Introduction
Inflammatory bowel disease (IBD) is a type of
chronic gastrointestinal tract-specific inflammation with unclear
etiology, that belongs to the category of autoimmune disease. The
incidence of IBD is increasing and it has become a common digestive
system disease and a major cause of chronic diarrhea (1). The disease is now considered to be
primarily mediated by an immune response, as well as involving
genetic and environmental factors. Dysfunction of immune regulation
may be the key factor in its pathogenesis (2,3). Th1
cells are considered to be closely related to IBD pathogenesis;
however, the nature of this relationship has not been fully
explained. A new CD4+ helper T cell subtype, Th17, has
been identified, which is named after the major cytokine it
secretes, interleukin (IL)-17. Previous studies have suggested that
Th17 cells are important in the pathogenesis of autoimmune
diseases, and these cells have therefore become a popular topic for
immunological studies (4,5). In the current study,
2,4,6-trinitrobenzenesulfonic acid (TNBS) was used to establish an
animal model of IBD, and the mutual relationship of Th17 and Th1
cells in the pathogenesis of IBD was explored using this model. In
addition, a monoclonal antibody against the Thl7 cytokine was used
to specifically block IL-17 in order to observe its effect on other
pro-inflammatory cytokines. The exploration of the expression of
cytokines by Th17 cells in IBD mice and investigation of the
mechanisms should provide new therapeutic directions for the
treatment of IBD.
Materials and methods
Materials
A total of 40 female BALB/C mice aged 8 weeks and
weighing ~25 g (from the SPF grade Laboratory Animal Center of
Harbin Medical University Public Health College) were used in this
study. The study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of Shengjing Hospital of China Medical
University.
Establishment of experimental model
After weighing and assigning an ID, the BALB/C mice
were randomly divided into the normal control and IBD model groups
comprising 5 and 35 mice, respectively. The normal control group
was normally fed. With reference to the literature, an IBD mice
model was established by enema using a solution of TNBS (Sigma, St.
Louis, MO, USA). Specifically, the mice were fasted for 24 h prior
to TNBS enema and anesthetized by an intraperitoneal injection of
0.08 ml 5% chloral hydrate. TNBS and 50% ethanol solution were
mixed at a 1:1 ratio and a total of 0.2 ml of the mixture was used
for the enema. The mice received a normal diet following the
procedure. The model group mice were sacrificed on days 1, 2, 3, 5,
7, 14 and 21 following the TNBS enema and the normal control mice
were sacrificed on day 3. Colon tissues with evident damage were
removed by dissection and preserved at -80°C for ELISA analysis. On
day 3, peripheral blood, spleen and mesenteric lymph nodes from the
normal and IBD mice were also obtained when sacrificing the
mice.
Peripheral blood mononuclear cells
(PBMCs)
Blood was drawn from the eyeballs of the mice,
placed in anticoagulant tubes and centrifuged in Ficoll-Hypaque
density gradient separation solution to obtain PBMCs.
Spleen mononuclear cells (SMCs)
Suspensions of mouse spleen cells were centrifuged
in Ficoll-Hypaque density gradient separation solution to isolate
the SMCs.
Culturing of SMCs
SMCs isolated from the mice 3 days after TNBS enema
were divided into 3 groups, each containing 1×106/ml
cells. The cells were placed into RPMI-1640 culture medium and 1
μg/ml anti-IL-17 antibody, 10 μg/ml anti-IL-17 antibody or an equal
amount of PBS were added. The same number of SMCs from the normal
control group were also placed in RPMI-1640 culture medium and an
equal amount of PBS was added. Cells from all 4 groups were
cultured at 37°C in a 5% CO2 incubator and removed after
24 h.
ELISA
Tissues were homogenized and centrifuged in order to
generate a supernatant. An ELISA procedure was performed following
the instructions provided with the kit (JingMei Biotechnology Co.,
Ltd., Shenzhen, China) and the absorbance value of each well was
read at 450 nm after terminating the reaction. A standard curve was
plotted with OD values as the y-axis and standard concentrations as
the abscissa. Sample concentrations were calculated according to
the corresponding OD value.
Western blot analysis
Western blot analysis was used to detect IL-17
protein expression in the PBMCs, SMCs, mesenteric lymph node cells
and colon tissues of the normal control mice and disease model mice
sacrificed on day 3. First, total protein was extracted from the
cells and tissues of each group and UV spectrophotometry was used
for protein quantification. Protein samples were then subjected to
SDS-PAGE and transferred onto PVDF membranes following
electrophoresis. The membranes were blocked at room temperature for
2 h and incubated with 1:800 rabbit anti-mouse anti-IL-17 primary
antibody at 4°C overnight. After washing with PBS, the membranes
were incubated with 1:1,000 goat anti-rabbit IgG/HRP secondary
antibody at room temperature for 2 h, washed 3 times, reacted with
chemiluminescence reagents for 5 min, exposed and then
developed.
RT-PCR
Total cellular RNA was extracted following the kit
instructions (Microgene Co., Shanghai, China) and UV
spectrophotometry was used to determine the RNA quality and
concentration in the samples. RNA was synthesized into cDNA by a
reverse transcription reaction for later PCR amplification. The
primers used for the internal control β-actin were: forward,
GAATACTCTATTGCCGATGGT and reverse, CGATGGGTTTGCGTTTG; amplification
length, 722 bp. The primers for tumor necrosis factor (TNF)-α were:
forward, TTACGCCTTTGAAGTTAGCAG and reverse, CGTCC AAATACATCGCAAC;
amplification length, 498 bp. For IL-6, the primers were: forward,
ACAACGGGTGGAAC ATTACC and reverse, TGGGTTTGCGTTTGTGAG;
amplification length, 422 bp. For interferon-γ (IFN-γ), the primers
were: forward, ACCCTCCTGGTTATTGAGCC and reverse,
TGGTAATGTTCCACCCGTTG; amplification length, 288 bp. The synthesized
primers were purchased from Shanghai Biotechnology Co., Ltd.
(Shanghai, China). The PCR products were subjected to
electrophoresis on a 1.5% agarose gel and analyzed using a UV gel
imaging analysis system. The relative expression levels of the
target genes were calculated as the ratio of the absorbance of the
target gene band to that of the internal control.
Statistical analysis
Experimental data were analyzed by ANOVA using SPSS
13.0 software and p<0.05 was considered to indicate a
statistically significant result.
Results
ELISA
Following the administration of TNBS by enema, ELISA
was used to detect the expression levels of IL-17 and IFN-γ in the
mouse colon tissues. The results revealed that the levels of IL-17
were significantly increased (p<0.05 compared with the normal
control group) on day 1 following the TNBS treatment, peaked on day
3 and decreased afterwards. The IL-17 levels in the model group
remained significantly different from those in the normal control
group until day 7 (p<0.05), but decreased to levels of no
significant difference from the control on days 14 and 21
(p>0.05). The levels of IFN-γ had not significantly increased on
day 1 (p>0.05 compared with the normal control group) and slowly
increased afterwards to a significant level on day 3 (p<0.05).
The IFN-γ level peaked on day 7 and subsequently began to decrease.
On days 14 and 21, the IFN-γ levels were significantly different
from those in the control group (p<0.05) (Table I). These results suggest that the
involvement of IL-17 in the TNBS-induced colitis occurred earlier
than that of IFN-γ, while the effect of IFN-γ in the target tissue
was sustained for longer since its expression remained detectable
even after the elevated expression of IL-17 had disappeared. Since
the expression of IL-17 reached its highest levels on day 3, the
model mice on day 3 after TNBS treatment were selected for further
study.
 | Table IELISA results of IL-17 and IFN-γ
levels in mouse colon tissues. |
Table I
ELISA results of IL-17 and IFN-γ
levels in mouse colon tissues.
| Group | IL-17 (pg/ml) | IFN-γ (pg/ml) |
|---|
| Day 0 | 89.4±23.7 | 54.6±16.5 |
| Day 1 | 118.7±29.7a | 60.8±18.9 |
| Day 2 | 160.1±34.7a | 67.4±20.1 |
| Day 3 | 219.4±57.8a | 78.6±19.8a |
| Day 5 | 187.5±44.6a | 90.6±30.4a |
| Day 7 | 159.8±36.8a | 109.8±29.8a |
| Day 14 | 98.4±18.7 | 90.6±20.1a |
| Day 21 | 90.8±24.6 | 77.8±26.3a |
Western blot analysis
As shown in Fig. 1,
western blot analysis was used to detect IL-17 protein expression
in the PBMCs, SMCs, mesenteric lymph node cells and colon tissues
of the normal control group and model group 3 days after TNBS
treatment. The results revealed that the IL-17 levels in the SMCs,
mesenteric lymph node cells and colon tissues of the model group
were significantly higher than those of the normal control group
(p<0.01) and were particularly high in colon tissues. The IL-17
levels in the PBMCs of the model group were not significantly
different from those of the control group (p>0.05) (Table II).
 | Table IIWestern blot analysis detection of
IL-17 protein levels in various tissues. |
Table II
Western blot analysis detection of
IL-17 protein levels in various tissues.
| Group | PBMCs | Mesenteric lymph
nodes | SMCs | Colon tissues |
|---|
| Model | 0.83±0.15 | 3.98±0.54a | 3.01±0.52a | 2.06±0.30a |
| Control | 1.03±0.18 | 0.82±0.13 | 1.02±0.19 | 0.28±0.06 |
RT-PCR
As shown in Fig. 2,
SMCs from the day 3 model group mice were treated with PBS, 1 μg/ml
anti-IL-17 antibody or 10 μg/ml anti-IL-17 antibody, respectively,
and the SMCs from the normal control group mice were treated with
PBS only. The cells were cultured for 24 h and RT-PCR was used to
detect the expression of TNF-α, IFN-γ and IL-6 mRNA. The TNF-α,
IFN-γ and IL-6 levels of the PBS model group were significantly
different from those of the normal PBS control group (p<0.05).
The TNF-α and IL-6 levels in the 1 μg/ml antibody-treated model
group were decreased compared with those of the PBS model group but
the differences were not statistically significant (p>0.05); the
IFN-γ levels in the 1 μg/ml antibody-treated model group were also
not significantly different from those in the PBS model group
(p>0.05). The TNF-α and IL-6 levels in the 10 μg/ml
antibody-treated model group were significantly different from
those in the PBS model group (p<0.05), while the difference in
IFN-γ levels between the 10 μg/ml antibody-treated model group and
the PBS model group was not statistically significant (p>0.05)
(Table III). The results
indicate that the expression levels of TNF-α, IFN-γ and IL-6 mRNA
increased in the SMCs of the TNBS-induced colitic mice and that the
application of an anti-IL-17 antibody reduced the secretion of
TNF-α and IL-6, but had no effect on IFN-γ secretion.
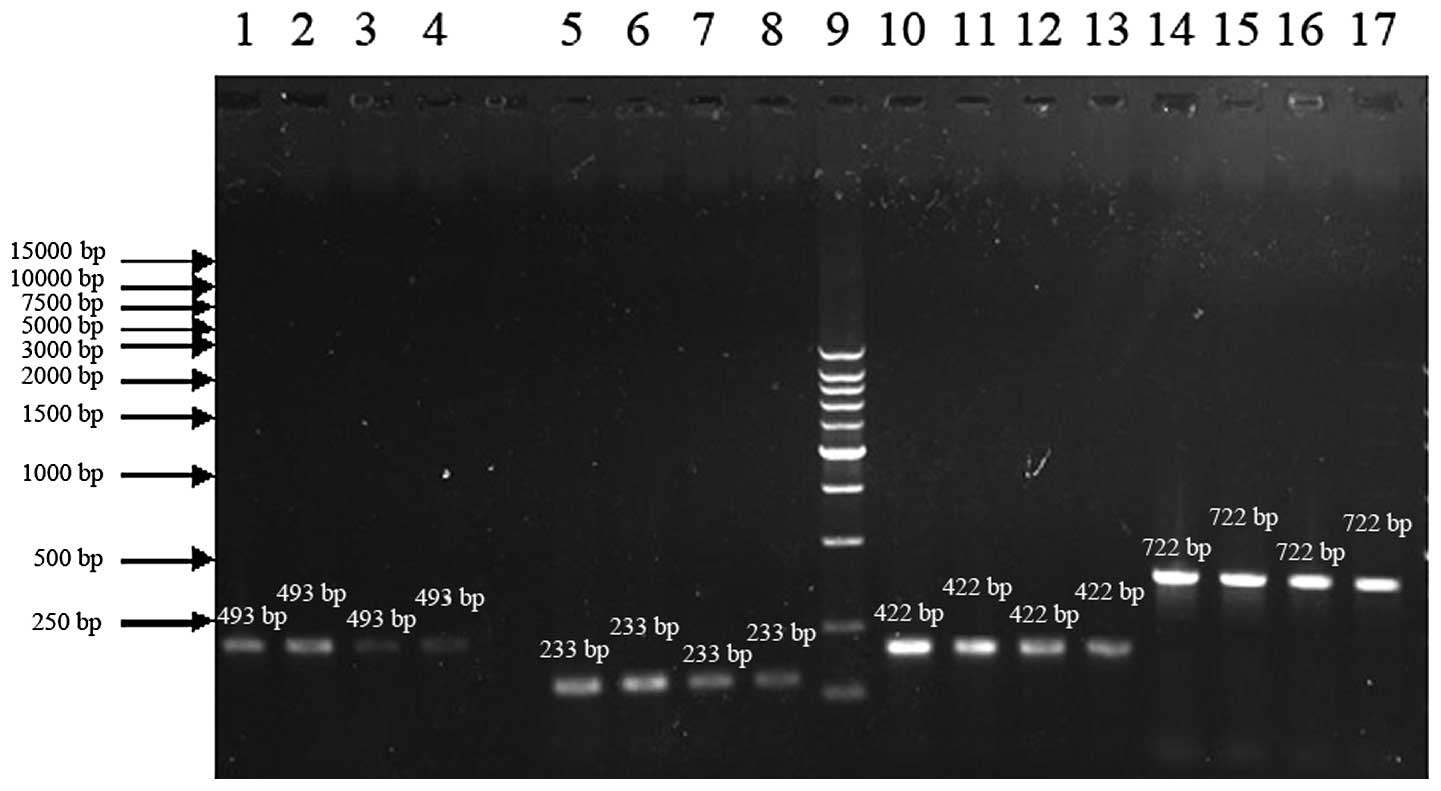 | Figure 2mRNA expression of TNF-α, IFN-γ and
IL-6 detected by RT-PCR. Lanes 1–4: TNF-α mRNA for the PBS model, 1
μg model, 10 μg model and PBS normal control groups, respectively.
Lanes 5–8: IFN-γ mRNA for the PBS model, 1 μg model, 10 μg model
and PBS normal control groups, respectively. Lane 9: Marker. Lanes
10–13: IL-6 mRNA for the PBS model, 1 μg model, 10 μg model and PBS
normal control groups, respectively. Lanes 14–17: β-actin mRNA for
the PBS model, 1 μg model, 10 μg model and PBS normal control
groups, respectively. |
 | Table IIIComparison of TNF-α, IFN-γ and IL-6
mRNA levels. |
Table III
Comparison of TNF-α, IFN-γ and IL-6
mRNA levels.
| Group | TNF-α | IFN-γ | IL-6 |
|---|
| PBS model | 0.54±0.11a | 0.53±0.10a | 0.92±0.18a |
| 1μg model | 0.49±0.12b | 0.52±0.13b | 0.84±0.20b |
| 10μg model | 0.29±0.06c | 0.50±0.12 | 0.77±0.15c |
| PBS control | 0.28±0.07 | 0.40±0.08 | 0.73±0.13 |
Discussion
It is currently considered that the pathogenesis of
IBD is related to a series of susceptibility genes, environmental
factors and immune system abnormalities (6). A number of factors of the intestinal
mucosal immune system, including cytokines and immune regulation,
are involved in the pathogenesis of IBD.
The role of CD4+ T cells in the
pathogenesis of IBD has been well-recognized. Previous studies have
suggested that the IL-12/IFN-γ axis-mediated Th1-type immune
response is closely related to the incidence of IBS; however, TNBS
is able to induce colitis in mice even following IFN-γ knockout or
anti-IFN-γ monoclonal antibody application (7), indicating that IFN-γ is not the key
factor in colitis induced by TNBS. Th17 cells have been confirmed
to be a CD4+ T-cell subtype. Previous studies have shown
that IL-23 is capable of stimulating memory CD4+ T cells
to produce IL-l7 and is involved in the proliferation of
Thl7/ThlL-17 cells and the maintenance of their survival (8). Related studies using mice revealed
that almost no Th17 cells were formed in the absence of IL-23
(9,10). It has been demonstrated that IL-17
expression is significantly increased in patients with a variety of
autoimmune diseases and animal models of these diseases, and its
functional status is closely related to the onset of numerous
autoimmune diseases (11). A
previous study found that the IL-23/Thl7 cell axis is closely
related to the pathogenesis of IBD (12). Blaschitz and Raffatellu (13) revealed that IL-17 was not expressed
in normal colonic mucosa, ischemic colitis or infectious colitis.
However, IL-17 expression was significantly increased in the
affected mucosa of active IBD patients. IL-17R knockout or
overexpression of IL-17R IgGl fusion protein has been reported to
significantly inhibit TNBS-induced colitis (14), indicating that Th17 cells are
significantly involved in the pathogenesis of IBD. However, further
studies focusing on Th17 cells revealed that Th17 cells are not the
only effector cells to induce organ-specific autoimmune diseases. A
previous study demonstrated that rats with a defective p19 subunit
of IL-23 were highly susceptible to hapten-induced colitis, which
may be related to the overproduction of IL-12 by intestinal
dendritic cells in an IL-23 deficient environment (15). Thus, it is likely that Th1 cells
and Th17 cells synergize to induce organ-specific autoimmune
diseases. Therefore, the mechanisms by which Th17 and Th1 cells
contribute to the pathogenesis of autoimmune diseases require
urgent investigation.
In the current study, TNBS was administered to
establish an IBD model and western blot analysis were used to
detect IL-17 expression in affected colon tissues, mesenteric lymph
node cells, SMCs and PBMCs. Compared with the normal control group,
the results revealed that IL-17 expression was significantly
increased in the affected local area, which is consistent with
previous reports (14). However,
the IL-17 expression levels in PBMCs did not increase, suggesting
that Th17 cells mainly act locally rather than systemically, which
further indicates that the immune response mediated by memory T
lymphocytes is tissue- and organ-specific (16). Since IL-17 is mainly produced by
memory T lymphocytes, its elevation locally may induce an
inflammatory response at a certain location and specific to this
location (17).
An ELISA method was used in this study to detect the
expression of the Th17 cytokine IL-17 and the Th1 cytokine IFN-γ in
mice. A specific anti-IL-17 monoclonal antibody was also applied to
block IL-17 and the effect on IFN-γ expression was evaluated. The
results revealed that the levels of IL-17 peaked prior to those of
IFN-γ while IFN-γ was sustained for longer in the target tissues,
even after the elevated expression levels of IL-17 had disappeared.
This is consistent with the results of Batten et al(18) in a study which tested the dynamic
expression of cytokines in the cerebrospinal fluid of an
experimental autoimmune encephalomyelitis (EAE) model. Our study
confirmed that Th17 and Th1 cells played important roles at
different stages of IBD, i.e., the effect of the Th17 cells was
produced sooner than that of the Th1 cells to initiate acute
inflammation, while the Th1 cells were involved in sustaining
inflammation subsequently. This suggests that the Th17 and Th1
cells are involved in the pathogenesis of IBD. In addition, an
anti-IL-17 antibody was not able to block IFN-γ secretion, further
suggesting that the Th17 and Th1 cells have a synergistic effect in
the pathogenesis of IBD (19).
Furthermore, pro-inflammatory cytokines play an
indispensable role in the pathogenesis of IBD (20). Among a large number of
pro-inflammatory cytokines, IL-6 and TNF-α are frequently used for
studying the pathogenesis of IBD (21,22),
as the expression levels of both are significantly increased in the
local mucosa of IBD patients. The application of anti-TNF-α
antibody has been revealed to significantly improve certain
pathological changes in the IBD mice model and reduce mortality, as
well as ameliorate the clinical symptoms of IBD patients (23,24).
In the current study, semi-quantitative RT-PCR confirmed that the
levels of TNF-α and IL-6 were increased in the SMCs of colitic
mice. In order to verify the relationship between Th17 cells and
these two cytokines, we added anti-IL-17 antibody to the cell
culture medium and found that the expression levels of IL-6 and
TNF-α decreased in direct proportion to the concentration of
antibody added. This result suggests that Th17 cells further induce
the onset of IBD by stimulating the production of the downstream
products TNF-α and IL-6.
The current study demonstrates that Th17 cells play
a key role in the early stage of IBD onset and that this effect is
tissue- and organ-specific, as well as direct-targeting. In
addition, Thl7 cells execute an inflammation-sustaining function
since blocking the secretion of cytokines by Thl7 reduces the
generation of IL-6 and TNF-α, indicating that the downstream
effects of IL-17/IL-17R pathway activation are important in the
pathogenesis of IBD. Therefore, further study on the mechanisms of
Th17 cells may help in the search for an effective treatment for
IBD.
References
|
1
|
Ouyang Q, Tandon R, Goh KL, Ooi CJ, Ogata
H and Fiocchi C: The emergence of inflammatory bowel disease in the
Asian Pacific region. Curr Opin Gastroenterol. 21:408–413.
2005.PubMed/NCBI
|
|
2
|
Jiang Y, Xia B, Jiang L, et al:
Association of CTLA-4 gene microsatellite polymorphism with
ulcerative colitis in Chinese patients. Inflamm Bowel Dis.
12:369–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermeire S: Genetic susceptibility and
application of genetic testing in clinical management of
inflammatory bowel disease. Aliment Pharmacol Ther. 24(Suppl 3):
2–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: an effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harrington LE, Mangan PR and Weaver CT:
Expanding the effector CD4 T-cell repertoire: the Th17 lineage.
Curr Opin Immunol. 18:349–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Podolsky DK: Inflammatory bowel disease. N
Eng J Med. 347:417–429. 2002. View Article : Google Scholar
|
|
7
|
Iwakura Y and Ishigame H: The IL-23/IL-17
axis in inflammation. J Clin Invest. 116:1218–1222. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park H, Li Z, Yang XO, et al: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar
|
|
10
|
Langrish CL, Chen Y, Blumenschein WM, et
al: IL-23 drives a pathogenic T cell population that induces
autoimmune inflammation. J Exp Med. 201:233–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada H: Th17 cells and human arthritic
diseases. Nihon Rinsho Meneki Gakkai Kaishi. 33:214–221. 2010.(In
Japanese).
|
|
12
|
Hue S, Ahern P, Buonocore S, et al:
Interleukin-23 drives innate and T cell- tmediated intestinal
inflammation. J Exp Med. 203:2473–2483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blaschitz C and Raffatellu M: Th17
cytokines and the gut mucosal barrier. J Clin Immunol. 30:196–203.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Zheng M, Bindas J,
Schwarzenberger P and Kolls JK: Critical role of IL-17 receptor
signaling in acute TNBS-induced colitis. Inflamm Bowel Dis.
12:382–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Becker C, Dornhoff H, Neufertet C, et al:
Cutting edge: IL-23 Eross-rugulates IL-12 production in T
cell-dependent experimental colitis. J Immunol. 177:2760–2764.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davidson NJ, Leach MW, Fort MM, et al: T
helper cell 1-type CD4+ T cells, but not B cells,
mediate colitis in interleukin 10-deficient mice. J Exp Med.
184:241–225. 1996.PubMed/NCBI
|
|
17
|
Fossiez F, Banchereau J, Murray R, Van
Kooten C, Garrone P and Lebecque S: Interleukin-17. Int Rev
Immunol. 16:541–551. 1998. View Article : Google Scholar
|
|
18
|
Batten M, Li J, Yi S, et al:
Interleukin-27 limits autoimmune encephalomyelitis by suppressing
the development of interleukin 17-producing T cells. Nat Immunol.
7:929–936. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elson CO, Cong Y, Weaver CT, et al:
Monoclonal anti-interleukin 23 reverses active colitis in a T
cell-mediated model in mice. Gastroenterology. 132:2359–2370. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Targan SR and Karp LC: Defects in mucosal
immunity leading to ulcerative colitis. Immunol Rev. 206:296–305.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy KP, Markowitz JE, Ruchelli ED,
Baldassano RN and Brown KA: Lamina propria and circulating
interleukin-8 in newly and previously diagnosed pediatric
inflammatory bowel disease patients. Dig Dis Sci. 52:365–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong DS, Angelo LS and Kurzrock R:
Interleukin-6 and its receptor in cancer: implications for
translational therapeutics. Cancer. 110:1911–1928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Van Assche G, Magdelaine-Beuzelin C,
D’Haens G, et al: Withdrawal of immunosuppression in Crohns disease
treated with seheduled infliximab maintaince: a randomized trial.
Gastroenterology. 134:1861–1868. 2008.PubMed/NCBI
|
|
24
|
Orlando A, Mocciaro F, Civitavecchia G,
Scimeca D and Cottone M: Minimizing infliximab toxicity in the
treatment of inflammatory bowel disease. Dig Liver Dis. 40(Suppl
2): S236–S246. 2008. View Article : Google Scholar : PubMed/NCBI
|
















