Introduction
The presence or development of multidrug resistance
(MDR) is the primary cause of chemotherapy failure in the majority
of types of cancer. Overexpression of P-glycoprotein (P-gp) is the
most frequent cause of MDR (1,2).
Cancers sensitive to drugs initially are usually observed to become
drug resistant due to the drug-induced upregulation of P-gp.
Decreased response to anticancer drugs and poor treatment outcomes
were associated with the overexpression of P-gp (3).
In our previous studies, we focused our emphasis on
the ‘reversing’ effect of certain promising chemicals through the
modulation of P-gp expression in MDR cancer cells. We reported for
the first time that certain naturally occurring agents, such as
honokiol and schisandrin B, are capable of reversing drug
resistance through downregulating or inhibiting P-gp in MCF-7/ADR
cells (4–6). However, with the progression of our
research, we discovered that once MDR had occurred, it is hard to
reverse, as the expression level of P-gp was markedly high and
certain related targets such as anti-apoptotic signaling were
activated (7,8).
P-gp elevation is usually the initial step leading
to final MDR (8). Therefore, it
would be ideal to prevent or block P-gp upregulation induced by
anticancer drugs prior to the formation of MDR. This hypothesis led
to us changing our research strategy. Our recently published report
indicated a novel function of curcumin: it is capable of preventing
acquired drug resistance induced by adriamycin (ADM) in K562 cells
(9). This discovery was awarded
the China patent in 2010 (X. Hu and D. Xu. The application of
curcumin as a cancer MDR preventor. China patent application No. ZL
2007 1 0070320.4). However, curcuminoids contain at least three
active forms: curcumin (Cur), demethoxycurcumin (D-Cur) and
bisdemethoxycurcumin (BD-Cur). The chemical structures of these
curcuminoids are shown in Fig. 1.
Therefore, in the present study, we further examined the most
active forms of curcuminoids present in turmeric as potent
preventers of drug resistance and explored the possible mechanism
underlying the preventive effect. We also developed an in
vitro model to test the long-term preventive effect of
curcumin.
Materials and methods
Reagents
Cur, D-Cur and BD-Cur were purchased from Ronghe Co.
(Shanghai, China), with a purity of >99%; RPMI-1640 and fetal
calf serum were purchased from Gibco-BRL (Invitrogen Co., Grand
Island, NY, USA); MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and
ADM were purchased from Sigma Chemical Co. (St. Louis, MO, USA);
antibodies were purchased from Cell Signaling Technology Inc.
(Danvers, MA, USA) and Santa Cruz Biotechnology Inc. (Santa Cruz,
CA, USA); primer pairs and probe were purchased from Sangon Co.
(Shanghai, China); TRIzol reagent and the RT-PCR kit were purchased
from Invitrogen.
Cell lines and culture conditions
Human leukemia cell line K562 was maintained in
RPMI-1640 containing 10% fetal calf serum. Cells were cultured at
37°C in a 5% CO2 humidified atmosphere.
RT-PCR determination of mdr1 mRNA
The mdr1 mRNA level was detected by
quantitative real-time RT-PCR. K562 cells were seeded into 24-well
plates at a density of 0.5×105/well with 5 μmol/l Cur,
D-Cur, BD-Cur or cyclosporin A (CsA) for 24 h and then co-incubated
with ADM (40 ng/ml) for another 48 h. In the vehicle control and
ADM alone group, only 0.5% DMSO was used to pretreat K562 cells.
Total RNA from K562 cells was isolated by TRIzol reagent according
to the manufacturer’s instructions. Total RNA was reverse
transcribed to cDNA and stored at -20°C. The sequence of TaqMan
probe and primer pairs for mdr1 mRNA were described
previously (10). Primers and
probe for GAPDH were purchased from PE Applied Biosystems (TaqMan
GAPDH Control Reagent kit; Foster City, CA, USA).
Quantitative real-time PCR was performed as
described previously (4). In
brief, mdr1 forward primer 5′-AGAAAG CGAAGCAGTGGTTCA-3′ and
mdr1 reverse primer 5′-CGAACTGTAGACAAACGATG-AGCTA-3′
amplified a 90-bp fragment from the mdr1 cDNA that was
detected by the TaqMan probe 5′-TGGTCCGACCTTTTCTGGCCTTAT CCA-3′.
The reaction was performed in triplicate for each RT product.
Samples were heated for 2 min at 50°C and 10 min at 95°C, followed
by 40 cycles of amplification for 15 sec at 95°C and 1 min at 60°C.
The fluorescent signal was determined using Sequence Detector™
software (PE Applied Biosystems), giving the threshold cycle number
(CT) at which PCR amplification reached a significant
threshold. The ΔCT value was defined as the difference
in CT value for the mdr1 and GAPDH mRNA.
Accordingly, ΔCT =(mdr1 mRNA CT) -
(GAPDH mRNA CT), and the relative mdr1
mRNA expression level was presented as 2−ΔCT. Thus, the
mRNA expression levels of mdr1 are expressed as
concentrations relative to GAPDH mRNA.
Flow cytometry (FCM) analysis of P-gp
expression
K562 cells were seeded into 6-well plates at a
density of 2×105/well and then pretreated with 5 μmol/l
Cur, D-Cur, BD-Cur or CsA for 24 h and then co-incubated with ADM
(40 ng/ml) for another 72 h. In the vehicle control and ADM alone
group, only 0.5% DMSO was used to pretreat K562 cells. For FCM
analysis, cells were incubated with FITC-conjugated mouse
anti-human monoclonal antibody (1:200 diluted) at 4°C for 1 h, then
washed twice with ice-cold PBS and the level of fluorescent
staining was analyzed using Beckman EPICS Flow Cytometry (Coulter
Electronics, Hialeah, FL, USA).
Cytotoxicity assay
Cells were seeded into 96-well plates, and then
pretreated with or without 5 μmol/l Cur, D-Cur and BD-Cur for 24 h,
followed by addition of 80 ng/ml ADM, and then co-incubated for
another 72 h. Finally, 20 μl MTT (5 mg/ml) was added to each well
and the cells were incubated for another 4 h. Following aspiration
of the culture medium, the resulting formazan was dissolved with
150 μl DMSO. The absorbance was read with a model ELX800 Micro
Plate Reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) at 570
nm.
Nuclear factor-κB (NF-κB) translocation
detection
K562 cells were seeded into 6-well plates at a
density of 1×106/well and then pretreated with 5 μmol/l
Cur, D-Cur or BD-Cur for 30 min and then co-incubated with ADM (100
ng/ml) for another 30 min. In the vehicle control and ADM alone
group, only 0.5% DMSO was used to pretreat K562 cells. For western
blot analysis, cells were harvested for isolation of nuclear and
cytoplasm extracts using the Nuclear Extract kit (Active Motif,
Carlsbad, CA, USA). The concentration of the proteins was then
determined by the standard BCA protein assay. Approximately 40 μg
of each sample was loaded in gels for the immunoblot assay
according to a standard protocol. The primary antibodies used were
mouse monoclonal anti-human NF-κB and rabbit polyclonal anti-human
lamin B and α-tubulin (Cell Signaling Technology Inc.), and the
secondary antibody used was HRP-conjugated anti-rabbit IgG (Santa
Cruz Biotechnology Inc.). The signal was visualized with
HzfRP-conjugated secondary antibodies using ECL Plus Western
Blotting Substrate (Thermo Fisher Scientific, Rockford, IL, USA).
Antibodies to α-tubulin (a cytosol-specific protein) and lamin B (a
nucleus-specific protein) were used as loading controls.
Culture of resistant cell lines
Resistant cell lines were cultured using the
protocol of Cocker et al(11). In the ADM alone group, K562 cells
were seeded into a 55-cm2 cell culture flask at a
density of 2×106 cells and incubated in culture medium
containing ADM at an initial concentration of 0.1 μg/ml. When the
cells grew to confluence, they were collected and centrifuged for 5
min at 2,000 rpm, then 2×106 cells were reseeded, and
the final concentration of ADM was doubled until the endpoint of
the culture.
In the combined group, 5 μmol/l Cur was used for
prevention of drug resistance. Cur was added to the culture medium
2 h prior to the ADM. The above two cell lines were analyzed for
P-gp expression with FCM.
Statistical analysis
Data were expressed as the means ± SD, and analyzed
using one-way ANOVA. Statistical analysis was performed with the
SPSS 11.0 statistical analysis software. P<0.05 was considered
to indicate a statistically significant difference.
Results
Preventive effects of Cur, D-Cur and
BD-Cur on mdr1 mRNA upregulation
Real-time RT-PCR was used to compare the different
preventive effects of Cur, D-Cur and BD-Cur on the mdr1 mRNA
upregulation caused by short-term exposure to ADM. As Fig. 2 shows, in the ADM alone group, the
mdr1 mRNA expression level increased markedly when induced
by ADM. In the combined treatment groups, the induced mdr1
mRNA expression was blocked. D-Cur was the most active of the three
curcuminoids for prevention of mdr1 mRNA upregulation,
followed by BD-Cur and then Cur. BD-Cur had similar preventive
potency on mdr1 mRNA expression as CSA. After statistical
analysis, we found that there were significant statistical
differences between the ADM alone group and four combined treatment
groups (P<0.05), and no statistical significance could be
observed between the Cur, D-Cur and BD-Cur combined groups and the
CsA combined group (P>0.05). The above results indicated that
Cur, D-Cur and BD-Cur were capable of blocking the upregulation of
mdr1 mRNA expression induced by ADM, and D-Cur was the most
active compound, followed by BD-Cur and then Cur. The preventive
effects of the three curcuminoids were similar to CsA, which was
the positive control used in the study.
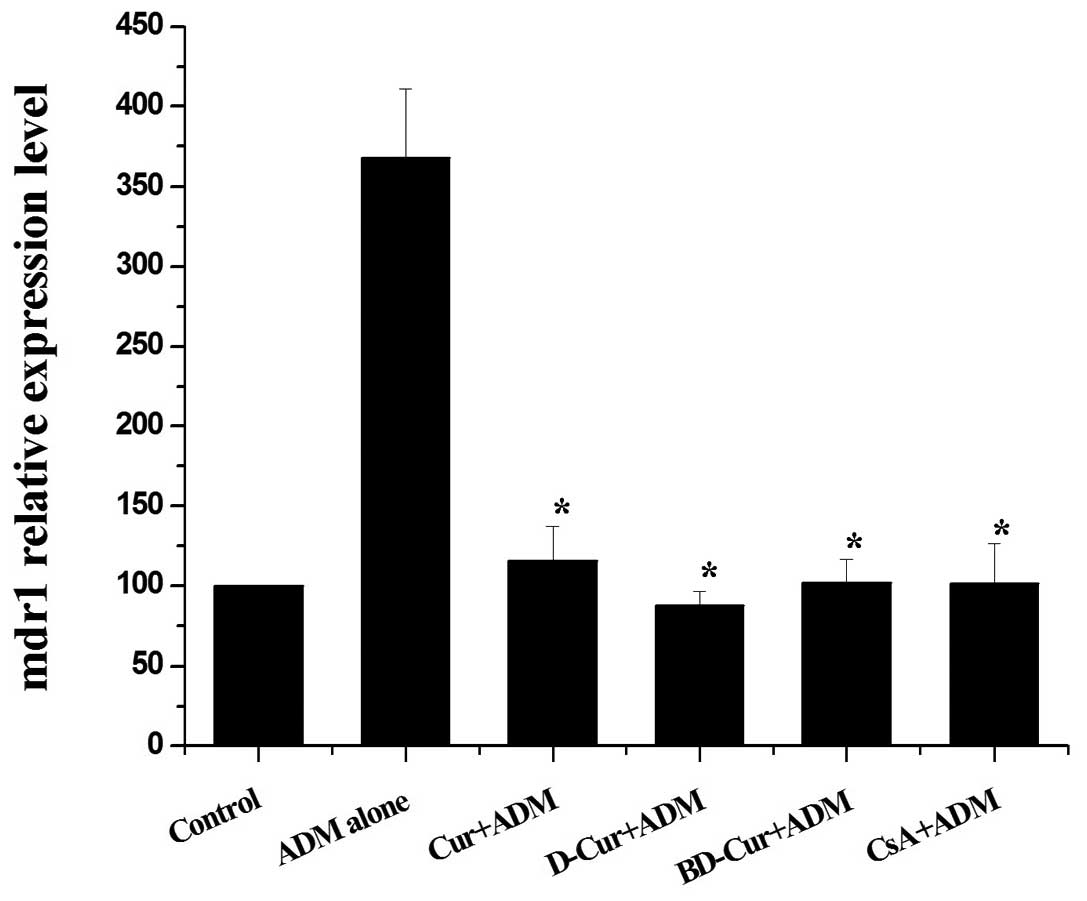 | Figure 2The preventive effects of Cur, D-Cur
and BD-Cur on mdr1 mRNA expression induced by ADM in K562
cells. K562 cells were pre-incubated with 5 μmol/l Cur, D-Cur,
BD-Cur or 5 μmol/l CsA (combined groups) or 0.5% DMSO (ADM alone
and control groups), respectively, for 24 h and then co-incubated
with 40 ng/ml ADM for another 48 h (combined groups and ADM alone
group). Finally, cells in the 6 groups were detected for
mdr1 mRNA levels by real-time RT-PCR. Data are shown as the
means ± SD of three independent experiments.
*Significantly different from the ADM alone group
(P<0.05). Cur, curcumin; D-Cur, demethoxycurcumin; BD-Cur,
bisdemethoxycurcumin; ADM, adriamycin; CsA, cyclosporin A. |
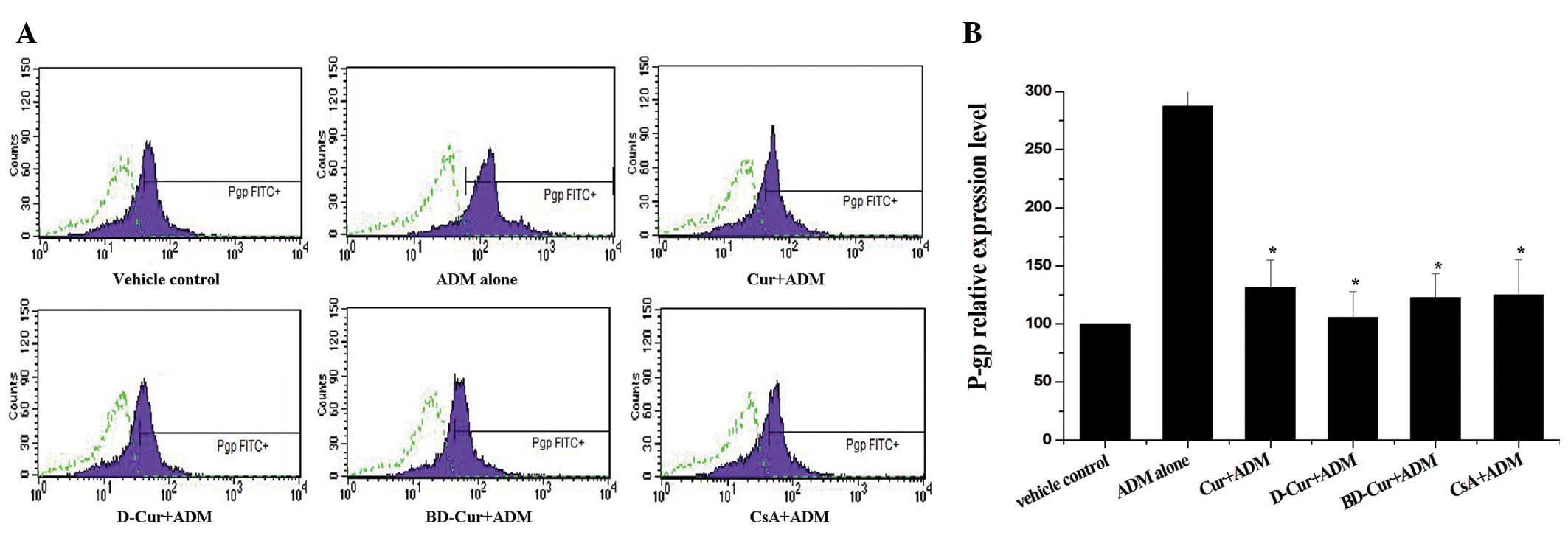
Preventive effects of Cur and its
derivatives on P-gp upregulation
Upregulation of P-gp was the most frequent reason
for intrinsic and acquired drug resistance. To prove whether the
preventive effect of curcuminoids on mdr1 mRNA upregulation
was also associated with its encoded protein, P-gp, we then further
separated the same groups and tested the P-gp expression by FCM. As
expected, a notably elevated P-gp expression level was also
observed after the short-term exposure to ADM alone (Fig. 3). However, in the combined
treatment groups, the P-gp upregulation induced by ADM was partly
blocked by Cur, D-Cur and BD-Cur. Similar to the previous RT-PCR
results, D-Cur had the most active preventive effect on P-gp
upregulation, followed by BD-Cur and then Cur. Statistical analysis
indicated a significant difference between the four combined
treatment groups and the ADM alone group (P<0.05). No
significant difference could be observed between the Cur, D-Cur and
BD-Cur combined groups and the CsA combined group (P>0.05).
These results suggested that short-term exposure of native K562
cells to ADM resulted in not only elevated mdr1 mRNA
expression (Fig. 2) but also
increased P-gp upregulation (Fig.
3). Cur and its derivatives could clearly prevent acquired
resistance triggered by ADM short-term exposure.
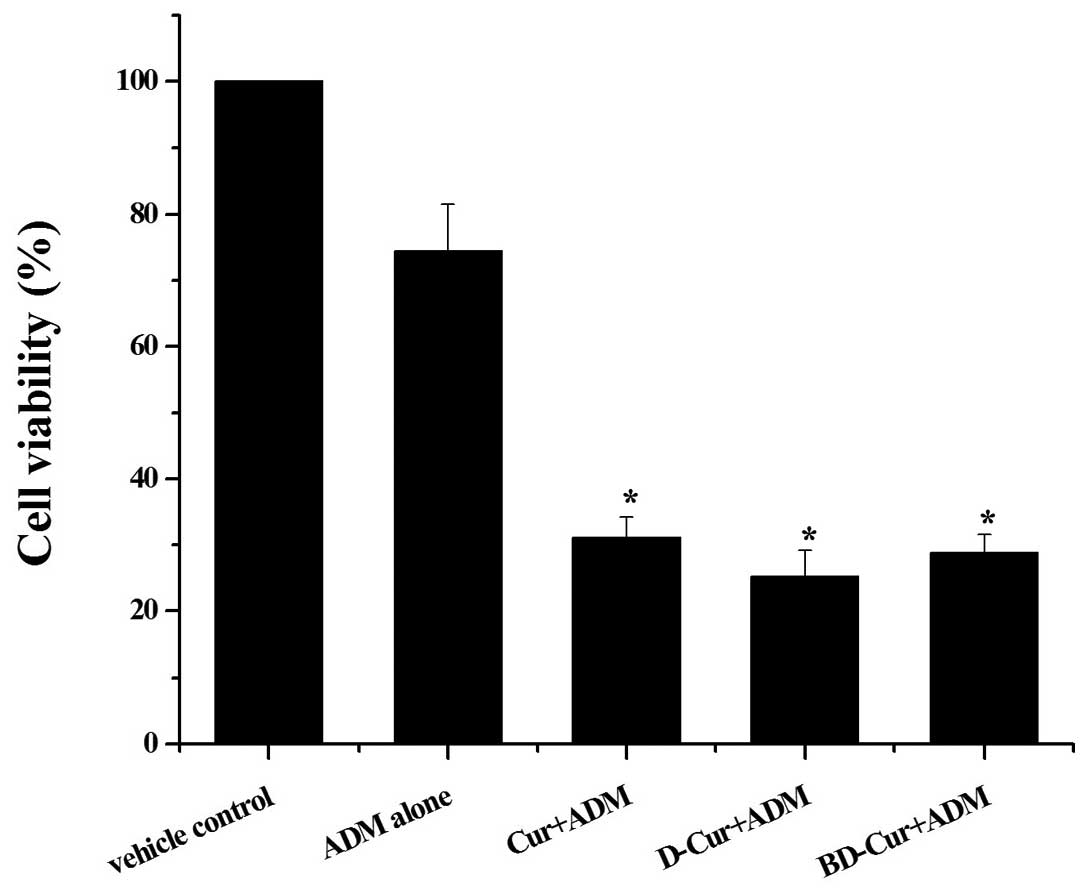
Effects of curcuminoids on the
sensitivities of K562 cells towards ADM
The results above demonstrated that Cur, D-Cur and
BD-Cur have the potency to block the expression of mdr1 mRNA
and its encoded P-gp induced by short-term exposure to ADM. To
further confirm the enhancing effects of the three curcuminoids on
ADM-induced cytotoxicity, we performed an MTT colorimetric assay to
assess cell viability. As shown in Fig. 4, pretreatment of K562 cells with
Cur, D-Cur and BD-Cur notably enhanced the sensitivity of native
K562 cells towards ADM. The mean cell viability detected by MTT
decreased sharply from 75% (ADM alone) to 31% (Cur-pretreated), 25%
(D-Cur-pretreated) and 29% (BD-Cur-pretreated), respectively.
Taking the above results together, we hypothesized that the
curcuminoid-enhanced ADM-induced cytotoxicity to K562 cells may be
partly due to the blocking of the upregulation of P-gp, the
transmembrane drug pump.
Western blot analysis detection of NF-κB
in nuclear and cytoplasm extracts
As a transcription factor, NF-κB exhibits
transcriptional activity when it is translocated into the nucleus.
We detected the expression of NF-κB in the nuclear and cytoplasm
extracts (Fig. 5) by western blot
analysis to explore the effect of curcuminoids on NF-κB nuclear
translocation. Following standardization with the internal control,
the densitometric units of nuclear NF-κB bands in the five groups
were 2.9, 12.3, 5.3, 8.1 and 4.0, respectively. Consistent with
mRNA and P-gp expression, nuclear NF-κB translocation was markedly
increased in the ADM alone group. However, in the
combined-treatment groups, nuclear NF-κB translocation was markedly
inhibited. Among the three curcuminoids, BD-Cur was the most active
for inhibiting the nuclear translocation of NF-κB induced by ADM,
followed by Cur and then D-Cur.
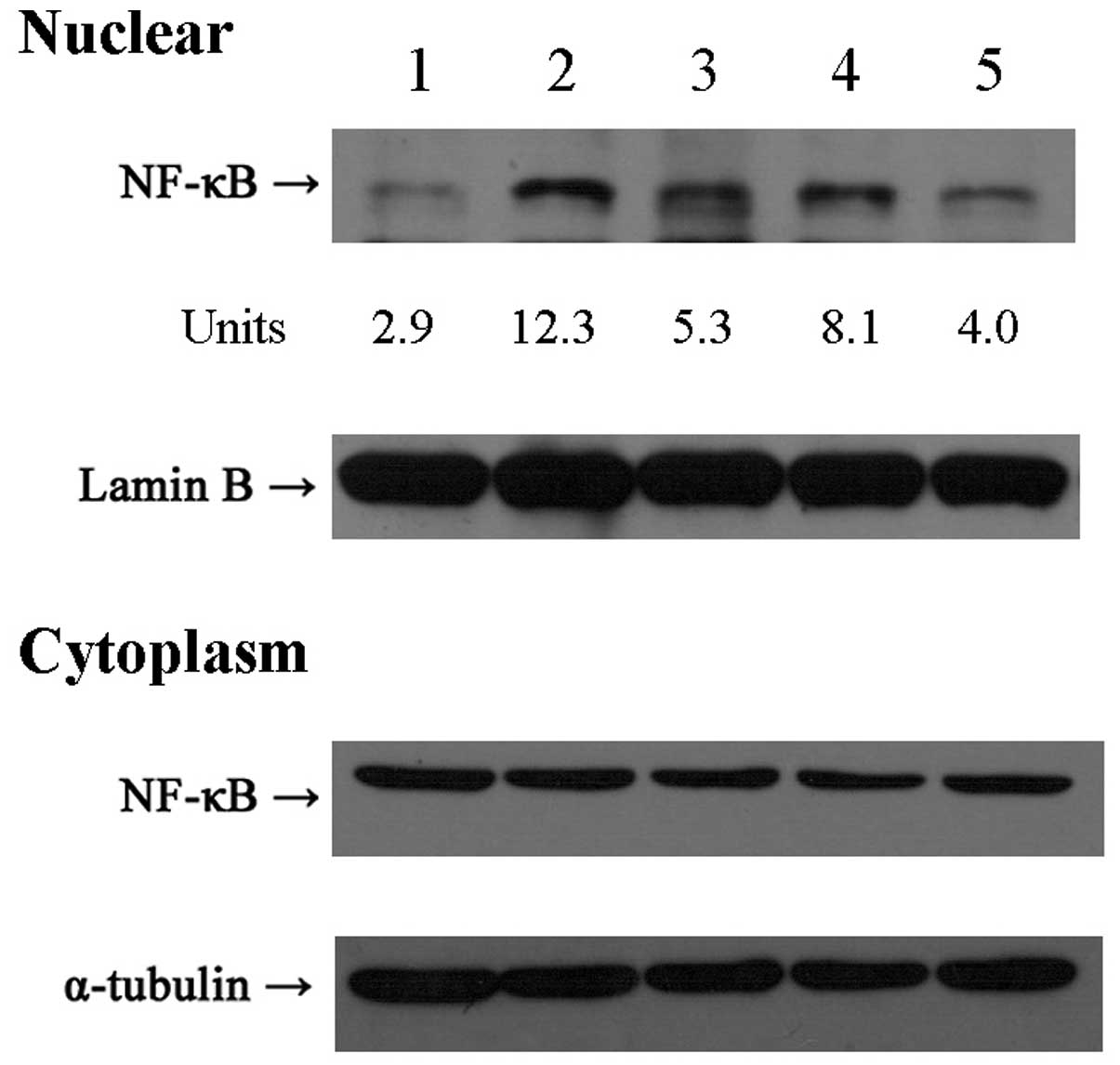 | Figure 5Western blot detection of NF-κB in
nuclear and cytoplasm extracts from K562 cells. K562 cells were
pretreated with 5 μmol/l Cur, D-Cur and BD-Cur (combined groups) or
0.5% DMSO (control and ADM alone group), respectively, for 30 min
and then co-incubated with 100 ng/ml ADM for another 30 min
(combined groups and ADM alone group). Cells were then collected
for the determination of NF-κB in nuclear and cytoplasm extracts by
western blot analysis. 1, Control; 2, ADM alone group; 3, 4 and 5,
corresponding Cur, D-Cur and BD-Cur combined groups. Cur, curcumin;
D-Cur, demethoxycurcumin; BD-Cur, bisdemethoxycurcumin; ADM,
adriamycin. |
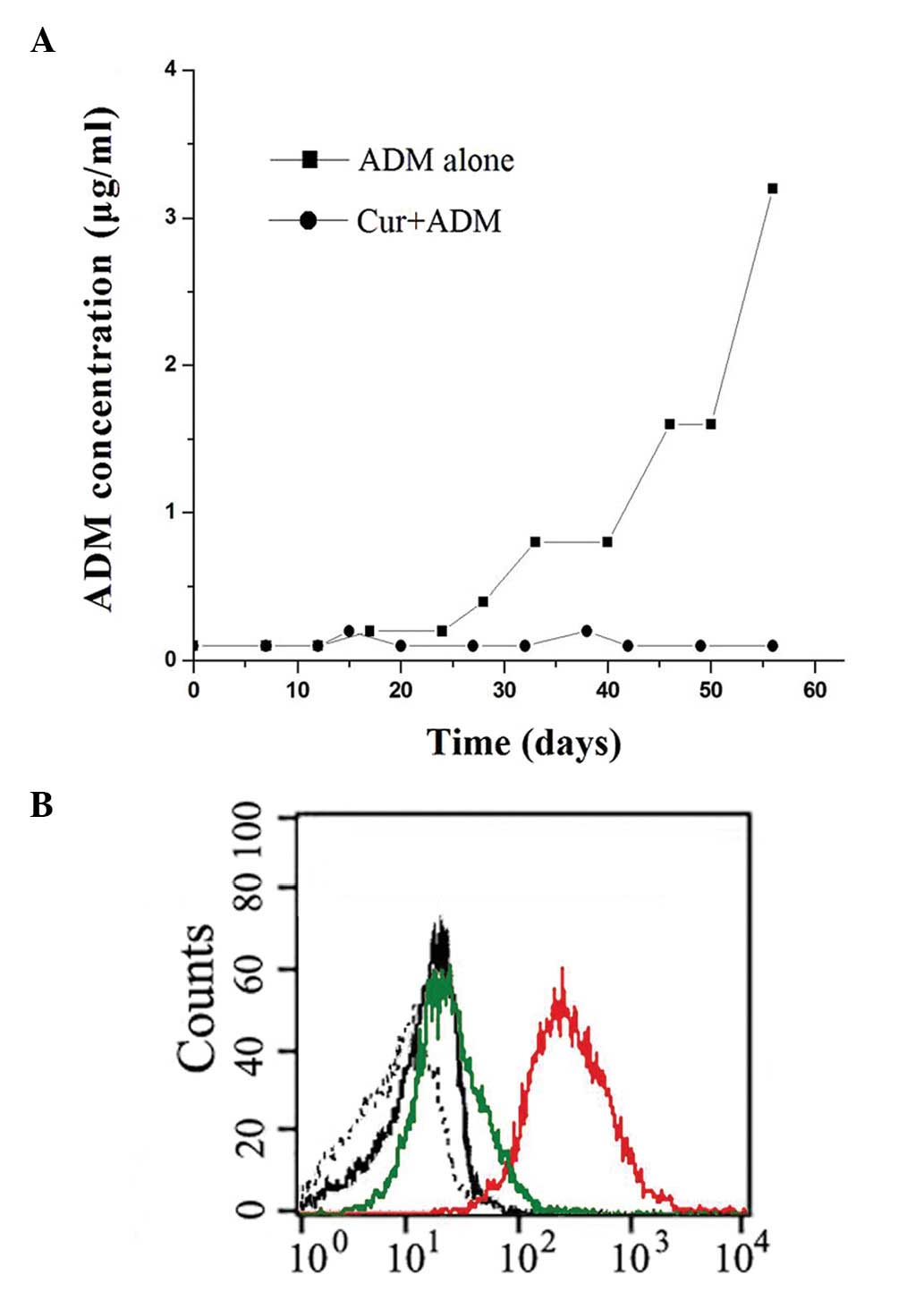
Effect of Cur on blocking the development
of the drug-resistant cell line
Cocker et al(11) had successfully developed a cell
line model for the acquisition of resistance to vincristine, and
tested several chemical agents for their capabilities to prevent
the acquisition of drug resistance. In our study, we conducted two
independent experiments simultaneously according to a set protocol.
As shown in Fig. 6A, the K562 cell
line acquired drug resistance rapidly in the ADM alone group during
the 56-day culture period, and finally, a resistant cell line
emerged. This cell line was capable of tolerating ADM at a final
concentration of 3.2 μg/ml, 32-fold higher than the initial
concentration. In the combined group, Cur was shown to have the
predominant preventive ability and the acquired resistance did not
develop during the same culture time. We further compared the P-gp
expression of the above two groups by FCM. Fig. 6B showed that ADM alone led to
marked upregulation of P-gp level (red), while combined Cur
pretreatment blocked the upregulation (green), and recovered the
P-gp level close to the vehicle control group level (black).
Discussion
The wild-type K562 cell line normally expresses very
low levels of mdr1 mRNA and P-gp, and has been characterized
extensively on the phenomenon of MDR, as well as the function of
P-gp (12,13). Therefore, we decided to use this
cell line to assess the preventive effect of Cur and its
derivatives on the expression of P-gp. CsA has been reported to
have the capacity to inhibit the induction of P-gp expression in
K562 cells (14), therefore it
appears to be a useful positive control for our present study.
Curcuminoids are a class of natural phenolic
coloring compounds found in the rhizomes of Curcuma longa
Linn., commonly known as turmeric. The major curcuminoids present
in turmeric are curcumin (Cur), demethoxycurcumin (D-Cur) and
bisdemethoxycurcumin (BD-Cur). Previous studies have suggested that
curcuminoids have enormous potency in the prevention and therapy of
cancer. Su et al reported that Cur is capable of inducing
apoptosis and cell cycle arrest in human colon carcinoma (15). Lin et al found that Cur
inhibits angiogenesis and tumor growth in ovarian cancer cells by
targeting the NF-κB pathway (16).
Notably, its two derivatives, D-Cur and BD-Cur, were proven to have
more potency than Cur in decreasing cell proliferation, inducing
apoptosis and inhibiting cancer invasion (17,18).
Additionally, researchers also reported that curcuminoids are
capable of reversing MDR by downregulating the expression of drug
resistance proteins such as P-gp (19,20)
and MRP1 (21,22). In conclusion, curcuminoids were
formerly considered as a class of promising anticancer or drug
resistance-reversing agents, but not as a novel class of
chemoresistance-preventing agents.
Recently, our latest report (9) demonstrated that Cur exhibits a novel
function of preventing chemoresistance. We found that ADM treatment
of K562 cells markedly elevated the expression of P-gp and induced
the occurrence of drug resistance. However, following Cur
pretreatment, the P-gp and mdr1 mRNA upregulation induced by
ADM was significantly inhibited. This result suggested that Cur has
dual function as a drug resistance inhibitor, not only ‘reversing’
but also ‘preventing’ acquired drug resistance. In the present
study, we have extended the above findings and compared the
preventive effect of each curcuminoid on the induction of drug
resistance. Similar to previous studies, we found that all the
three curcuminoids are capable of preventing the acquired drug
resistance induced by ADM in K562 cells, but the preventative
effect of each curcuminoid was different.
Historically, many studies concerning cancer MDR
have proved that numerous chemicals have the potency to reverse MDR
in drug-resistant cell lines. However, only a few of them, such as
CsA and PSC833, are capable of working as a dual drug resistance
modulator, not only reversing MDR in drug-resistant cells, but also
preventing chemoresistance in native cells (14,23).
In our previous studies, we reported for the first time that two
naturally occurring compounds, honokiol and schisandrin B, are
capable of reversing MDR through downregulating (4) and inhibiting P-gp (5) separately, but neither of them work as
a drug resistance preventer (data not shown). In the present study,
we introduced Cur and its derivatives as a novel class of
chemoresistance preventer with the dual function of MDR
modulation.
The mechanism of curcuminoids as bi- rather than
mono-modulators was unclear until now, but may be partly related to
the NF-κB nuclear translocation and activation, after which the
downstream target genes, such as mdr1, were regulated
(12,24). NF-κB has now been implicated in
carcinogenesis, invasion, metastasis and the development of drug
resistance in cancer cells. As a transcription factor, NF-κB
exhibits transcriptional activity when it is translocated into the
nucleus. Several studies demonstrated that mdr1 gene
expression could be regulated by NF-κB (25). Cur is an efficient inhibitor of
NF-κB since it inhibits NF-κB activation in multiple human
carcinomas. Lin et al reported that Cur inhibits tumor
growth and angiogenesis in ovarian carcinoma by targeting the NF-κB
pathway (16). Kunnumakkara et
al suggested that Cur potentiates the antitumor effects of
gemcitabine in pancreatic cancer by suppressing proliferation,
angiogenesis, NF-κB and NF-κB-regulated gene products (26). In our present study, results from
western blot analysis showed that ADM treatment of K562 cells
apparently increased the nuclear translocation of NF-κB. However,
after pretreatment with Cur or its derivatives, the nuclear NF-κB
translocation was inhibited markedly. This result demonstrated that
Cur and its derivatives could inhibit the ADM-induced increase of
NF-κB nuclear translocation and activation, and may be part of the
mechanism underlying the preventive effect of curcuminoids on
acquired drug resistance.
In the majority of research, Cur was found to be the
most active of the three derivatives present in turmeric (26–28),
while in certain studies BD-Cur exhibited the highest activity
(20). Certain data also suggested
that the mixture of all three is more potent than either one alone
(20). Our present results did not
closely correspond with the previous reports: results from RT-PCR
and FCM showed that D-Cur was the most active of the curcuminoids
for prevention of mdr1 mRNA and P-gp upregulation, followed
by BD-Cur and then Cur. However, results from western blot analysis
showed that BD-Cur was the most active agent in inhibiting the
ADM-induced increase of nuclear NF-κB translocation, followed by
Cur and then D-Cur. This ranking did not correlate with the trend
of the drug resistance-preventing activity of the three
curcuminoids and supports the theory that the activity of
preventing drug resistance was not necessarily proportional to the
activity of inhibiting NF-κB translocation.
Previously, Cocker et al had established a
preclinical model for the development of drug resistance to
vincristine (11). Therefore, in
our study, we conducted two independent experiments simultaneously
according to a set protocol. After 56 days of culture, native K562
cells acquired drug resistance rapidly in the ADM alone group and
could tolerate ADM at a 32-fold higher level than the initial
culture concentration. However, in the combined group, after 5
μmol/l Cur pretreatment, the development of drug-resistant cells
was blocked during the same culture time and the ADM-induced P-gp
upregulation was markedly reduced. This experiment strongly
indicated that the development of drug resistance may be
preventable using curcuminoids not only after short-term but also
after long-term exposure of native cancer cells to ADM. Thus,
chemoresistance prevention should be considered in the early course
of chemotherapy, particularly among patients whose cancers are
newly diagnosed and potentially drug sensitive. Therefore, in the
clinic, treatment combining chemotherapy drugs with
resistance-preventing agents may reduce the appearance of resistant
MDR subclones and lead to a longer disease-free survival.
As for the potency of curcuminoids in preventing
P-gp-mediated drug resistance, it is at least comparable to CsA.
However, curcuminoids were superior to CsA, since at effective
concentrations, curcuminoids do not have severe side effects like
CsA, such as immunosuppression and renal toxicity (23). In conclusion, curcuminoids are a
novel class of potent chemoresistance-preventing agents with low
toxicity, high safety and potential for wide clinical use.
Acknowledgements
The authors acknowledge financial support from the
National Natural Science Foundation of China (No. 30901741) and the
Zhejiang Provincial Natural Science Foundation of China (No.
2080308).
References
|
1
|
Cole SP, Bhardwaj G, Gerlach JH, et al:
Overexpression of a transporter gene in a multidrug-resistant human
lung cancer cell line. Science. 258:1650–1654. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schinkel AH and Jonker JW: Mammalian drug
efflux transporters of the ATP binding cassette (ABC) family: an
overview. Adv Drug Deliv Rev. 55:3–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu D, Lu Q and Hu X: Down-regulation of
P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by
honokiol. Cancer Lett. 243:274–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan Q, Lu Q, Zhang K and Hu X:
Dibenzocyclooctadiene lingnans: a class of novel inhibitors of
P-glycoprotein. Cancer Chemother Pharmacol. 58:99–106. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun M, Xu X, Lu Q, Pan Q and Hu X:
Schisandrin B: a dual inhibitor of P-glycoprotein and multidrug
resistance-associated protein 1. Cancer Lett. 246:300–307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakaeda T, Nakamura T, Hirai M, et al:
MDR1 up-regulated by apoptotic stimuli suppresses apoptotic
signaling. Pharm Res. 19:1323–1329. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: the early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006.PubMed/NCBI
|
|
9
|
Xu DTW and Shen H: Curcumin prevents
induced drug resistance: a novel function? Chin J Cancer Res.
23:218–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schiedlmeier B, Kuhlcke K, Eckert HG, Baum
C, Zeller WJ and Fruehauf S: Quantitative assessment of retroviral
transfer of the human multidrug resistance 1 gene to human
mobilized peripheral blood progenitor cells engrafted in nonobese
diabetic/severe combined immunodeficient mice. Blood. 95:1237–1248.
2000.
|
|
11
|
Cocker HA, Tiffin N, Pritchard-Jones K,
Pinkerton CR and Kelland LR: In vitro prevention of the emergence
of multidrug resistance in a pediatric rhabdomyosarcoma cell line.
Clin Cancer Res. 7:3193–3198. 2001.PubMed/NCBI
|
|
12
|
Shen H, Xu W, Chen Q, Wu Z, Tang H and
Wang F: Tetrandrine prevents acquired drug resistance of K562 cells
through inhibition of mdr1 gene transcription. J Cancer Res Clin
Oncol. 136:659–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yague E, Armesilla AL, Harrison G, et al:
P-glycoprotein (MDR1) expression in leukemic cells is regulated at
two distinct steps, mRNA stabilization and translational
initiation. J Biol Chem. 278:10344–10352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu XF, Slater A, Wall DM, Parkin JD,
Kantharidis P and Zalcberg JR: Cyclosporin A and PSC 833 prevent
up-regulation of MDR1 expression by anthracyclines in a human
multidrug-resistant cell line. Clin Cancer Res. 2:713–720.
1996.PubMed/NCBI
|
|
15
|
Su CC, Lin JG, Li TM, et al:
Curcumin-induced apoptosis of human colon cancer colo 205 cells
through the production of ROS, Ca2+ and the activation
of caspase-3. Anticancer Res. 26:4379–4389. 2006.PubMed/NCBI
|
|
16
|
Lin YG, Kunnumakkara AB, Nair A, et al:
Curcumin inhibits tumor growth and angiogenesis in ovarian
carcinoma by targeting the nuclear factor-kappaB pathway. Clin
Cancer Res. 13:3423–3430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yodkeeree S, Chaiwangyen W, Garbisa S and
Limtrakul P: Curcumin, demethoxycurcumin and bisdemethoxycurcumin
differentially inhibit cancer cell invasion through the
down-regulation of MMPs and uPA. J Nutr Biochem. 20:87–95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tamvakopoulos C, Dimas K, Sofianos ZD, et
al: Metabolism and anticancer activity of the curcumin analogue,
dimethoxycurcumin. Clin Cancer Res. 13:1269–1277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anuchapreeda S, Leechanachai P, Smith MM,
Ambudkar SV and Limtrakul PN: Modulation of P-glycoprotein
expression and function by curcumin in multidrug-resistant human KB
cells. Biochem Pharmacol. 64:573–582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Limtrakul P, Anuchapreeda S and Buddhasukh
D: Modulation of human multidrug-resistance MDR-1 gene by natural
curcuminoids. BMC Cancer. 4:132004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chearwae W, Wu CP, Chu HY, Lee TR,
Ambudkar SV and Limtrakul P: Curcuminoids purified from turmeric
powder modulate the function of human multidrug resistance protein
1 (ABCC1). Cancer Chemother Pharmacol. 57:376–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Revalde JL, Reid G and Paxton JW:
Modulatory effects of curcumin on multi-drug resistance-associated
protein 5 in pancreatic cancer cells. Cancer Chemother Pharmacol.
68:603–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egashira M, Kawamata N, Sugimoto K, Kaneko
T and Oshimi K: P-glycoprotein expression on normal and abnormally
expanded natural killer cells and inhibition of P-glycoprotein
function by cyclosporin A and its analogue, PSC833. Blood.
93:599–606. 1999.PubMed/NCBI
|
|
24
|
Su CC, Chen GW, Lin JG, Wu LT and Chung
JG: Curcumin inhibits cell migration of human colon cancer colo 205
cells through the inhibition of nuclear factor kappa B/p65 and
down-regulates cyclooxygenase-2 and matrix metalloproteinase-2
expressions. Anticancer Res. 26:1281–1288. 2006.
|
|
25
|
Amiri KI and Richmond A: Role of nuclear
factor-kappa B in melanoma. Cancer Metastasis Rev. 24:301–313.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar
|
|
27
|
Ahsan H, Parveen N, Khan NU and Hadi SM:
Pro-oxidant, anti-oxidant and cleavage activities on DNA of
curcumin and its derivatives demethoxycurcumin and
bisdemethoxycurcumin. Chem Biol Interact. 121:161–175. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sandur SK, Pandey MK, Sung B, et al:
Curcumin, demethoxycurcumin, bisdemethoxycurcumin,
tetrahydrocurcumin and turmerones differentially regulate
anti-inflammatory and anti-proliferative responses through a
ROS-independent mechanism. Carcinogenesis. 28:1765–1773. 2007.
View Article : Google Scholar
|