
IH764-3 groups, the peritoneal cavity was opened and the common
bile duct was completely ligated (double-ligated) with 3-0 silk and
cut between the ligatures. In the control group, animals underwent
a sham surgery that consisted of exposure but no ligation of the
common bile duct. All surgeries were performed under aseptic
conditions. The monomer IH764-3 was diluted to 8 g/l with saline.
On the day of the surgery, the rats in the IH764-3 group were
injected intraperitoneally with IH764-3 (40 mg/kg/day) and the rats
in the control and model groups were administered normal saline.
Post-operatively, at 14 days, all animals were anesthetized,
sacrificed and their livers harvested. The liver tissue specimens
were fixed in 4% phosphate-buffered paraformaldehyde (Shijiazhuang
Huarui Scientific and Technological Co., Ltd., Shijiazhuang, China)
and stained with hematoxylin and eosin (H&E), Masson’s
trichrome (MT) and immunohistochemical staining. Other specimens
were snap-frozen in liquid nitrogen and stored at −80°C for the
analysis of mRNA and proteins.
Histopathology
The liver specimens were fixed for 12–24 h in 4%
phosphate-buffered paraformaldehyde and then embedded in paraffin
for light microscopic examination. Tissue sections (5 μm thick)
were stained with H&E for morphological evaluation and MT
staining for assessment of the degree of fibrosis. The collagen
expression levels were measured using a Motic Med 6.0 digital
video-image analysis system (Motic China Group Co., Ltd, Xiamen,
China) and are expressed as optical density values.
Immunohistochemical detection of
α-SMA
All immunohistochemical studies were performed on
5-μm thick, paraformaldehyde-fixed, paraffin-embedded liver tissue
sections using the SP technique. The sections were deparaffinized
in xylene, rehydrated in graded ethanol and rinsed three times
(5-min washes) with 0.01 M phosphate-buffered saline (PBS).
Endogenous peroxidase activity was quenched with 3% hydrogen
peroxide in methanol for 30 min at room temperature. All subsequent
washings of the sections were with PBS (three changes, 5 min each).
Following antigen retrieval using 0.1 M citrate buffer (pH 6.0) in
a microwave oven at 98°C (20 min), the sections were immediately
cooled to room temperature and then blocked with 10% goat serum at
37°C for 30 min. Superfluous goat serum was blotted with a piece of
filter paper and the sections were then incubated overnight at 4°C
with the primary antibody (mouse anti-α-SMA monoclonal antibody) at
a dilution of 1:100. After washing the sections, the
biotin-conjugated rabbit anti-mouse secondary antibody (1:100
dilution) was added and the sections were incubated at 37°C for 30
min. Sections were washed and then incubated with
streptavidin-peroxidase complex (l:200 dilution) at 37°C for 30
min. Following another wash, diaminobenzidine solution was used as
a substrate for peroxidase, yielding a brown-colored positive
reaction. The negative control samples were processed under the
same conditions, except that normal mouse serum (1:100 dilution)
was used in place of the primary antibody. The α-SMA-positive
expression levels were measured using a Motic Med 6.0 digital
video-image analysis system and are expressed as optical density
values.
TUNEL and α-SMA immunohistochemical
double staining
The sections were deparaffinized in xylene,
rehydrated in graded ethanol and rinsed three times (5-min washes)
with 0.01 M PBS. The sections were then incubated with 3% acetic
acid (pH 2.5) for 10 min at room temperature, rinsed three times
(2-min washes) with distilled water (DW) and digested with protease
K at 37°C for 10 min. The sections were rinsed three times (2-min
washes) in triethanolamine-buffered saline (TBS) and, following the
addition of the TUNEL reaction mixture, were incubated overnight at
4°C. The sections were then washed and blocked with confining
liquid at 37°C for 30 min. The biotin-conjugated anti-digoxin
antibody (Digibind; 1:100 dilution) was added and the sections were
incubated at 37°C for 30 min. The sections were washed and then
incubated with SABC-AP (l:100 dilution) at 37°C for 30 min.
Following another wash, the sections were stained with BCIP/NBT.
The immunohistochemical staining of α-SMA was then detected
according to the preceding method. The negative control samples
were processed under the same conditions, with the exception of the
terminal deoxynucleotidyl transferase labeling. The nuclei of the
apoptotic HSCs were stained blue-black by BCIP/NBT in the TUNEL
assay and the cytoplasms of the activated HSCs were stained brown
by DAB in the α-SMA immunohistochemical staining assay. Thus, the
cells with brown cytoplasms and blue-black nuclei were apoptotic
activated HSCs. The apoptotic rate was determined from the ratio of
the area of apoptotic activated HSCs to the total area of activated
HSCs.
Preparation of hepatic tissue protein and
western blots
Approximately 100 mg rat liver tissue was collected,
rinsed twice with ice-cold PBS, homogenized and transferred into an
Eppendorf tube. Cytoplasmic proteins were then extracted using
modified RIPA lysis buffer [50 mmol/l Tris-HCl (pH 7.5), 100 mmol/l
NaCl, 1% NP-40, 0.5% sodium deoxycholate, 2 μg/ml Leupeptin, 1%
SDS, 2 mmol/l EDTA, 1 mmol/l PMSF, 50 mmol/l HEPES and 100 mmol/l
sodium orthovanadate]. The protein concentrations were determined
by Coomassie Brilliant Blue staining. The proteins, fractionated by
8 or 10% SDS-PAGE, were transferred to a nitrocellulose filter
membrane. After blocking, the membrane was incubated with rabbit
anti-FAK polyclonal antibody (1:500), rabbit anti-p-FAK (Tyr397)
polyclonal antibody (1:150), rabbit anti-ERK polyclonal antibody
(1:500), mouse anti-p-ERK monoclonal antibody (1:200) and goat
anti-β-actin polyclonal antibody (1:300) overnight at 4°C. The
membranes were incubated at room temperature for 2 h with
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (1:5000), goat anti-mouse IgG antibody (1:3000) and rabbit
anti-goat IgG antibody (1:5000). The protein bands were
subsequently detected by enhanced chemiluminescence (ECL). The
images were procured using Kodak ID Image Analysis software. The
results are presented as the ratio of the integral optical density
(IOD) of the target protein to that of β-actin.
RT-PCR
The total RNA of the hepatic tissue was extracted
with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer’s instructions and then reverse transcribed
with the oligo-dT primer. Primer express 5.0 was used to design the
following primers: FAK forward primer, 5′-ACT TGG ACG CTG TAT TGG
AG-3′ and reverse, 5′-CTG TTG CCT GTC TTC TGG AT-3′ (833 bp
amplicon); ERK forward primer, 5′-GCT GAC CCT GAG CAC GAC CA-3′ and
reverse, 5′-CTG GTT CAT CTG TCG GAT CA-3′ (451 bp amplicon); and
β-actin forward primer, 5′-AGC TGA GAG GGA AAT CGT GCG-3′ and
reverse, 5′-GTG CCA CCA GAC AGC ACT GTG-3′ (300 bp amplicon). The
primers were synthesized by Saibaisheng Gene Co., Ltd. (Beijing,
China).
The RT-PCR was performed using 2 μg total RNA, 1 μl
upstream primers (10 pmol/μl), 1 μl downstream primers (10
pmol/μl), 1 μl dNTP, 10 μl 5X reaction buffer AMV/Tfl, 1 μl AMV
reverse transcriptase (5 U/μl), 1 μl RNA Tfl DNA polymerase and 2
μg DNA template made up to a total volume of 50 μl with DEPC water
(Kangwei Corporation, Beijing, China). RT was carried out at 41°C
for 45 min. The PCR conditions were as follows: initial
pre-denaturation at 94°C for 2 min, denaturation at 94°C for 40
sec, annealing at 52°C for 1 min, polymerization at 72°C for 1.5
min (35 cycles) and terminal extension at 72°C for 10 min. The
products were resolved using 1.5% agarose gels containing 0.5 mg/ml
ethidium bromide. The optical density was assayed using a gel image
analysis system. The ratio of target gene to β-actin gene in each
group was semi-quantitatively determined.
Statistical treatment
Measurement data are expressed as the mean ±
standard deviation (mean ± SD). The apoptotic rate is expressed as
a percentage. Analysis was carried out using SPSS 18.0 software
(SPSS, Inc., Chicago, IL, USA). The comparison of mean variability
among all groups was conducted by one-way ANOVA and two group
comparisons were made using the LSD test. P<0.05 was considered
to indicate a statistically significant result.
Results
Established BDL-induced liver fibrosis
model
The rats actively recovered from 1 to 2 h following
BDL. The rats had light yellow urine the next day and had developed
yellow coloration in the thinner parts of the skin, including the
ear, and dark yellow urine on days 5–7 after the surgery.
Throughout the experiment, 4 rats died in the model group and 2
rats died of internal hemorrhage caused by intraperitoneal
injection of the drug in the IH764-3 group.
IH764-3 ameliorated BDL-induced liver
fibrosis
H&E and Masson’s trichrome staining revealed a
histologically normal phenotype in the control group. These
sections appeared to have structurally intact hepatic lobules, an
orderly arrangement of hepatic plates, no hepatic cell swelling, no
proliferation of the bile duct and little connective tissue in the
portal area.
In the model group, the liver sections revealed that
the normal arrangement of hepatic plates had disappeared, the
structure of the lobules was disordered and the small bile ducts of
the portal area had invasively proliferated into the lobules.
Further, in addition to an enlarged portal area, there was marked
fibroplasia around the infiltrating small bile ducts and in the
hepatic lobules. MT staining demonstrated that the collagen area
density in the model group was significantly higher than in the
control group (16.72±1.15 vs. 3.47±0.23%, P<0.01) and in the
IH764-3 group, although there were no marked changes in the
structure of the hepatic lobules and the small bile ducts still
proliferated, the collagen fibers in the portal area slightly
increased in number. The collagen area density in the IH764-3 group
was also markedly decreased compared with the model group
(12.60±0.95 vs. 16.72±1.15%, P<0.01).
In summary, the swelling of hepatic cells, fatty
degeneration, necrosis, regeneration and fibrosis were observed in
the BDL-induced rat model and treatment with IH764-3 substantially
decreased the extent of liver fibrosis (Fig. 1).
IH764-3 inhibited the proliferation of
HSCs
Given that α-SMA is an activated HSC marker,
immunohistochemical staining of α-SMA was used to quantify the
activation and proliferation of HSCs. In the liver tissues of the
rats in the control group, α-SMA was occasionally detected in
vascular smooth muscle cells and its expression levels were low. In
the model group, with the development of liver fibrosis, the
α-SMA-positive cells in the liver tissue increased significantly
and were distributed mainly in the portal area, fibrous septa,
hepatic sinusoids and proliferated peripheral cells of the bile
duct. In the IH764-3 group, the positive expression of α-SMA in the
liver tissues of the rats was clearly decreased, particularly in
the portal area and hepatic sinusoids.
The immunohistochemical results indicate that the
area of α-SMA-positive cells in the rat liver tissues of the model
group was significantly increased compared with the control group
(22.65±2.16 vs. 5.88±1.46%, P<0.01) and markedly reduced in the
IH764-3 group compared with the model group (12.92±2.45 vs.
22.65±2.16%, P<0.01), but remained higher than in the control
group (P<0.01, Fig. 2).
IH764-3 induced the apoptosis of
activated HSCs
HSC apoptosis was detected by TUNEL and α-SMA
immunohistochemical double staining. The nuclei of the apoptotic
HSCs were colored blue-black by BCIP/NBT in the TUNEL assay, and
the cytoplasms of the activated HSCs were colored brown by the
immunohistochemical staining of α-SMA. Therefore, the cells with
brown cytoplasms and blue-black nuclei were apoptotic activated
HSCs.
In the control group few apoptotic HSCs were
detected in the rat liver tissue, and in the model group, the areas
of apoptotic HSCs and activated cells increased, indicating that
activation and apoptosis were concurrent in the HSCs. In the
IH764-3 group, the apoptotic activated HSCs increased significantly
compared with the model group. These activated HSCs were
distributed mainly in the portal area, hepatic sinusoids and
proliferated peripheral cells of the bile duct. The apoptotic rates
of activated HSCs in the rat liver tissues in the control, model
and IH764-3 groups were 0.01±0.02, 4.72±0.37 and 34.8±4.5%,
respectively (P<0.01; Fig.
3).
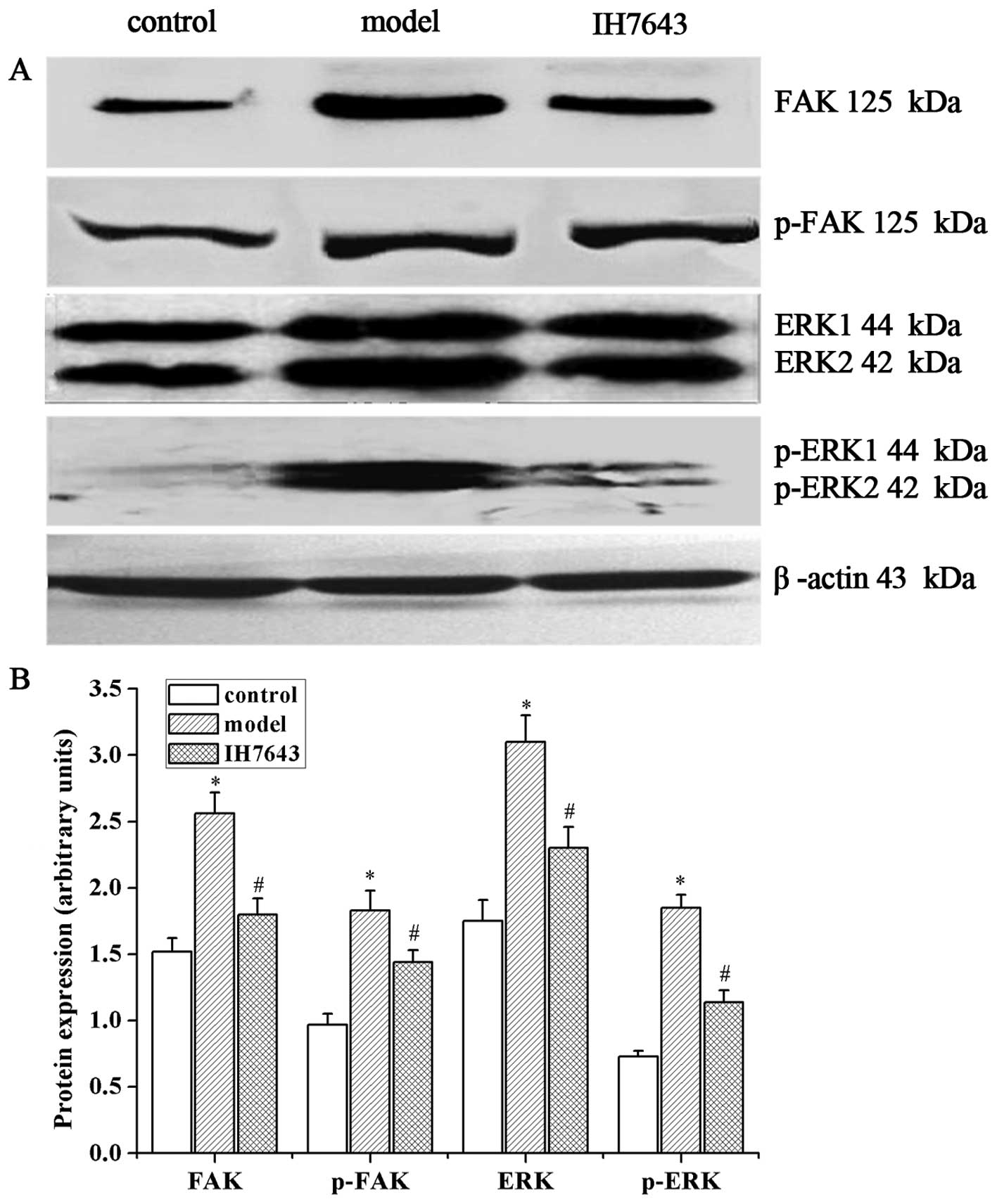
IH764-3 inhibited the expression of FAK
and p-FAK (Tyr397) proteins and FAK mRNA in rat liver tissue
Our previous study has shown that FAK regulates HSC
proliferation, and that its expression is increased during
progressive liver fibrosis in BDL-treated rats (14,15).
Therefore, to further explore the mechanisms by which the monomer
IH764-3 affects HSC apoptosis, we measured the levels of FAK and
p-FAK (Tyr397).
Western blotting revealed that the protein
expression levels of FAK and p-FAK (Tyr397) in the model group were
higher than in the control group (2.56±0.16 vs. 1.52±0.10 and
1.83±0.15 vs. 0.97±0.10, P<0.01) and were downregulated in the
IH764-3 group compared with the model group (1.80±0.12 vs.
2.56±0.16 and 1.44±0.99 vs. 1.83±0.15, P<0.01, Fig. 4). RT-PCR similarly demonstrated a
significant increase in FAK mRNA in the model group compared with
the control group (0.83±0.06 vs. 0.46±0.03, P<0.01), and a
marked reduction in the IH764-3 group compared with the model group
(0.69±0.04 vs. 0.83±0.06, P<0.01; Fig. 5A and C).
IH764-3 inhibited the expression of ERK
and p-ERK proteins and ERK mRNA in rat liver tissue
As ERK is the key downstream signaling molecule of
FAK, we determined the expression levels of ERK and p-ERK in our
study. The expression levels of ERK and p-ERK proteins were
significantly increased in the model group compared with the
control group (3.10±0.20 vs. 1.75±0.16 and 1.85±0.10 vs. 0.73±0.04,
P<0.01). The treatment of rats with IH764-3 significantly
decreased the levels of ERK and p-ERK in liver tissue (2.30±0.16
vs. 3.10±0.20 and 1.14±0.09 vs. 1.85±0.10, P<0.01, Fig. 4) compared with the model group.
Furthermore, the mRNA levels of ERK in the model group were higher
than in the control group (1.86±0.15 vs. 0.92±0.08, P<0.01), and
treatment with IH764-3 reduced the mRNA level of ERK in the rat
liver tissues (1.08±0.09 vs. 1.86±0.15, P<0.01, Fig. 5B and C).
Discussion
Liver fibrosis is considered to be reversible
through the apoptosis of activated HSCs and the degradation of ECM
proteins (16). However, an ideal
approach for the clinical treatment of liver fibrosis is lacking.
Accumulated data have shown that Salvia miltiorrhiza
ameliorates liver fibrosis induced by carbon tetrachloride
(CCl4) or dimethylnitrosamine (DMN) in rats (17,18).
Salvia miltiorrhiza extract has also been shown to exert
anti-fibrosis activity in vitro by mediating TGF-β/Smad
signaling in myofibroblasts (19).
Furthermore, in our earlier studies, we revealed that the monomer
IH764-3, a water-soluble extract of Salvia miltiorrhiza,
inhibits HSC activation and proliferation and induces the apoptosis
of HSCs stimulated by H2O2 in vitro
(9–11), revealing that IH764-3 may be able
to affect liver fibrosis through the regulation on HSCs, the key
cells of liver fibrosis.
Cholestatic fibrosis induced by BDL in rats, which
is similar to human cholestatic liver injury, is a common method
for producing experimental cirrhosis and is a suitable experimental
model of human liver disease. Cholestatic liver injury induced by
biliary obstruction causes acute hepatocellular injury and leads to
progressive fibrogenesis. Although the mechanism of cholestatic
liver injury is not well understood, oxidative stress or
proinflammatory cytokines play an key role in the development of
hepatocellular injury and fibrogenesis (20). In the current study, we selected
the rat BDL model of liver fibrosis, employing H&E and MT
staining to assess the degree of fibrosis. Notably, the monomer
IH764-3 had beneficial effects on intrahepatic fibrogenesis. The
administration of IH764-3 substantially decreased the extent of the
hepatocyte necrosis and degeneration and collagen deposition in the
liver tissues, indicating that it exhibited in vivo
antifibrotic effects that mitigated the BDL-induced liver
fibrogenesis.
It is known that HSCs exist in the space of Disse in
a relatively small quantity, in a quiescent state and expressing no
α-SMA, and their main function is to store vitamin A (21). The quiescent HSCs are activated to
myofibroblasts expressing α-SMA when stimulated by various
cytokines and inflammatory mediators, and have been shown to
migrate to and proliferate in sites of liver injury (22,23),
synthesize ECM components and upregulate the expression levels of
α-SMA and collagen matrices (24).
Hence, α-SMA indirectly represents the degree of HSC activation and
proliferation. In the normal rat livers of the control group, α-SMA
was occasionally detected in vascular smooth muscle cells, and its
expression level was low, revealing that few HSCs were activated.
Following BDL, the area of tissue expressing α-SMA spread to the
portal area and revealed the presence of more activated HSCs.
Immunohistochemical staining demonstrated that the expression
levels of α-SMA in the model group were significantly increased. In
the IH764-3 group, significantly decreased expression levels of
α-SMA, particularly in the portal area and hepatic sinusoids, were
observed compared with the model group, which suggests that the
monomer IH764-3 may act as an antifibrotic agent by inhibiting HSC
activation and proliferation in vivo.
It has been reported that activated HSCs are
eliminated mainly through apoptosis, since it is difficult for them
to return to quiescence (5,6). In
fact, the augmentation of HSC apoptosis is known to promote the
resolution of fibrosis (25–27).
Hence, the induction of apoptosis in activated HSCs is a critical
event in the treatment of liver fibrosis. Our previous studies have
demonstrated that the monomer IH764-3 is capable of inducing HSC
apoptosis activated by H2O2 in a
dose-dependent manner in vitro (11). In the present study, we observed
the effect of IH764-3 on HSC apoptosis in vivo by TUNEL and
α-SMA immunohistochemical double staining. Our results revealed
that the apoptotic rate of activated HSCs in the rat liver tissues
of the IH764-3 group (34.8±4.5%) was higher than in the model group
(4.72±0.37%). These results are consistent with in vitro
studies of IH764-3 and suggest that the induction of apoptosis in
HSCs is one of the antifibrotic mechanisms of IH764-3.
It is well known that FAK, a non-receptor protein
tyrosine kinase, is involved in various cellular processes,
including adhesion, migration, proliferation and apoptosis
(28–30). The MAPK signaling pathway regulates
diverse cellular events including proliferation, growth,
differentiation and apoptosis (31,32).
A number of studies have revealed that the MAPK pathway is involved
in the pathogenesis of fibrosis (33,34).
As one of the key kinases in the MAPK pathway, ERK1 has also been
shown to be implicated in the development of hepatic fibrosis
(35,36). FAK and ERK1 promote proliferation
and migration in a variety of cells. Studies have shown that FAK
activation causes a cascade reaction through the MAPK pathway.
Previous studies have confirmed that the downregulation of FAK by
short hairpin RNA technology or the endogenous inhibitor decreases
the expression level of p-FAK (Tyr397), inhibits HSC proliferation
and induces HSC apoptosis in vitro, which indicates an
involvement of the FAK-ERK signal transduction pathway (37–39).
We also have revealed that the expression levels of FAK and ERK1
increase during progressive liver fibrosis in BDL-treated rats and
that in an in vitro experiment, the monomer IH764-3
downregulated FAK and ERK1 in HSCs stimulated by
H2O2 (11–13,15).
In the present study, we demonstrated that FAK and ERK1 were
decreased at the translation and transcription levels by the
monomer IH764-3, which indicates that IH764-3 inhibits the FAK-ERK
signal transduction pathway and that this is the mechanism by which
IH764-3 inhibits HSC proliferation and induces HSC apoptosis.
In summary, the monomer IH764-3 significantly
ameliorates experimental liver fibrosis by inhibiting HSC
proliferation and inducing HSC apoptosis. The relevant mechanism
involves inhibition of the FAK-ERK signal transduction pathway.
These results provide evidence that IH764-3 is an attractive agent
for the treatment of liver fibrosis.
Acknowledgements
This study was supported by the Department of
Science and Technology of Hebei Province (09966107D) and the
Traditional Chinese Medicine Drug Administration of Hebei Province
(No. 2007061). The authors would like to thank the Foundations for
their support.
Abbreviations:
|
BDL
|
bile duct ligation
|
|
HSC
|
hepatic stellate cells
|
|
α-SMA
|
α-smooth muscle actin
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
UTP-nick end labeling
|
|
FAK
|
focal adhesion kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
References
|
1
|
Povero D, Busletta C, Novo E, di Bonzo LV,
Cannito S, Paternostro C and Parola M: Liver fibrosis: a dynamic
and potentially reversible process. Histol Histopathol.
25:1075–1091. 2010.PubMed/NCBI
|
|
2
|
Friedman SL: Mechanisms of hepatic
fibrogenesis (Review). Gastroenterology. 134:1655–1669. 2008.
View Article : Google Scholar
|
|
3
|
Jiao J, Friedman SL and Aloman C: Hepatic
fibrosis (Review). Curr Opin Gastroenterol. 25:223–229. 2009.
View Article : Google Scholar
|
|
4
|
Friedman SL: Hepatic stellate cells:
protean, multifunctional, and enigmatic cells of the liver
(Review). Physiol Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lakner AM, Walling TL, McKillop IH and
Schrum LW: Altered aquaporin expression and role in apoptosis
during hepatic stellate cell activation. Liver Int. 31:42–51. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kisseleva T and Brenner DA: Hepatic
stellate cells and the reversal of fibrosis (Review). J
Gastroenterol Hepatol. 21:S84–S87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moon S, Shin S, Kim S, Oh HE, Han S, Lee S
and Kim K: Role of Salvia miltiorrhiza for modulation of
Th2-derived cytokines in the resolution of inflammation. Immune
Netw. 11:288–298. 2011.
|
|
8
|
You Z, Xin Y, Liu Y, Han B, Zhang L, Chen
Y, Chen Y, Gu L, Gao H and Xuan Y: Protective effect of Salvia
miltiorrhizae injection on N(G)-nitro-d-arginine induced nitric
oxide deficient and oxidative damage in rat kidney. Exp Toxicol
Pathol. 64:453–458. 2012.
|
|
9
|
Liu L, Jiang HQ and Zhang XL: The effect
and mechanism of Salvia miltiorrhiza monomer IH764-3 on
proliferation and collagen synthesis of hepatic stellate cells
stimulated by H2O2. Zhongguo Ying Yong Sheng
Li Xue Za Zhi. 19:78–81. 2003.(In Chinese).
|
|
10
|
Zhang XL, Liu L and Jiang HQ: Salvia
miltiorrhiza monomer IH764-3 induces hepatic stellate cell
apoptosis via caspase-3 activation. World J Gastroenterol.
8:515–519. 2002.
|
|
11
|
Fang SM, Li CS, An JY, Dun ZN, Yao DM, Liu
L and Zhang XL: The role of extracellular signal-regulated kinase
in induction of apoptosis with Salvia miltiorrhiza monomer
IH764-3 in hepatic stellate cells. Zhongguo Ying Yong Sheng Li Xue
Za Zhi. 27:402–406. 2011.(In Chinese).
|
|
12
|
Liu L, Jiang HQ, Zhang XL and Zhao DQ:
Effect of Salvia miltiorrhiza monomer IH764-3 on MMP-13 and
TIMP-1 by downregulating the expression of focal adhesion kinase in
hepatic stellate cell stimulated by H2O2.
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 23:482–486. 2007.(In
Chinese).
|
|
13
|
Zhang XL, Liu JM, Yang CC, Zheng YL, Liu
L, Wang ZK and Jiang HQ: Dynamic expression of extracellular
signal-regulated kinase in rat liver tissue during hepatic
fibrogenesis. World J Gastroenterol. 12:6376–6381. 2006.PubMed/NCBI
|
|
14
|
Huo XX, Zhang XL, Shen JG and Wei J:
FAK-related non-kinase plasmid transfection inhibited hepatic
stellate cell proliferation stimulated by fibronection. Clin J
Hepatol. 15:547–548. 2007.PubMed/NCBI
|
|
15
|
Zhang XL, Huo XX, Shen JA, Wei J and Jiang
HQ: Focal adhesion kinase tyrosine phosphorylation promotes rat
hepatic fibrogenesis and its possible mechanism. Basic &
Clinical Medicine. 27:143–147. 2007.(In Chinese).
|
|
16
|
Elsharkawy AM, Oakley F and Mann DA: The
role and regulation of hepatic stellate cell apoptosis in reversal
of liver fibrosis (Review). Apoptosis. 10:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu YC, Lin YL, Chiu YT, Shiao MS, Lee CY
and Huang YT: Antifibrotic effects of Salvia miltiorrhiza on
dimethylnitrosamine-intoxicated rats. J Biomed Sci. 12:185–195.
2005.
|
|
18
|
Lee TY, Wang GJ, Chiu JH and Lin HC:
Long-term administration of Salvia miltiorrhiza ameliorates
carbon tetrachloride-induced hepatic fibrosis in rats. J Pharm
Pharmacol. 55:1561–1568. 2003.
|
|
19
|
Yang Y, Yang S, Chen M and Zhang X, Zou Y
and Zhang X: Compound Astragalus and Salvia miltiorrhiza
extract exerts anti-fibrosis by mediating TGF-beta/Smad signaling
in myofibroblasts. J Ethnopharmacol. 118:264–270. 2008.
|
|
20
|
Maeda K, Koda M, Matono T, Sugihara T,
Yamamoto S, Ueki M, Murawaki Y, Yamashita N and Nishiyama S:
Preventive effects of ME3738 on hepatic fibrosis induced by bile
duct ligation in rats. Hepatol Res. 38:727–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geerts A: History, heterogeneity,
developmental biology, and functions of quiescent hepatic stellate
cells (Review). Semin Liver Dis. 21:311–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bataller R and Brenner DA: Hepatic
stellate cells as a target for the treatment of liver fibrosis
(Review). Semin Liver Dis. 21:437–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury
(Review). J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arthur MJ: Fibrogenesis II:
Metalloproteinases and their inhibitors in liver fibrosis (Review).
Am J Physiol Gastrointest Liver Physiol. 279:G245–G249.
2000.PubMed/NCBI
|
|
25
|
Chor JS, Yu J, Chan KK, Go YY and Sung JJ:
Stephania tetrandra prevents and regresses liver fibrosis
induced by carbon tetrachloride in rats. J Gastroenterol Hepatol.
24:853–859. 2009. View Article : Google Scholar
|
|
26
|
Tao LL, Cheng YY, Ding D, Mei S, Xu JW, Yu
J, Ou-Yang Q, Deng L, Chen Q, Li QQ, et al: C/EBP-α ameliorates
C(Cl4)-induced liver fibrosis in mice through promoting
apoptosis of hepatic stellate cells with little apoptotic effect on
hepatocytes in vitro and in vivo. Apoptosis. 17:492–502. 2012.
|
|
27
|
Wang X, Ikejima K, Kon K, Arai K, Aoyama
T, Okumura K, Abe W, Sato N and Watanabe S: Ursolic acid
ameliorates hepatic fibrosis in the rat by specific induction of
apoptosis in hepatic stellate cells. J Hepatol. 55:379–387. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murata T, Naomoto Y, Yamatsuji T, Okawa T,
Shirakawa Y, Gunduz M, Nobuhisa T, Takaoka M, Sirmali M, Nakajima
M, et al: Localization of FAK is related with colorectal
carcinogenesis. Int J Oncol. 32:791–796. 2008.PubMed/NCBI
|
|
29
|
Xia J, Lv N, Hong Y, Li C, Tao X, Chen X
and Cheng B: Increased expression of focal adhesion kinase
correlates with cellular proliferation and apoptosis during
4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis. J Oral
Pathol Med. 38:524–529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu G, Meng X, Jin Y, Bai J, Zhao Y, Cui
X, Chen F and Fu S: Inhibitory role of focal adhesion kinase on
anoikis in the lung cancer cell A549. Cell Biol Int. 32:663–670.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lewis TS, Shapiro PS and Ahn NG: Signal
transduction through MAP kinase cascades. Adv Cancer Res.
74:49–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Karreth FA and Tuveson DA: Modelling
oncogenic Ras/Raf signalling in the mouse. Curr Opin Genet Dev.
19:4–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Secker GA, Shortt AJ, Sampson E, Schwarz
QP, Schultz GS and Daniels JT: TGFβ stimulated re-epithelialisation
is regulated by CTGF and Ras/MEK/ERK signalling. Exp Cell Res.
314:131–142. 2008.
|
|
34
|
Ma FY, Sachchithananthan M, Flanc RS and
Nikolic-Paterson DJ: Mitogen activated protein kinases in renal
fibrosis (Review). Front Biosci (Schol Ed). 1:171–187. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smart DE, Green K, Oakley F, Weitzman JB,
Yaniv M, Reynolds G, Mann J, Millward-Sadler H and Mann DA: JunD is
a profibrogenic transcription factor regulated by Jun N-terminal
kinase-independent phosphorylation. Hepatology. 44:1432–1440. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiang H, Lin Y, Zhang X, Zeng X, Shi J,
Chen YX, Yang MF, Han ZG and Xie WF: Differential expression genes
analyzed by cDNA array in the regulation of rat hepatic
fibrogenesis. Liver Int. 26:1126–1137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen JG, Zhang XL and Huo XX: The role of
FAK-ERK signal transduction pathway in apoptosis of hepatic
stellate cell. Zhonghua Gan Zang Bing Za Zhi. 16:849–853. 2008.(In
Chinese).
|
|
38
|
Shen JG, Zhang XL, Huo XX and Wei J: The
role of focal adhesion kinase-extracellular signal regulated kinase
signal transduction pathway in proliferation of hepatic stellate
cell. J Gastroenterol Hepatol. 21:A3742006.
|
|
39
|
An J, Zheng L, Xie S, Dun Z, Hao L, Yao D,
Shih DQ and Zhang X: Down-regulation of focal adhesion kinase by
short hairpin RNA increased apoptosis of rat hepatic stellate
cells. APMIS. 119:319–329. 2011. View Article : Google Scholar : PubMed/NCBI
|