Introduction
Chlamydophila pneumoniae (Cpn) is a pathogen
that causes respiratory infections such as pneumonia, asthma,
chronic pharyngitis, chronic bronchitis and cardiovascular diseases
(1–5). Although its pathogenic mechanism
remains unclear, Cpn may result from its toxic protein secretion.
The typeIII secretion system (T3SS) is an independent secretion
system since its effector proteins may change the cytoskeleton
formation, destroy the signal transduction pathways, inhibit
apoptosis and interfere with transcriptional regulation (6–9).
Screening and identification of the Cpn T3SS effector proteins have
become a research hotspot globally (10–12).
Genes encoding T3SS effector proteins are believed to be positioned
close to their chaperones (13).
Gene Cpn lcrH1 is Cpn homologous to Yersinia and adjacent to
Cpn0425 (14,15). Since Cpn0425 is located in the
T3SS-encoding gene family, it is expected to be one of the Cpn T3SS
effector proteins. In this study, the Cpn0425 protein was cloned,
expressed and purified in vitro, and its localization and
immune activities in infected cells were investigated to further
study the screening, identification and pathogenic action of Cpn
T3SS effector proteins.
Materials and methods
Plasmids and reagents
The pGEX 6p-2 plasmid, E. coli Bl21 strains
and THP-1 cell lines were provided by the Institute of Pathogenic
Biology, Nanhua University (China). AxyPrep PCR kit was purchased
from Axygen Biosciences (Union City, CA, USA), GST purification
resin was obtained from Novagen (Madison, WI, USA) and the BCA
protein concentration assay kit was purchased from Biyuntian
Company (Jiangsu, China). The assay kit for the TNF-α and IL-6
cytokines were provided by Jingmei Biotech Co., Ltd. (Shenzhen,
China).
Construction of pGEX-6p-2/Cpn0425
The Cpn AR-39 genome was used as the template to
amplify Cpn0425 coding genes by PCR. Recombinant plasmid
pGEX-6p-2/Cpn0425 was transformed into vector E. coli Bl21
for enzyme digestion and sequencing.
Expression, purification and
identification of recombinant proteins
Recombinant protein GST-Cpn0425 was induced by IPTG
and purified by GST purification resin. Following the expression
and purity analysis of 10% SDS-PAGE, the purified product was
identified by western blotting.
Preparation of serum
The purified GST-Cpn0425 fusion protein was
emulsified with the same amount of Freund’s complete adjuvant, and
intraperitoneally injected into 12immune 6-week-old BALB/c mice.
Fourteen days after the initial immunization, fusion protein with
incomplete Freund’s adjuvant was applied 3 times every 10 days to
strengthen immunity. Seven days after the last immunization, a
blood sample was taken. The serum was separated to obtain
polyclonal antibodies. Indirect immunofluorescence assay (IFA) was
performed to detect the location and immune activity of the Cpn0425
protein in Cpn-infected Hep-2 cells.
IFA for Chlamydia protein
Cell growth solution (1 ml), containing
8×104 Hep-2 cells, was added to each well of the 24-well
tissue culture plates and cultured for 18 h. The medium was
discarded when the cells grew into a monolayer. Cells were
inoculated with Cpn AR-39, followed by the addition of 2mg/l
cycloheximide solution. Cells were then incubated for 72h. The
chlamydial infection solution was aspirated from the wells and
fixed with 4% paraformaldehyde. Rabbit anti-CT polyclonal antibody
Cpn AR-39 was double-stained with dilutions (1:200, 1:1000 and
1:5000) of mouse anti-Cpn 0425 antibody, and incubated at 37°C for
1 h. After washing, Cy2-labeled goat anti-rabbit IgG and
Cy3-labeled goat anti-mouse IgG were added to the solution. The
nucleus was marked by Hoechst and observed under a fluorescence
microscope after incubation at 37°C for 1 h and washing.
ELISA
Savyon GST-Cpn0425 protein was diluted by 0.05mol/l,
pH 7.4 carbonate buffer, and 100 μg of the solution was then added
to each well of the microtiter plates for the detection of Cpn
standard serum. Negative and positive controls were established
with tetramethylbenzidine (TMB) as the chromogenic substrate, and
A450 values were measured by microplate reader.
Additionally, 20 cases of Cpn-positive serum and 10 cases of
Cpn-negative serum were determined by the established indirect
ELISA methods. The test serum was diluted at 1:100, and a
A450 value +2 S was considered as the cut-off value.
When the A450 value was higher than the cut-off level,
the value was regarded as positive, otherwise it was negative. The
results were compared with the test results of the SeroCP™ IgG
ELISA diagnostic kit from Savyon Diagnostics (Ashdod, Israel) and
the application value of the recombinant protein antigens in the
serological diagnosis of Cpn was preliminarily evaluated.
Results
Induced expression of the recombinant
plasmid pGEX6p-2/Cpn0425
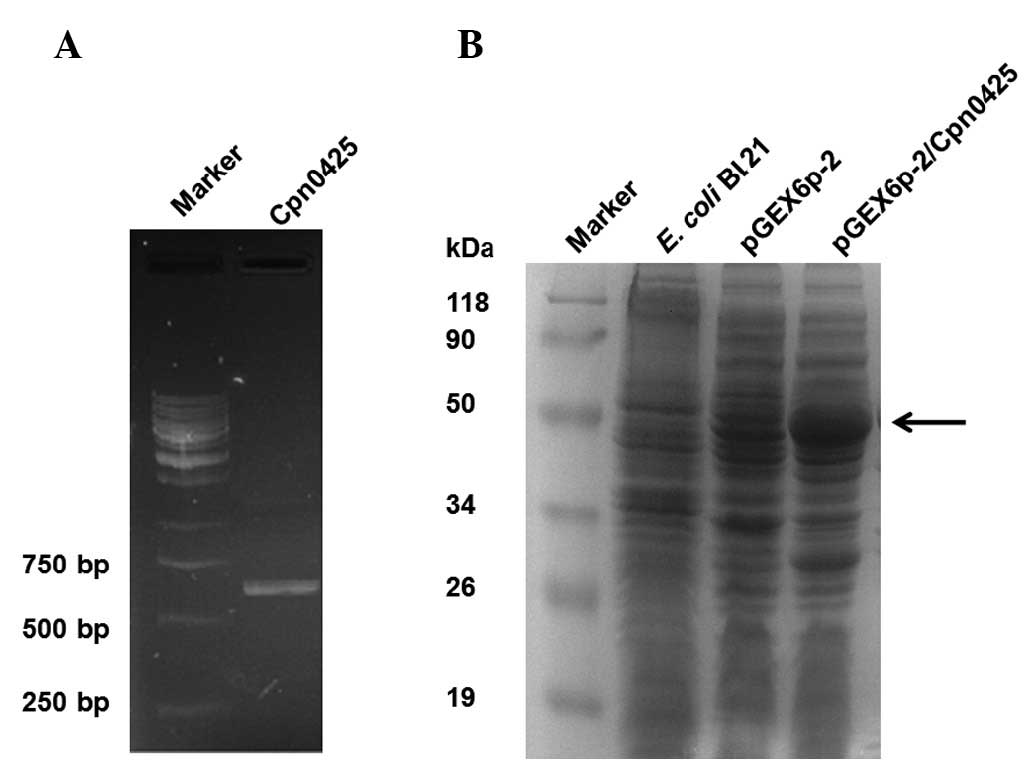
PCR products were separated on agarose gels, which
showed a clear and specific band located approximately at 350bp
(Fig. 1A). This result was
consistent with the expected fragment size of the Cpn0425 products
(588 bp).
The recombinant plasmid pGEX6p-2/Cpn0425 was
transformed into the E. coli Bl21 host strain for induced
expression. Results of the SDS-PAGE showed an obvious band of the
induced protein at approximately 50 kDa. This result was consistent
with the expected molecular weight of Cpn0425 (Fig. 1B).
Western blot analysis of the recombinant
protein GST-Cpn0425
After the induced expression of 0.2 mol/l IPTG for 4
h, results showed that the recombinant protein was present mainly
in the supernatant, but little in the precipitation. This result
indicates that pGEX6p-2/Cpn0425 was expressed mainly in soluble
form, and only a little in the inclusion bodies. GST of the
recombinant protein GST-Cpn0425 was purified and analysed by 12%
SDS-PAGE. The purification of fusion proteins was found to have
been obtained in the corresponding positions with Mr of
approximately 50 kDa. Bands below the fusion protein were
considered as fusion protein degradation products (Fig. 2A).
Rat anti-Cpn AR39 polyclonal antibody was the
primary antibody and was detected by western blot analysis for the
induced expression of GST-Cpn 0425, which showed obvious specific
bands, at approximately 50 kDa of Mr, whereas no bands
were observed of the uninduced bacteria (Fig. 2B).
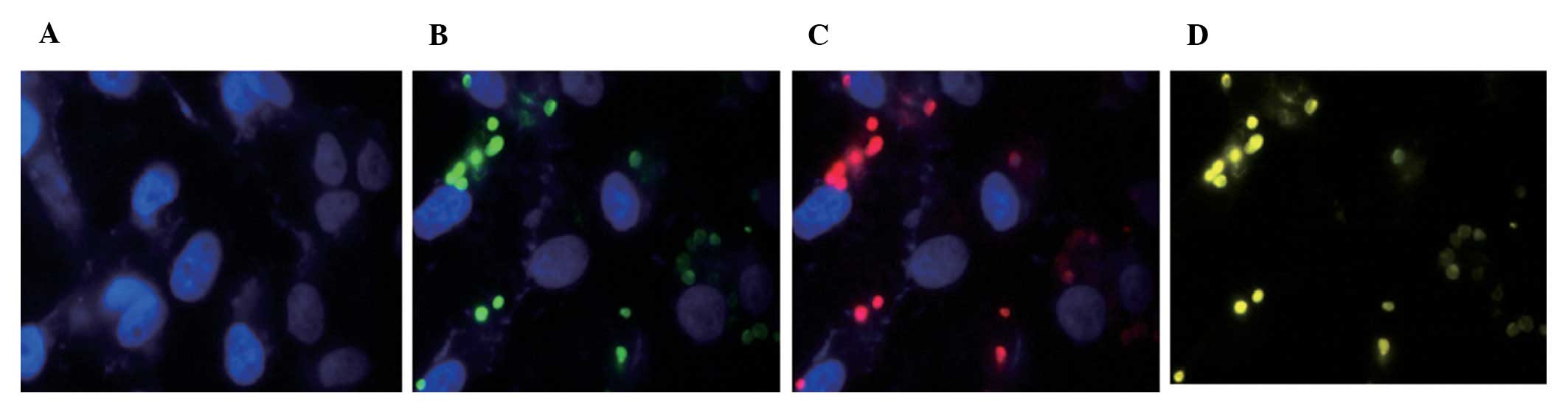
Positioning of the Cpn0425 protein in
infected cells
After the infection of Cpn AR-39 in Hep-2 cells for
72 h, cells were fixed and the fusion protein polyclonal antibody
serum was considered as the primary antibody. IFA results showed
that the Cpn 0425 protein was present in intracellular inclusions
(Fig. 3). Indirect ELISA was
applied for the detection of Cpn IgG antibodies in the serum of
Cpn-infected patients (Table
I).
 | Table IELISA detection of serum from the
Chlamydophila pneumoniae (Cpn)-infected patients using the
recombinant Cpn0425 as antigen. |
Table I
ELISA detection of serum from the
Chlamydophila pneumoniae (Cpn)-infected patients using the
recombinant Cpn0425 as antigen.
| Serum of patients
(Cpn IgG) | Indirect ELISA
results |
|---|
|
|---|
| Positive | Negative | Total |
|---|
| Positive | 17 | 3 | 20 |
| Negative | 0 | 10 | 10 |
| Total | 17 | 13 | 30 |
Discussion
In this experiment, the expression proteins of the
clone and transformation vector pGEX-6P-2 were fusion proteins,
which facilitated to further separate the purified protein. In
addition, the selection of the expression bacteria highly expressed
the key factors of fusion proteins (16). Expression bacteria applied in this
study was E. coli Bl21, a commonly used expression strain
that generally strongly expresses target genes together with a
strongly expressed vector pGEX. The Bl21 strain lacks Lon and OmpT
protease products, thus reducing the impact of protease degradation
in the host cells. In addition, this strain helps to increase the
yield of soluble protein and facilitates the efficient expression
of soluble GST fusion protein. GST fusion protein was purified by
GST purification resin (Merck Novagen). The resin is of high
specificity for the GST purification filter and of specific
adsorption of the GST fusion protein, which subsequently improves
the purity of the target protein. Purification is achieved through
the affinity of GST and glutathione (GSH), and the purified protein
obtained maintains its natural biological activity since only a
natural GST protein has affinity characteristics.
Polyvalent mouse immune serum was obtained using
purified recombinant protein-immunized BALB/c mice. Its high
specific antibody titer was determined by indirect ELISA. This
revealed the high immunogenicity of the recombinant
protein-stimulated BALB/c mice that produced a strong humoral
immune response, resulting in the gradual increase of the immune
effect. Due to its high immunogenicity, the fusion protein antibody
obtained in this experiment may be applied in western blotting,
immunofluorescence and other types of research techniques.
Seventeen out of 20 cases showed a positive reaction
to the fusion protein and serum from patients with respiratory
tract infections, suggesting that Cpn0425 may be produced in the
natural infection. Since this endogenous protein exhibits
immunogenicity, it stimulates the body to respond differently.
Thus, Cpn0425 may be used as a new chlamydia-immune diagnostic
reagent, and may even be applied in the laboratory to diagnose Cpn
infection (17).
Three color marker staining was applied in the IFA
staining process: DNA was stained blue by Hoechst, whole CT cell
was stained green by Cy2-labeled fluorescent antibody, while target
protein positioning was stained red by the Cy3-labeled fluorescent
antibody. When target proteins were distributed around bacteria,
green and red fluorescence were overlapped and the IFA results
appeared to be yellow or orange. If the target proteins were at the
inclusion body membrane (17–20),
it was red around the inclusion for the membrane staining in IFA
results. IFA results in this study showed that the Cpn0425 protein
was located inside the inclusion bodies in infected cells,
indicating that Cpn0425 was not an effector protein in the
Chlamydia type III secretion system.
In conclusion, the cell localization of the CT0425
protein, present in inclusion bodies with strong immune activity,
in C. trachomatis-infected cells was successfully
desmonstrated in this study through IFA. Additional studies are
required to investigate the structure and biological function of
CT0425.
Acknowledgements
This project is funded by the Doctoral Fund of the
Ministry of Education (200805400002) of the Health Department of
the Province of Hunan (B2011-040).
References
|
1
|
Lui G, Ip M, Lee N, et al: Role of
‘atypical pathogens’ among adult hospitalized patients with
community-acquired pneumonia. Respirology. 14:1098–1105. 2009.
|
|
2
|
Damy SB, Higuchi ML, Timenetsky J, et al:
Mycoplasma pneumoniae and/or Chlamydophila pneumoniae
inoculation causing different aggravations in cholesterol-induced
atherosclerosis in apoE KO male mice. BMC Microbiol. 9:1942009.
View Article : Google Scholar
|
|
3
|
Papaetis GS, Anastasakou E and Orphanidou
D: Chlamydophila pneumoniae infection and COPD: more
evidence for lack of evidence? Eur J Intern Med. 20:579–785. 2009.
View Article : Google Scholar
|
|
4
|
Song JH, Thamlikitkul V and Hsueh PR:
Clinical and economic burden of community-acquired pneumonia
amongst adults in the Asia-Pacific region. Int J Antimicrob Agents.
38:108–117. 2011.PubMed/NCBI
|
|
5
|
Jackson RW, Vinatzer B, Arnold DL, Dorus S
and Murillo J: The influence of the accessory genome on bacterial
pathogen evolution. Mob Genet Elements. 1:55–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mota LJ: Cornelis. The bacterial injection
kit: type III secretion systems. Ann Med. 37:234–249. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koizumi Y, Toma C, Higa N, Nohara T,
Nakasone N and Suzuki T: Inflammasome activation via intracellular
NLRs triggered by bacterial infection. Cell Microbiol. 14:149–154.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diaz MR, King JM and Yahr TL: Intrinsic
and extrinsic regulation of type III secretion gene expression in
pseudomonas aeruginosa. Front Microbiol. 2:892011.PubMed/NCBI
|
|
9
|
Dean P: Functional domains and motifs of
bacterial type III effector proteins and their roles in infection.
FEMS Microbiol Rev. 35:1100–1125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SH, Kwon SJ, Lee SJ, Kim YC, Hwang
KY, Kang YH and Lee KJ: Identification of immunogenic antigen
candidate for Chlamydophila pneumoniae diagnosis. J Proteome
Res. 8:2933–2943. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tammiruusu A, Penttilä T, Lahesmaa R,
Sarvas M, Puolakkainen M and Vuola JM: Intranasal administration of
chlamydial outer protein N (CopN) induces protection against
pulmonary Chlamydia pneumoniae infection in a mouse model.
Vaccine. 25:283–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalman S, Mitchell W, Marathe R, et al:
Comparative genomes of Chlamydia pneumoniae and C.
trachomatis. Nat Genet. 21:385–389. 1999. View Article : Google Scholar
|
|
13
|
Chen CQ, Wu YM, Li ZY, et al: Chlamydia
trachomatis outer membrane protein expression of recombinant
protein purification and immunological identification. Chin Lab
Med. 28:697–699. 2005.
|
|
14
|
Lugert R, Kuhns M, Polch T and Gross U:
Expression and localization of type III secretion-related proteins
of Chlamydia pneumoniae. Med Microbiol Immunol. 193:163–171.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Timms P, Good D, Wan C, Theodoropoulos C,
Mukhopadhyay S, Summersgill J and Mathews S: Differential
transcriptional responses between the interferon-gamma-induction
and iron-limitation models of persistence for Chlamydia
pneumoniae. J Microbiol Immunol Infect. 42:27–37.
2009.PubMed/NCBI
|
|
16
|
Chen C, Chen D, Sharma J, et al: The
hypothetical protein CT813 is localized in the Chlamydia
trachomatis inclusion membrane and is immunogenic in women
urogenitally infected with C. trachomatis. Infect Immun.
74:4826–4840. 2006.PubMed/NCBI
|
|
17
|
Huang Z, Feng Y, Chen D, et al: Structural
basis for activation and inhibition of the secreted chlamydia
protease CPAF. Cell Host Microbe. 4:529–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pirbhai M, Dong F, Zhong Y, et al: The
secreted protease factor CPAF is responsible for degrading
pro-apoptotic BH3-only proteins in Chlamydia
trachomatis-infected cells. J Biol Chem. 281:31495–31501. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li ZY, Wang SP, Wu YM, et al: Immunization
with chlamydial plasmid protein pORF5 DNA vaccine induces
protective immunity against genital chlamydial infection in mice.
Sci China C Life Sci. 51:973–980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Toh H, Miura K, Shirai M, et al: In
silico inference of inclusion membrane protein family in
obligate intracellular parasites chlamydiae. DNA Res. 10:9–17.
2003. View Article : Google Scholar
|