Introduction
In recent years, with the emergence of
drug-resistant or multidrug-resistant strains of Mycobacterium
tuberculosis (MTB), the resurgence of tuberculosis has become a
public health and social problem of worldwide concern. According to
statistics released by the WHO in 2007, the global annual TB
incidence is approximately 9.27 million cases and approximately 130
million people succumbed to the disease. The traditional BCG
vaccine is very unstable and the protective immunity it provides to
individuals from different parts of the world differ widely
(1). Therefore, it is essential
that studies concerning new tuberculosis vaccines and diagnostic
reagents are carried out.
A new generation of DNA vaccines has been developed
and has shown significant advantages in the prevention and
treatment of a variety of infectious diseases and cancers (2). One prerequisite for the development
of an effective vaccine is the selection of a highly immunogenic
antigen. MTB culture filtrate proteins (CFPs) have been used as
antigen compositions for TB prevention and in diagnostic reagents
(3,4). It has been found that the Ag85
complex is the major secreted protein antigen of MTB, accounting
for 30% of the total secreted proteins (5). The antigen-specific response of
CD4+ and CD8+ T cells to an Ag85A DNA vaccine
in BALB/c mice demonstrated that the Ag85A DNA vaccine was able to
produce a strong and broad T-cell response and CTL activity
(6). MTB secretes MTB protein 64
(MPT64), which is a major secreted protein in the early
culture filtrate of MTB, accounting for 8% of total secreted
proteins. The MPT64 protein has superoxide dismutase activity and a
strong cellular immune activity with a specificity higher than that
of tuberculin purified protein derivative (PPD) (7). In 1999, Kamath et al reported
that the immunization of mice with MPT64 DNA vaccines induced
spleen cells to produce IFN-γ and also induced MPT64-specific CTL
activity (8).
In order to combine the characteristics of the Ag85
and MPT64 proteins, a DNA vaccine was constructed and expressed in
the present study. The recombinant proteins were purified and then
validated by enzyme-linked immunospot (ELISPOT) assay.
Materials and methods
Materials
Pfu (Pyrococcus furiosus) Taq DNA polymerase,
dNTPs, buffer and MgCl2 were purchased from Shanghai
Sangon Biotech Co., Ltd. (Shanghai, China). The EcoRI and
BamHI restriction enzymes, T4 DNA ligase kit and DNA markers
were purchased from Takara Bio, Inc. (Shiga, Japan). The PCR
product purification kit was purchased from Omega Bio-Tek, Inc.
(Norcross, GA, USA). The gel extraction and plasmid extraction kits
were purchased from Shanghai Huashun Bioengineering Co., Ltd.
(Shanghai, China). The bacterial genomic DNA extraction kit was
purchased from Shanghai Sangon Biotech Co., Ltd (Shanghai, China).
The human IFN-γ capture and IFN-γ detection antibodies were
purchased from R&D Systems (Minneapolis, MN, USA).
Phytohemagglutinin (PHA) was acquired from Sigma (St. Louis, MO,
USA).
Animals
A total of 24 6-week-old special pathogen-free
female C57BL/6 mice were purchased from the Experimental Animal
Center of Jilin University, China. The mice were given free access
to food and water throughout the study. The study was approved by
the Institutional Animal Care and Use Committee of Jilin
University, Changchun, China.
Genomic DNA extraction
Small amounts of MTB H37Rv were inoculated in 7H9
liquid medium and cultured at 37°C for two weeks. The liquid
culture (3 ml) was centrifuged at 12,300 rpm for 1 min. The pellet
was resuspended in 50 μl lysis buffer (5 μl 10X PCR buffer, 5 μl
45g/l Tween-20, 5 μl 45 g/l NP-40, 0.5 μl 20 g/l protease K and
34.5 μl water) and kept at 55°C for 1 h and then in a boiling water
bath for 10 min to inactivate proteinase K. Following
centrifugation at 12,300 rpm for 1 min, the supernatant was
extracted twice with phenol/chloroform/isoamyl alcohol, followed by
extraction twice with phenol/chloroform. The extract was
precipitated using pre-cooled ethanol and then frozen at −80°C for
10 min. The precipitate was dissolved in 50 μl TE and stored at
−20°C.
Primers
The Ag85A and MPT64 primers were synthesized by
Invitrogen Life Technologies (Carlsbad, CA, USA). According to the
gene coding sequences of MTB H37Rv Ag85A and MPT64, four primers
were designed: P1, MPT64 forward primer
5′-GGAATTCCATGGTGCGCATCAAGATCTT-3′ containing EcoRI
restriction sites and start codon; P2, MPT64 reverse primer
5′-GGATCCCTAGGCCAGCATCGAGTC-3′ containing BamHI sites and
stop codon; P3, Ag85A reverse primer
5′-AGGATCCATGTTTTCCCGGCCGGGCTTG-3′, containing BamHI
restriction sites and start codon; and P4, Ag85A reverse primer
5′-GGAATTCCTATGTTCGGAGCTAGGCGCCCTGGG-3′, containing EcoRI
restriction sites and stop codon. The PCR reaction system consisted
of: 5 μl 10X buffer, 1 μl dNTPs (2.5 mM), 2 μl DNA, 0.5 μl (50 μM)
primer and 0.5 μl (2 units) Taq DNA polymerase, with water added to
50 μl. The reaction conditions were as follows: 95°C for 5 min,
95°C for 45 sec, 60°C for 30 sec and 72°C for 60 sec, for 32
cycles, then 72°C for 10 min. The PCR products were identified
using 0.8% agarose gel electrophoresis.
Ag85A/MPT64 cloning plasmid
construction
Double-digested plasmid pcDNA3.1(+) and the PCR
product were ligated by T4 DNA ligase at 22–24°C overnight and then
transformed into DH5α competent cells. The cells were cultured in
2YT medium containing 100 mg/l ampicillin. The blue/white method
was used for screening positive clones. White colonies were
selected randomly and inoculated into 4 ml liquid 2YT medium
containing ampicillin at 37°C with agitation overnight. Plasmid DNA
was extracted using the alkaline lysis method. The plasmid DNA was
digested with BamHI and EcoRI. The Ag85A (978 bp) and
MPT64 (738 bp) fragments were detected using 0.8% agarose gel
electrophoresis. Positive clones were sequenced by Shanghai Sangon
Biotech Co.
Ag85A/MPT64 eukaryotic expression plasmid
construction
Positive clones of the Ag85A/MPT64 recombinant
plasmid were digested with BamHI and EcoRI and the
products were analyzed using 0.8% agarose gel electrophoresis. A
PCR product of size 1716 bp was recovered using the PCR product
purification kit and then dissolved in 35 μl water and stored at
−40°C. At the same time, an eukaryotic expression plasmid was
prepared using the same method. Double-digested plasmid and DNA
were ligated at 22–24°C overnight and then transformed into BL21
competent cells. The cells were cultured in 2YT medium containing
100 mg/l ampicillin. The blue/white method was used for screening
positive clones. Positive clones were identified by BamHI
and EcoRI double digestion.
DNA vaccine preparation and animal
immunization
Following the mass culture of BL21 cells containing
the Ag85A/MPT64 plasmid, the plasmid was extracted and then
purified using phenol/chloroform/isoamyl alcohol (9). The concentration of plasmid was
adjusted to 1.5 g/l with sterile saline. Eight BALB/c mice were
injected with 60 μl 8.5g/l pivacaine in the side of the thigh.
Three weeks after pretreatment, the mice were immunized once with
50 μg Ag85A/MPT64 plasmid. The vector was injected intramuscularly
in a 60 μl volume. The control mice were immunized using the blank
plasmid and sterile saline, respectively (8 mice per group).
ELISPOT
Anticoagulant (3–4 ml) was taken from the mouse tail
vein, and after centrifugation, the lymphocyte layer was separated
from mouse serum, followed by purification of blood mononuclear
cells and adjustment of the cell concentration. The positive
control (PHA), negative control (normal saline), blank control
(plasmid) and different dilutions of the cell suspension were added
to a pre-packed IFN-γ capture monoclonal antibody microplate. The
cells were cultured at 37°C in 5% CO2 overnight. The
supernatant was discarded and the plates were washed with PBST. The
detection antibody was added to each well and the plate was
incubated at 37°C for 2 h. After washing, the substrate solution
was added to each well and the plate was incubated for a further 30
min. The plates were washed, the reaction was terminated and the
results were observed.
Results
Ag85A/MPT64 gene cloning
A specific band was amplified from a template of MTB
H37Rv genomic DNA by PCR. Following double digestion, the PCR
products were cloned into the plasmid and then transformed into
DH5α competent cells. White colonies were selected randomly and the
plasmids were extracted and double-digested. A 978-bp fragment and
a 738-bp fragment were obtained (Fig.
1A). Bi-directional sequencing results were consistent with
those previously reported (10).
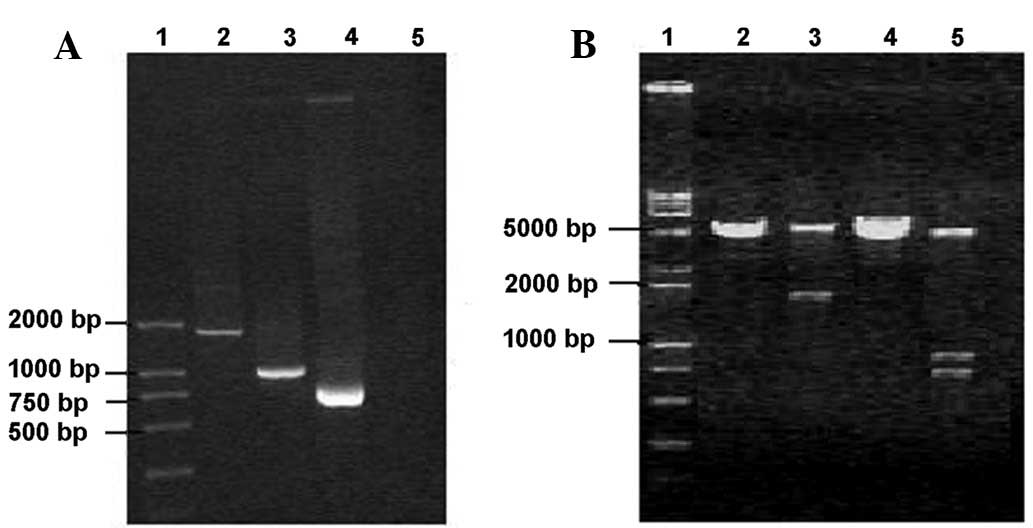 | Figure 1Detection of PCR products and vector
restriction sites using agarose gel electrophoresis. (A)
Electrophoresis results of the PCR products. Lane 1, DNA marker; 2,
PCR fragment of Ag85A and MPT64 fusion gene; 3, PCR product of
Ag85A gene; 4, PCR product of MPT64 gene; and 5, negative control.
(B) Electrophoresis results for vector digestion products. Lane 1,
DNA marker; 2, product of plasmid pcDNA3.1 (+) by single
EcoRI digestion; 3, product of positive clone by single
EcoRI digestion; 4, product of plasmid pcDNA3.1 (+) by
double digestion; and 5, product of positive clone by double
digestion. |
Ag85A/MPT64 vector construction
Plasmid DNA was extracted after positive clones were
selected and cultured. Following double enzyme digestion, a 1716-bp
fragment was recovered and then ligated with the plasmid fragment
that was digested using the same enzyme. Positive clones in the
transformed plates were selected randomly. A ~1716-bp fragment was
obtained after the plasmid was digested with EcoRI and
~978-bp and 738-bp fragments were obtained after the plasmid was
double-digested (Fig. 1B). The
nucleotide sequence of the recombinant gene encoding Ag85A/MPT64
had no mutations, and the gene was correctly inserted into the
vector.
ELISPOT
As shown in Fig. 2,
the numbers of ELISPOT spots for the mice immunized with
Ag85A/MPT64 were significantly greater than for the negative and
blank controls. The vaccine-stimulated production of immunoglobulin
(Ig) and cytokines may be screened effectively by ELISPOT assay
(11), which is important for
vaccine research. The soluble protein in the cells forms clearly
visible spots through a color reaction and these spots may be
directly and manually counted under a microscope. The spots may
also be counted by an ELISPOT analysis system and screened by
high-throughput screening, which is far more efficient than other
detection methods.
Discussion
A 4,400-kb fragment of MTB contains more than 4,000
genes and these genes encode proteins that may cause strong
cellular and humoral immune responses. Therefore, these genes,
including HSP, MPT64, Ag85, Esat-6 and PstS-3, may be considered as
candidate genes for DNA vaccines (12).
The Ag85 complex includes three subunits, Ag85A,
Ag85B and Ag85C, with relative molecular weights of
30×103–32×103 kDa, which exist in the MTB and
BCG cell walls and culture filtrates. The Ag85 content is highest
in the MTB cell wall proteins and secreted proteins and comprises
31–45% of the total protein (13).
Ag85A and PstS-3 DNA vaccines have been used to treat C57BL/6J
mouse tuberculosis infection and it was found that DNA vaccines can
prevent the recurrence of latent tuberculosis infection in mice and
shorten the cycle of conventional chemotherapy. Significant
reductions in the load of MTB in the lung and spleen occurred when
a Ag85A DNA vaccine was used alone or in combination with
chemotherapy to treat multidrug-resistant tuberculosis in mice for
2 months (14). MPT64 is a major
secretory protein of the MTB complex group and is also a key T-cell
antigen. MPT64 and ESAT6 DNA vaccines were used to immunize mice
and, by observing the pathological changes in the lungs of the
MTB-infected mice 4 weeks after abdominal challenge to evaluate the
protective strength of the DNA vaccine, it was found that the
changes in the MPT64 and ESAT6 DNA vaccine groups were similar to
those in the BCG group. This is because most of the mice
experienced the proliferative response and a small number of the
mice experienced a tissue reaction.
Ag85 is abundant in the cell wall and culture
filtrates of MTB and BCG cells. The mycolic acid transferase
activity of Ag85 has an important role in the later stages of the
synthesis of the MTB cell wall. Ag85A, Ag85B and Ag85C have highly
homologous sequences (13). The
Ag85/MPT64 protein is capable of stimulating the body to produce a
large amount of IFN-γ and induces strong T-cell proliferation. Mice
were able to resist MTB attack following immunization with
Ag85/MPT64 (15). It has also been
reported that high levels of Ag85/MPT64 antibodies were detected in
the serum following the infection of individuals with TB bacilli
(16).
Antigens are a feasible alternative to BCG. When BCG
enters the bladder, it combines with the tumor cell surface
fibronectin (FN) or bladder epithelial cells and causes an immune
response. This combination confers the characteristics of the Ag85
complex, known as the fibronectin-binding antigen (12,13).
The Ag85 protein was expressed in some regions of the bladder
following the infusion of a eukaryotic expression vector into the
bladder. Therefore, it appears that the bladder wall absorbs the
eukaryotic expression vector DNA vaccine.
An Ag85A/MPT64 DNA vaccine has been successfully
constructed in this study and it was confirmed that specific
antibodies against MTB were produced following animal immune
challenge.
ELISPOT assays are more specific and sensitive than
TST, which is helpful for the screening and monitoring tuberculosis
infection of patients undergoing anti-tumor necrosis factor (TNF)
treatment (17). A IFN-γ test
using TB-specific antigens enables the efficient diagnosis of
latent TB infection (LTBI) in HIV-infected individuals. ELISPOT
assays with their high sensitivity and specificity play an
important role in TB screening. Due to the high incidence of
tuberculosis and BCG vaccination in China, investigations may be
carried out using this technology among tuberculosis-exposed
populations to exclude the effect of TST and PPD false positives
and false negatives and for the early diagnosis of
tuberculosis-infected individuals According the degree of reaction,
preventive treatment may be carried out if necessary, which will
reduce the incidence and the source of infection.
References
|
1
|
Kaufmann SH: Is the development of a new
tuberculosis vaccine possible? Nat Med. 6:955–960. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fuller DH, Rajakumar PA, Wilson LA, et al:
Introduction of mucosal protection against primary, heterologous
simian immunodeficiency virus by a DNA vaccine. J Virol.
76:3309–3317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Havlir DV, Wallis RS, Boom WH, Daniel TM,
Chervenak K and Ellner JJ: Human immune response to
Mycobacterium tuberculosis antigens. Infect Immun.
59:665–670. 1991.
|
|
4
|
Weldingh K, Rosenkrands I, Jacobsen S,
Rasmussen PB, Elhay MJ and Andersen P: Two-dimensional
electrophoresis for analysis of Mycobacterium tuberculosis
culture filtrate and purification and characterization of six novel
proteins. Infect Immun. 66:3492–3500. 1998.PubMed/NCBI
|
|
5
|
Malin AS, Huygen K, Content J, et al:
Vaccinia expression of Mycobacterium tuberculosis-secreted
proteins: tissue plasminogen activator signal sequence enhances
expression and immunogenicity of M. tuberculosis Ag85.
Microbes Infect. 2:1677–1685. 2000.
|
|
6
|
Romano M, Roupie V, Wang XM, et al:
Immunogenicity and protective efficacy of tuberculosis DNA vaccines
combining mycolyl-transferase Ag85A and phosphate transport
receptor PstS-3. Immunology. 118:321–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oettinger T, Holm A and Hasløv K:
Characterization of the delayed type hypersensitivity-inducing
epitope of MPT64 from Mycobacterium tuberculosis. Scand J
Immunol. 45:499–503. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamath AT, Feng CG, Macdonald M, Briscoe H
and Britton WJ: Differential protective efficacy of DNA vaccines
expressing secreted proteins of Mycobacterium tuberculosis.
Infect Immun. 67:1702–1707. 1999.PubMed/NCBI
|
|
9
|
Tehranchian S, Akbarzadeh T, Fazeli MR,
Jamalifar H and Shafiee A: Synthesis and antibacterial activity of
1-[1,2,4-triazol-3-yl] and
1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5h)ones.
Bioorg Med Chem Lett. 15:1023–1025. 2005.
|
|
10
|
Wang Z, Potter BM, Gray AM, Sacksteder KA,
Geisbrecht BV and Laity JH: The solution structure of antigen MPT64
from Mycobacterium tuberculosis defines a new family of
beta-grasp proteins. J Mol Biol. 366:375–381. 2007.PubMed/NCBI
|
|
11
|
Bastos RG, Ueti MW, Knowles DP and Scoles
GA: The Rhipicephalus (Boophilus) microplus Bm86 gene plays a
critical role in the fitness of ticks fed on cattle during acute
Babesia bovis infection. Parasit Vectors. 3:1112010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi SS, Chung E and Jung YJ: Newly
identified CpG ODNS, M5-30 and M6-395, stimulate mouse immune cells
to secrete TNF-alpha and enhance Th1-mediated immunity. J
Microbiol. 48:512–517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Klerk LM, Michel AL, Bengis RG, Kriek
NP and Godfroid J: BCG vaccination failed to protect yearling
African buffaloes (Syncerus caffer) against experimental
intratonsilar challenge with Mycobacterium bovis. Vet
Immunol Immunopathol. 137:84–92. 2010.PubMed/NCBI
|
|
14
|
Liang Y, Wu X, Zhang J, et al: The
treatment of mice infected with multi-drug-resistant
Mycobacterium tuberculosis using DNA vaccines or in
combination with rifampin. Vaccine. 26:4536–4540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ingolotti M, Kawalekar O, Shedlock DJ,
Muthumani K and Weiner DB: DNA vaccines for targeting bacterial
infections. Expert Rev Vaccines. 9:747–763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai H, Tian X, Hu XD, Zhuang YH and Zhu
YX: Combined DNA vaccines formulated in DDA enhance protective
immunity against tuberculosis. DNA Cell Biol. 23:450–456. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takeno M, Murakami S and Ishigatsubo Y:
Tuberculosis associated with anti-TNF therapy. Nippon Rinsho.
65:1308–1313. 2007.(In Japanese).
|
















