Introduction
Ultrasound-mediated microbubble fragmentation
technology is used to transport remedial exogenous genes or drugs
into impaired organs or tissues and create biological effects. This
technology has become an extremely promising gene delivery tool for
myocardial ischemia gene therapy (1–4).
Previous studies have mainly focused on the effects of ultrasound
irradiation parameters on gene transfer efficiency (5–7),
however, this study was designed to explore the effects of other
parameters, including microbubble concentration, gene dosage,
cell-microbubble mixing mode and the presence of fetal bovine serum
(FBS), and how to match these parameters to achieve good gene
delivery. Furthermore, therapeutic gene integrity post ultrasound
irradiation was also tested, as it is fundamental for gene
expression. The ultimate aim is to provide an objective basis for
future angiogenesis research of microbubble/ultrasound-mediated
hAng-1 gene delivery into ischemic myocardium in vivo.
Materials and methods
Preparation of SonoVue microbubble/hAng-1
gene suspension
The eGFP-hAng-1 construct was generated by PCR with
a high-fidelity thermostable DNA polymerase (Fermentas Co., Glen
Burnie, MD, USA) based on the pAAVAVNGPT1 plasmid (designed and
presented by Wu’s Lab, College of Life Science, Wuhan University,
Wuhan, China). The suspension of SonoVue microbubbles was prepared
according to the manufacturer’s instructions. The SonoVue
microbubble was produced by Bracco Co. (Geneva, Switzerland), with
a diameter of 2.5 μm and coated with phospholipids on the surface.
The mixture of the suspension of the hAng-1 plasmid and SonoVue
microbubbles was kept at 4°C for 15 min with oscillation to ensure
the plasmid and microbubbles were in full contact and adhered to
one other. The study was approved by the ethics committee of Renmin
Hospital of Wuhan University, Wuhan, China.
Determination of optimal ultrasound
exposure
Human embryonic kidney 293T cells were maintained in
DMEM with 10% FBS. For transfection, cells were seeded at
1×106 cells/well in 6-well plates and transfected with
10% μl/μl microbubble suspension containing 10 μg/ml hAng-1 gene
via ultrasound irradiation (8–10).
During transfection, the ultrasound probe was placed under the
6-well plate at a distance of 3–5 mm, and irradiation frequency was
ranged from 0.5–2 MHz with continuous wave (UGT2007 ultrasound
irradiation machine, Ultrasonic Research Institute, Chongqing
Medical School, China). The irradiation intensity was set to 0.5,
1.0, 1.5 and 2.0 W/cm2 with exposure time set to 10, 20,
30, 60 and 120 sec. Transfected cells were exposed to ultrasound
irradiation over various time courses, and transfection efficiency
and cell viabilities were used to determine the optimal ultrasound
exposure parameters.
Determination of optimal microbubble
concentration
To determine the optimal matching parameters between
microbubble concentration and ultrasound exposure, the microbubble
concentration was set to 5, 10, 20, 30 and 40% (μl/μl) under the
ultrasound exposure dosage of 1.5, 3.0, 4.5 and 6.0
W/cm2 × sec (intensity × time). Microbubble
concentration was calculated as microbubble solution
volume/(microbubble solution volume + reaction system volume;
μl/μl). The plasmid DNA concentration was 10 μg/ml. hAng-1 gene
delivery rate and cell death under these conditions were used to
determine the optimal microbubble concentration.
Determination of optimal plasmid DNA
concentration
To determine the optimal concentration of the hAng-1
gene in cell suspension, plasmids were prepared at 2.5, 5, 10, 15,
20 and 30 μg/ml. hAng-1 gene delivery rate and cell death under
optimal microbubble conditions and ultrasound radiation parameters
remained as above.
Determination of mixed mode of cells and
microbubble
To determine the effect of mixed mode on
transfection efficiency and cell viabilities, 293T cells and
microbubbles carrying the hAng-1 gene were cultured in adherence
and suspension modes. In suspension mode, the microbubbles carrying
the gene were added into cultured 293T cells in 6-well plates at
the moment of inoculation, followed by ultrasound irradiation.
Under adherence mode, 293T cells at 1×106/ml
concentration were seeded in 6-well plates (total number of cells
remained constant under both modes). Following adhesion, the
microbubble suspension carrying the gene was added, followed by
ultrasound irradiation. All other experimental parameters remained
constant between the two modes.
Effect of FBS on transfection system
To determine the effect of FBS on transfection
efficiency and cell viability, the transfection efficiency of 293T
cells, transfected with the hAng-1 plasmid at 15 μg/ml
concentration via ultrasound irradiation with 8% FBS was compared
with that in the absence of FBS. All other experimental parameters
followed the above optimal conditions and remained constant between
the two groups.
Control group
Cells were transfected with microbubble suspension
carrying the gene hAng-1 as the experimental group, and cells were
transfected with only the hAng-1 gene as the control group. All
other experimental parameters remained constant.
Determination of gene transfection
efficiency and cell viability
Fluorescence quantity and intensity of green
fluorescent protein (GFP) under the fluorescence microscope and
percentage of eGFP positive cells via flow cytometry were used to
determine the transfection efficiency. Cell viability was
determined by 0.4% trypan blue staining and the cell counting
method. Cell morphology and structure were compared using an
inverted microscope under the conditions stated.
Detection of hAng-1 mRNA
hAng-1 gene expression at the mRNA level was
determined by quantitative RT-PCR. The primer sequence used for
detection was designed, upstream, 5′-TGCCATTACCAGTCAGAGG-3′, and
downstream, 5′-CAAGCATCAAACCACCATC-3′, using Primer Premier 5.0
software and the product was analyzed by 1% agarose gel
electrophoresis.
Detection of hAng-1 protein
hAng-1 gene expression at the protein level was
determined by western blot analysis. Cell lysis was performed 48 h
following hAng-1 gene transfection and hAng-1 protein was detected
using a primary antibody against hAng-1, purchased from Abcam Co.
(Cambridge, MA, USA; Angiopoietin 1 antibody, ab8451).
Analysis of DNA integrity following
microbubble-mediated gene transfer
To determine the integrity of DNA after ultrasound
irradiation, the hAng-1 solution was analyzed by agarose gel
electrophoresis following ultrasound irradiation. The result was
compared with that of the hAng-1 gene solution wtihout ultrasonic
irradiation.
Statistical analysis
SPSS 11.0 was used for statistical analysis. Data
are expressed as the mean ± SD, and one-way ANOVA and post hoc
tests were used to analyze the significance of hAng-1 gene
transfection efficiency and cell viability under the above various
experimental conditions.
Results
Ultrasound exposure parameters
Gene transfection efficiency increased with
enhancement of irradiation intensity and extension of irradiation
time. However, further increases were not obtained at irradiation
intensity and duration beyond 1.5 W/cm2 and 30 sec. In
addition, cell viability decreased significantly beyond these
settings (Tables I and II). Taking into account the transfection
efficiency and cell survival rate, 1.5 W/cm2 and 30 sec
were set as optimal conditions in our experiments.
 | Table IEffect of ultrasonic irradiation
intensity on efficiency of gene transfer and cell viability (mean ±
SD). |
Table I
Effect of ultrasonic irradiation
intensity on efficiency of gene transfer and cell viability (mean ±
SD).
| Intensity
(W/cm2) | Transfection
efficiency | Cell viability |
|---|
| 0.5 | 5.2±1.9 | 92.8±2.0 |
| 1.0 | 10.1±0.9 | 88.9±2.4 |
| 1.5 | 15.5±1.0a | 83.2±2.2 |
| 2.0 | 14.2±2.3a | 68.1±4.6b |
 | Table IIEffect of ultrasonic irradiation
duration on efficiency of gene transfer and cell viability (mean ±
SD). |
Table II
Effect of ultrasonic irradiation
duration on efficiency of gene transfer and cell viability (mean ±
SD).
| Duration (sec) | Transfection
efficiency | Cell viability |
|---|
| 10 | 2.7±0.7 | 91.7±2.1 |
| 20 | 9.5±0.4 | 84.9±1.9 |
| 30 | 15.9±0.9a | 82.6±2.6 |
| 60 | 11.8±1.2 | 75.0±2.1b |
| 120 | 8.7±1.0 | 64.6±3.8b |
Microbubble concentration
Gene delivery efficiency was relatively low at a
microbubble concentration of 5 and 10% (μl/μl), efficiency
increased significantly at 20 and 30% concentrations under 3.0
W/cm2 × sec, reaching the highest level at 4.5
W/cm2 × sec. No significant differences were identified
with respect to gene delivery efficiency between these two
microbubble concentrations under the ultrasound irradiation of 3.0
W/cm2 × sec (14.04±1.04 vs.13.92±0.97%, P>0.05) and
4.5 W/cm2 × sec (19.66±1.67 vs. 17.54±1.41%, P>0.05).
However, the gene transfection efficiency was significantly
decreased at 40% microbubble concentration under any ultrasound
exposure condition (P<0.01). Cell viability was significantly
decreased at microbubble concentrations >30% (P<0.01;
Fig. 1). Therefore, 20%
microbubble concentration was determined as optimal for
experiments.
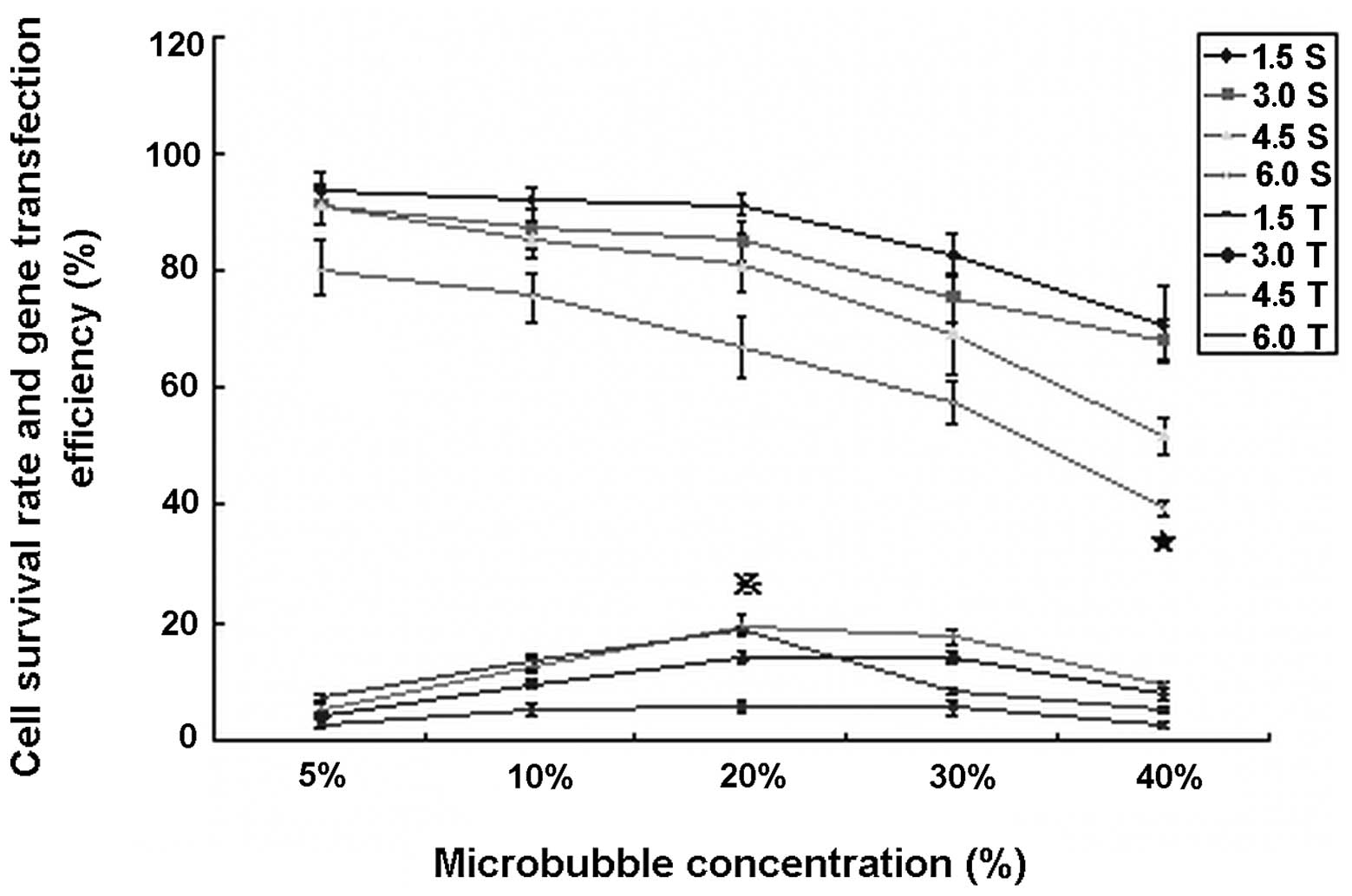 | Figure 1Gene delivery efficiency and cell
viability under various microbubble concentrations and ultrasonic
irradiation doses of 1.5, 3.0, 4.5 and 6.0 W/cm2 × sec.
Ultrasonic irradiation doses were 1.5, 3.0, 4.5 and 6.0
W/cm2 × sec. Gene transfection efficiency was relatively
low, especially at 5 and 40% microbubble concentration. Cell
viability was kept within a safe range with the exception of 40%
microbubble concentration (P<0.001). ★, cell survival rate was
significantly lower than that of any other microbubble
concentration (P<0.001); , gene transfection efficiency under 4.5
W/cm2 × sec was significantly higher than that in any
other conditions, with the exception of 6.0 W/cm2 × sec
at 20% microbubble concentration (P<0.001); however, the latter
cell survival rate was severely decreased (P<0.001). 1.5S, 3.0S,
4.5S and 6.0S, cell survival rate at 1.5, 3.0, 4.5 and 6.0
W/cm2 × sec, respectively; 1.5T, 3.0T, 4.5T and 6.0T,
gene transfection efficiency under 1.5, 3.0, 4.5 and 6.0
W/cm2 × sec, respectively. |
DNA concentration
Gene transfection efficiency was low at DNA
concentrations of 2.5 μg/ml (1.08±0.35%) and 5 μg/ml (3.98±1.13%);
it increased significantly at DNA concentrations of 10 μg/ml
(9.14±1.56%) under ultrasound irradiation of 1.5 W/cm2
and 30 sec (ultrasound exposure dose, 4.5 W/cm2 × sec)
with 20% microbubble concentration. Thereafter, the efficiency
reached a plateau level at DNA concentrations of 15, 20 and 30
μg/ml. No significant differences were identified among the above
concentrations (18.74±1.57, 18.52±1.29 and 19.02±2.10,
respectively, all P>0.05). No significant differences were
identified in cell viability between the various DNA concentrations
(Fig. 2). In our experiments, 15
μg/ml was determined to be the optimal plasmid concentration.
FBS
No significant differences were identified in gene
transfection efficiency between the group containing 8% FBS and the
group in its absence (19.2±1.1 vs. 19.7±0.9%, P>0.05). In
addition, no significant differences in cell viability were
identified between the two groups (86.3±2.1 vs. 86.6±1.7%,
P>0.05; results not shown).
Mixing mode of cell and microbubble
The gene transfection efficiency was 8–15% under the
cell adherence mode (10.4±1.2%); however, the cell survival rate
was significantly decreased following ultrasound irradiation
(65.4±2.9%). Comparatively, gene delivery efficiency reached almost
20% (19.7±0.7%, P<0.01) under cell suspension mode without a
significant decrease in cell viability (76.6±2.1%, P<0.01).
Therefore, the cell suspension mode was the optimal mode in our
experiments.
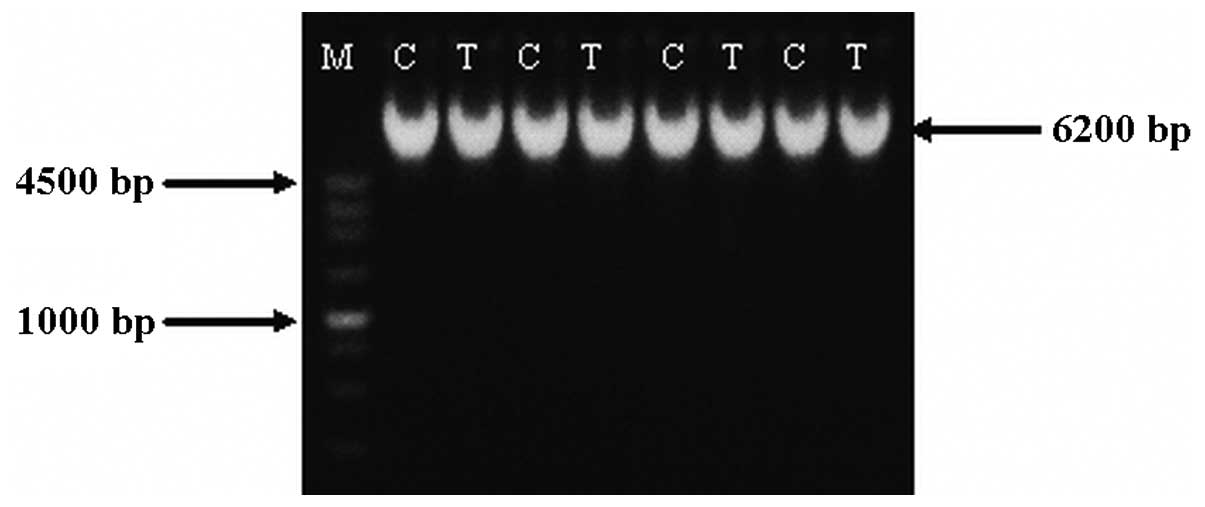
Integrity of hAng-1 DNA
Agarose gel electrophoresis revealed a band
corresponding to the hAng-1 gene that underwent ultrasound
irradiation at the same position as a band produced for the hAng-1
plasmid which had not undergone ultrasound irradiation. This
indicates that ultrasound irradiation used in this experiment did
not cause the hAng-1 gene DNA chain to break, and therefore did not
affect integrity (Fig. 3).
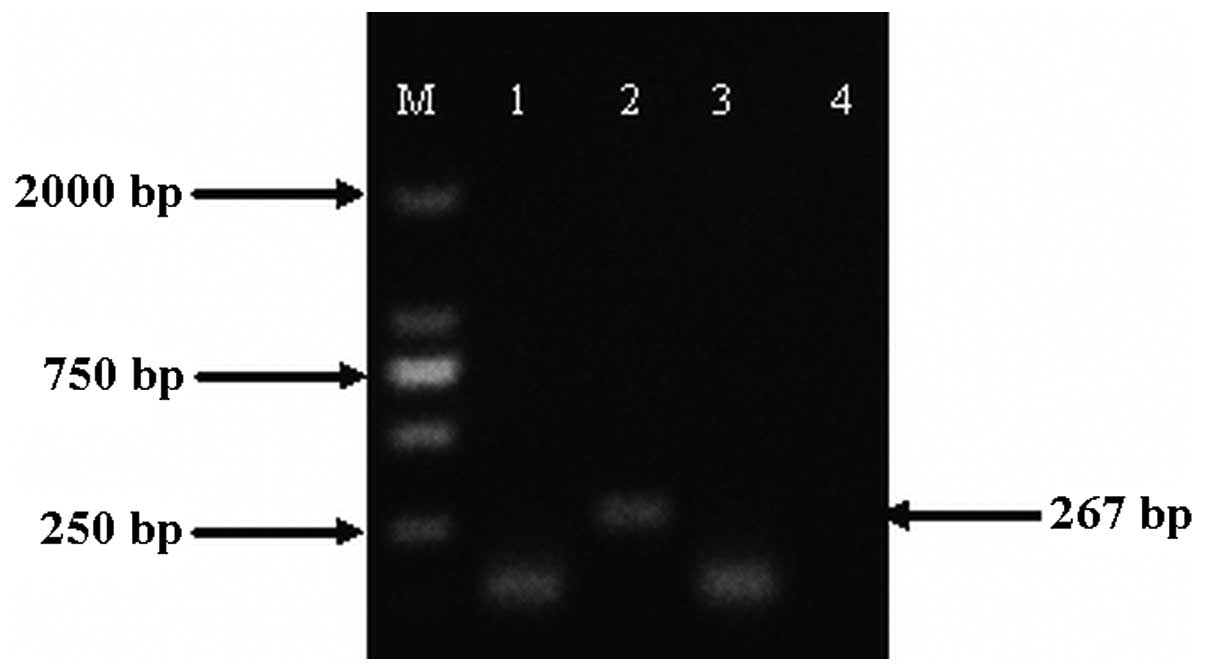
Determination of mRNA of hAng-1 gene
Following 48-h transfection, there was marked
eGFP-C3-hAng-1 mRNA expression in the
ultrasound/microbubble-mediated gene delivery group compared with
the control group, demonstrating that the eGFP-C3-hAng-1 gene is
expressed via the ultrasound/microbubble-mediated method (Fig. 4).
Determination of hAng-1 protein
hAng-1 protein was detected using SDS-PAGE/western
blot analysis in the ultrasound/microbubble-mediated gene delivery
group but was absent in the control group. This implied that the
delivery process via ultrasound/microbubbles does not affect the
gene eGFP-C3-hAng-1a translation into the functional protein
(Fig. 5).
Discussion
Optimal gene transfection conditions are utilized to
enhance gene expression levels and restrict cell death to 5% or
less (11). Previous studies have
discussed the effect of ultrasound exposure and irradiation
intensity on gene delivery (12,13);
however, the influential factors are more extensive than discussed.
Contrast agents, target cell and tissue type, corpuscular
physiological status and uptake capacity of cells to DNA, etc., are
the most common factors affecting the efficiency of gene transfer
(14). Determining how to match
these parameters to achieve optimal conditions is critical for the
improvement of gene delivery and expression.
The present study aimed to investigate hAng-1 gene
delivery efficiency and cellular viability under various
conditions. The study mainly focused on contrast agent, DNA
concentration, mixing mode of microbubble and cells and the
presence of FBS. With regard to contrast agent, we identified that
when the SonoVue microbubble was used as a gene transport medium,
with a gradual increase in ultrasound irradiation intensity to 1.5
W/cm2 and exposure time to 30 sec, and a microbubble
concentration of 20%, a progressive increase in hAng-1 gene
transfer was observed. Cell viability was gradually decreased,
although it remained at an acceptable range. The above three
parameters had similar effects on gene delivery and cell viability.
However, hAng-1 gene delivery did not continue to rise with
continued increase of levels of the above parameters. This
indicates that for a certain type of cell or tissue, particular
gene transfection efficiency cannot be increased after reaching
saturated levels. Beyond this level, the gene transfection
efficiency decreased rather than increased, likely due to extensive
cell death. Under these conditions, the ultrasound/microbubble gene
delivery may become unsafe. It should be noted that under
conditions of transfection saturation, a small increase in
ultrasound exposure or the microbubble concentration improves gene
transfection minimally, and at the cost of a large number of cell
deaths. Therefore, to obtain the optimal transfection parameters,
instead of maximal transfection efficiency, we should select lower
levels of ultrasound radiation intensity, exposure duration and
microbubble concentration within the plateau phase parameters to
achieve higher transfection efficiency and ensure the safety of
ultrasound/microbubble as a gene carrier.
In addition, we revealed that the mixed mode of
microbubbles and cells also affected transfection efficiency. In
preliminary experiments, we observed that in order to obtain high
transfection efficiency, cell and microbubble contact must be
sufficient. Thus, we added the microbubble solution with a
concentration of 20% microbubble solution per μl into the adherent
cells in a 6-well plate after removing the culture medium, and then
performed ultrasound irradiation. Although this method improved the
transfection efficiency, it reduced cell viability notably and
generated unstable results and poor reproducibility. The causes may
be: i) the microbubble always floats on the surface of the
transfection system due to buoyancy effects, thus it cannot be in
contact with adherent cells, ultimately decreasing the effects of
the microbubble on cells; ii) ultrasonic irradiation causes
detachment of adherent cells from the bottom of the 6-well plate,
resulting in reduced cell activity. Therefore, we mixed the
concentrated cell suspension with the microbubble solution. We
identified that this method not only increased contact opportunity
between microbubbles and cells, but also reduced the negative
effects on cell activity, thereby, significantly increasing Ang-1
gene transfection and decreasing cell injury.
Previous studies used fresh culture medium without
serum during gene transfection, changing to culture medium
containing serum post-transfection during the in vitro
ultrasound/microbubble gene delivery experiment (15–17).
The ultimate aim of the present study was to apply this new gene
delivery system in vivo, therefore it was necessary to
investigate the system in the presence of FBS, to determine whether
the microbubble/ultrasound-mediated gene transfer system performed
an effective gene transfection. The results of this study indicate
that the presence of FBS has no effects on the hAng-1 gene
transfection efficiency and cell viability. The therapeutic gene
was previously reported to be administered by intravenous
injection, therefore, successful transfection of the plasmid DNA
in vitro in the presence of FBS may be of greater value for
application of the ultrasound-directed gene transfer to
cardiovascular disease therapy in vivo.
Successful gene transfection not only requires cells
to take up the exogenous gene, but also maintain normal function of
expression and translation of the ingested gene. The heat and
micro-jet generated by cavitation effects may cause the plasmid DNA
to fragment (18,19), endanger the integrity of DNA and
affect its normal expression and functions. Therefore, we
investigated DNA integrity under optimal transfection conditions.
We identified that under optimal conditions, the
ultrasound/microbubble-mediated gene transfection system did not
affect DNA integrity in vitro. Furthermore, detection of
hAng-1 mRNA by quantitative RT-PCR and hAng-1 protein by
SDS-PAGE/western blot analysis provided evidence that the
transferred hAng-1 gene was expressed, and suggested normal
function inside the cells.
Current studies demonstrate relatively low
transfection efficiency, which is a common challenge for all
non-viral gene delivery methods (20,21).
The main causes may be correlated with lack of effective protection
of the plasmid when entering the cytoplasm and possible degradation
of the plasmid by DNase in the cytoplasm before entering the
nucleus (22). It is vital to
successfully optimize transfection parameters of the hAng-1 gene as
an angiogenesis gene for cardiac ischemic diseases therapy in
humans, including thorough understanding of the effects of the
system on blood flow and ultrasound energy attenuation following
penetration of skin and muscle tissue (23). The above optimal parameters
function as a significant reference, but currently remain
unsuitable for experiments in vivo.
To summarize, for a particular type of contrast
agent, the exogenous gene, and the target tissue or cell, it is
necessary to determine the specific optimal transfection conditions
for utilization of the ultrasound/microbubble gene transfer system.
Gene transfection efficiency is determined by multiple factors and
interactions. Further studies are required to clarify the
biological effects and functional mechanisms.
Acknowledgements
This study was supported by a grant from the
National Natural Science Fundation of China (30600141).
References
|
1
|
Korpanty G, Chen S, Shohet RV, et al:
Targeting of VEGF-mediated angiogenesis to rat myocardium using
ultrasonic destruction of microbubbles. Gene Ther. 12:1305–1312.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inagaki H, Suzuki J, Ogawa M, Taniyama Y,
Morishita R and Isobe M: Ultrasound-microbubble-mediated NF-kappaB
decoy transfection attenuates neointimal formation after arterial
injury in mice. J Vasc Res. 43:12–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamashita T, Sonoda S, Suzuki R, et al: A
novel bubble liposome and ultrasound-mediated gene transfer to
ocular surface: RC-1 cells in vitro and conjunctiva in vivo. Exp
Eye Res. 85:741–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen ZP, Brayman AA, Chen L and Miao CH:
Ultrasound with microbubbles enhances gene expression of plasmid
DNA in the liver via intraportal delivery. Gene Ther. 15:1147–1155.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larina IV, Evers BM and Esenaliev RO:
Optimal drug and gene delivery in cancer cells by
ultrasound-induced cavitation. Anticancer Res. 25:149–156.
2005.PubMed/NCBI
|
|
6
|
Duvshani-Eshet M, Adam D and Machluf M:
The effects of albumin-coated microbubbles in DNA delivery mediated
by therapeutic ultrasound. J Control Release. 112:156–166. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nie F, Xu HX, Tang Q and Lu MD:
Microbubble-enhanced ultrasound exposure improves gene transfer in
vascular endothelial cells. World J Gastroenterol. 12:7508–7513.
2006.PubMed/NCBI
|
|
8
|
Taniyama Y, Tachibana K, Hiraoka K, et al:
Development of safe and efficient novel nonviral gene transfer
using ultrasound: enhancement of transfection efficiency of naked
plasmid DNA in skeletal muscle. Gene Ther. 9:372–380. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo DP, Li XY, Sun P, et al:
Ultrasound/microbubble enhances foreign gene expression in ECV304
cells and murine myocardium. Acta Biochim Biophys Sin. 36:824–831.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Tachibana K and Kuroki M and Kuroki
M: Gene transfer with echo-enhanced contrast agents: comparison
between Albunex Optison and Levovist in mice - initial results.
Radiology. 229:423–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miller DL, Dou C and Song J: DNA transfer
and cell killing in epidermoid cells by diagnostic ultrasound
activation of contrast agent gas bodies in vitro. Ultrasound Med
Biol. 29:601–607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YS, Davidson E, Reid CN and McHale AP:
Optimising ultrasound-mediated gene transfer (sonoporation) in
vitro and prolonged expression of a transgene in vivo: potential
applications for gene therapy of cancer. Cancer Lett. 273:62–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinoshita M and Hynynen K: Key factors
that affect sonoporation efficiency in in vitro settings: the
importance of standing wave in sonoporation. Biochem Biophys Res
Commun. 359:860–865. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki R, Takizawa T, Negishi Y, Utoguchi
N and Maruyama K: Effective gene delivery with novel liposomal
bubbles and ultrasonic destruction technology. Int J Pharm.
354:49–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rahim A, Taylor SL, Bush NL, ter Haar GR,
Bamber JC and Porter CD: Physical parameters affecting
ultrasound/microbubble-mediated gene delivery efficiency in vitro.
Ultrasound Med Biol. 32:1269–1279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taniyama Y, Tachibana K, Hiraoka K, et al:
Development of safe and efficient novel nonviral gene transfer
using ultrasound: enhancement of transfection efficiency of naked
plasmid DNA in skeletal muscle. Circulation. 105:1233–1239.
2002.PubMed/NCBI
|
|
17
|
Guo DP, Li XY, Sun P, et al:
Ultrasound-targeted microbubble destruction improves the low
density lipoprotein receptor gene expression in HepG2 cells.
Biochem Biophys Res Commun. 343:470–474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O’Brien WD Jr: Ultrasound-biophysics
mechanisms. Prog Biophys Mol Biol. 93:212–255. 2007.
|
|
19
|
Kimmel E: Cavitation bioeffects. Crit Rev
Biomed Eng. 34:105–161. 2006. View Article : Google Scholar
|
|
20
|
Glover DJ, Lipps HJ and Jans DA: Towards
safe non-viral therapeutic gene expression in humans. Nat Rev
Genet. 6:299–310. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hurtado Picó A, Wang X, Sipo I, et al:
Viral and nonviral factors causing nonspecific replication of
tumor- and tissue-specific promoter-dependent oncolytic
adenoviruses. Mol Ther. 11:245–256. 2005.
|
|
22
|
Mehier-Humbert S, Bettinger T, Yan F and
Guy RH: Ultrasound-mediated gene delivery: Kinetics of plasmid
internalization and gene expression. J Control Release.
104:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki R, Takizawa T, Negishi Y, et al:
Tumor specific ultrasound enhanced gene transfer in vivo with novel
liposomal bubbles. J Control Release. 125:137–144. 2008. View Article : Google Scholar : PubMed/NCBI
|