Introduction
The vascular endothelial growth factor (VEGF) family
is considered to be one of the most important pro-angiogenic
factors during therapeutic angiogenesis. The VEGF family has been
shown to not only reduce the infarction area, ameliorate cardiac
function and increase angiogenesis in ischemic tissues during
animal experiments (1–3), but also improve the ischemic symptoms
of patients in partial phase I and II clinical trials (4,5).
However, there are some potential risks associated with the
application of genes for therapeutic angiogenesis. For instance,
overexpression and ectopic expression of genes may lead to angioma
and promote carcinogenesis. Therefore, gene expression should be
further regulated to enhance the safety and effectiveness of gene
therapy.
Hypoxia response elements (HREs) are upstream
enhancers of gene promoters under hypoxic regulation. They are able
to induce the upregulation of target genes under hypoxic
conditions. Multiple copies of HRE promoters have been found to
increase the expression of target genes under hypoxic conditions,
while the same genes were barely expressed in the presence of
normal concentrations of oxygen, which increases the safety of gene
therapy (6). Gene transport to
target organs is important, but has been difficult to achieve to
date. A number of studies have indicated that cultivated human
endothelial progenitor cells (EPCs) or other sources of EPCs are
able to reduce the area of myocardial infarction, providing a good
strategy for treating patients with coronary atherosclerotic heart
diseases, especially end-stage coronary heart diseases (7). Moreover, due to stem cell homing, the
transportation of foreign genes into target organs may be realized,
which improves the targeting effect of gene therapy. The VEGF gene
is able to promote stem cell homing, stem cell differentiation and
angiogenesis in ischemic tissues as well as improve the functions
of ischemic tissues.
The effect of promoting angiogenesis using
pro-angiogenic gene-modified stem cells under hypoxic regulation
remains undefined. Thus, hypoxia promoter 6HRE-regulated plasmid
VEGF165 was constructed in this study and transfected
into rat bone marrow-derived EPCs following in vitro
isolation and cultivation to investigate the effect of
6HRE-VEGF165 gene-modified EPCs on angiogenesis in
ischemic myocardium and cardiac function following acute myocardial
infarction (AMI). Furthermore, the differences in the effects on
therapeutic angiogenesis in the ischemic myocardium and on the
cardiac function of rats with AMI following transplantation of
6HRE-VEGF165 gene-modified EPCs, VEGF165
gene-modified EPCs and normal EPCs were compared. No similar
studies have been reported previously.
Materials and methods
Recombinant construction and
identification of plasmids pCMV-VEGF165 and
p6HRE-CMV-VEGF165
The single-copy HRE gene sequence from the pGEM-T
vector (pGEM-T vector with HRE-CMV-mp gene; Shanghai Boya Corp.,
Shanghai, China) was connected to the 6-copy gene sequence using
isocaudomers BamHI and BglII (Takara Corp., Tokyo,
Japan) to synthesize a 6HRE-VEGF165 gene expression
plasmid (p6HRE-CMV-VEGF165-EGFP) and non-6HRE-regulated
control plasmid (pCMV-VEGF165-EGFP) by molecular cloning
techniques. The recombinant plasmids were then identified using
restriction enzyme digestion, polymerase chain reaction (PCR) and
DNA sequencing. The primers were as follows. 6HRE primer sequences:
upstream, 5′-CGTACACGCCTA CCAGAT-3′; downstream,
5′-CCGCACTAGTGATTGGAT-3′, 300 bp in length. VEGF165
primer sequences: upstream, 5′-GCCACCATGAACTTTCTGCTG-3′;
downstream, 5′-GGATCCTCACCGCCTCGGCTTGT-3′, 630 bp in length.
Isolation, cultivation and identification
of EPCs
EPCs were isolated from the femoral bone marrow of
80–100 g SPF male Sprague-Dawley rats (anesthetized with
pentobarbital sodium, 30 mg/kg i.p.; provided by the Laboratory
Animal Center of Chongqing Medical University). The cells were
adjusted to a density of 2–4×106 cells/well and
inoculated onto a 24-well plate precoated with fibronectin (FN;
Chemi-Con, Inc., Temecula, CA, USA) and cultured in M199 culture
medium (HyClone Laboratories, Inc., Logan, UT, USA) containing 20%
FCS, 40 μg/ml BPE (HyClone Laboratories, Inc.) and 1 ng/ml
FGF-basic (PeproTech Inc., Rocky Hill, NJ, USA). The cells were
incubated in a 37°C, 5% CO2, humidity-saturated cell
culture incubator (Forma Scientific, Inc., Marietta, OH, USA). The
culture medium was exchanged after 3 days of cultivation for the
first time, and the medium was then exchanged every 2 days to
observe the morphological changes of the cells. At days 5 and 11 of
cultivation, cells were obtained for the detection of the specific
cell surface antigens CD133 (Abcam, Inc., Cambridge, MA, USA), CD34
(Millipore Corp., Billerica, MA, USA) and the VEGF receptor (VEGFR;
Millipore Corp.), and the phagocytosis of Dil-ac-LDL (Invitrogen
Corp., Carlsbad, CA, USA) by the cells using immunofluorescent
cytochemical staining (Leica, Mannheim, Germany). The rat
experiments were approved by the local institutional animal
research committee [SCXK (Yu) 2007-0001].
The successfully cultured EPCs were allocated to one
of three groups: EPCs transfected with the recombinant plasmid
p6HRE-CMV-VEGF165-EGFP (group HV), EPCs transfected with
the recombinant plasmid pCMV-VEGF165-EGFP (group V) and
control EPCs transfected with the plasmid pCMV-MCS-EGFP (group E,
EPCs control group). The gene transfection with plasmids
pCMV-VEGF165 and p6HRE-CMV-VEGF165 was
conducted using liposome Lipofectamine 2000 (Invitrogen Corp.); 24
h after gene transfection, the positive rate of transfection was
calculated by flow cytometry (Becton-Dickinson Co., Franklin Lakes,
NJ, USA) as follows: number of positive cells carrying
fluorescence/total number of detected cells × 100%.
Dil-ac-LDL-labeled EPCs
EPCs cultured for 10 days were added to Dil-ac-LDL
(10 μg/ml) and incubated for 5 h. The culture medium was then
exchanged and the cells were observed under an inverted
fluorescence microscope to determine the ratio of fluorescently
labeled cells. Successfully labeled EPCs were digested with 0.25%
trypsin, centrifuged, resuspended in PBS and adjusted to a density
of 2×107 cells/ml. The EPC suspension (0.5 ml) was
injected via the tail vein into rats with a successfully
established model of AMI. Three days after cell transplantation,
the rats were sacrificed. Heart specimens were removed from the
rats, rinsed with PBS and cut into frozen sections (10 μm) to
enable observation of the location of the fluorescence-labeled
cells in the cardiac sections under an inverted microscope.
Establishment of the rat model with AMI
and cell transplantation
MI was induced in adult male Sprague-Dawley rats
weighing 180–230 g (anesthetized with pentobarbital sodium, 30
mg/kg i.p.; provided by the Laboratory Animal Center of Chongqing
Medical University) by permanent ligation of the left anterior
descending coronary artery (8).
The animal study was approved by the Chongqing Medical University
ethics review board [SCXK (Yu) 2007-0001]. The rats with a
successfully established model of AMI were divided into 4 groups
(each n=10) and injected via the tail vein with Dil-ac-LDL
fluorescence-labeled p6HRE-CMV-VEGF165-transfected EPCs
(2×107 cells/ml), pCMV-VEGF165-transfected
EPCs (2×107 cells/ml), EPCs (2×107 cells/ml)
or 0.5 ml normal saline. The sham surgery group was regarded as the
control (n=10). One week later, 4 rats (one randomly selected from
each group) were sacrificed by cervical dislocation and their
hearts were rapidly removed. The myocardial tissues near the left
ventricular infarction were excised, rinsed with pre-cooled normal
saline and DEPC, and equally divided into 2 samples for reverse
transcription-PCR (RT-PCR) and western blot detection of the
VEGF165 gene and protein expression levels. At week 4,
cardiac echocardiography was performed on the remaining rats to
evaluate cardiac function and cardiac tissues. The sacrificed rats
were rinsed with normal saline and fixed in 4% paraformaldehyde in
preparation for further use.
Detection of VEGF165 gene
expression under hypoxia promoter regulation
EPCs successfully transfected with
p6HRE-CMV-VEGF165 and pCMV-VEGF165 were
placed in an N2 incubator containing 1% O2 36
h after transfection. Following 12-h hypoxic incubation, the EPCs
were removed from the incubator and allocated to either a
transfection control group or a normal oxygen concentration
incubation control group. The levels of VEGF165 mRNA and
protein expression were detected using RT-PCR and western blotting,
respectively. The levels of VEGF165 mRNA and protein
expression in the ischemic myocardium 1 week after cell
transplantation were also detected using the same assays.
The mRNA in the EPCs and the ischemic myocardium was
extracted and reversely transcribed into cDNA with reference to the
total DNA extracted standard protocol. The absorbance ratio between
VEGF165 and β-actin (β-actin upstream primer,
5′-CCCATCTATGAGGGTTACGC-3′; downstream primer,
5′-TTTAATGTCACGCACGATTTC-3′, 150 bp in length) was calculated
following analysis using the software Quantity One 4.4.0 and
represented the relative expression level.
Total protein was extracted from the EPCs and the
ischemic myocardium with reference to the protein extracted
standard protocol; 20 μl supernatant was obtained to determine the
protein concentration using bicinchoninic acid, the wavelength was
measured using a microplate reader and the protein concentration
was calculated from the standard curve. The VEGF165
protein was isolated using 120 ml/l SDS-PAGE gel electrophoresis,
electrotransferred onto a PVDF membrane and blocked with 50 g/l
evaporated skimmed milk. After 1-h incubation at room temperature,
the blocking buffer was removed and the protein was combined with
rabbit anti-rat VEGF primary antibody (Millipore Corp., 1:500). The
protein was then added to secondary antibody anti-rabbit
immunoglobulin IgG (1:1000, Zhongshan Jin Qiao Biotechnology Co.,
Ltd., Beijing, China). Enhanced chemiluminescence was applied for
color development and the grayscale of the band was analyzed using
a gel image analysis system.
Hematoxylin and eosin (H&E) and
immunohistochemical staining of myocardial tissues
Infarct size (IS) was determined by H&E
staining. In brief, sections of the left ventricle were immersed in
fixative solution, dehydrated and then embedded in paraffin.
Afterwards, 5‐mm-thick histological slices were obtained and
stained with H&E. The endocardial and epicardial circumferences
of the infarcted tissue and the left ventricle were determined
using image analysis software (Image-Pro Plus 4.5). The IS was
calculated as (endocardial + epicardial circumference of the
infarcted tissue)/(endocardial + epicardial circumference of the
left ventricle) and expressed as a percentage. The capillary
density of the heart was detected by immunohistochemical staining
using a 2-Step Immunohistochemistry Detection kit (Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China). Specific
procedures in line with the operation manual were followed. The
working concentration of rabbit anti-rat VIII primary antibodies
was 1:50. The primary antibodies were replaced by PBS buffer as the
negative control.
Statistical processing
All measurement data were expressed as the mean ± SD
and statistically analyzed using SPSS 13.0 statistical software.
The means of mRNA and protein expression levels among different
groups were compared by 1-way analysis of variance (ANOVA) and
multiple comparisons between each pair of groups were conducted
using the 2-sided Tukey’s test. P<0.05 was considered to
indicate a statistically significant result.
Results
Delete
Identification results for in vitro
cultivation of EPCs
EPCs isolated and cultured in vitro adhered
to the plate after 3 days of cultivation and displayed a radial and
proliferative growing state 7 days later. Immunofluorescent
cytochemical staining revealed that the EPC surface antigens CD34
and CD133 stained positive on day 5, but the expression of CD34 was
more intense than that of CD133 (Fig.
1). By day 11, EPC surface antigen CD133 was essentially
unexpressed, but CD34 (green) and VEGFR (red) were stained
positive. In addition, the cells phagocytized Dil-ac-LDL and the
positive cells displayed red fluorescence under the inverted
microscope (Fig. 2). Flow
cytometry carried out 24 h later detected EPCs successfully
cultured using cationic liposomes and revealed the expression of
green fluorescence protein by 40% of the EPCs following
transfection.
Detection of VEGF165 gene
expression
Detection of VEGF165 mRNA
and protein expression under in vitro regulation of the hypoxia
promoter
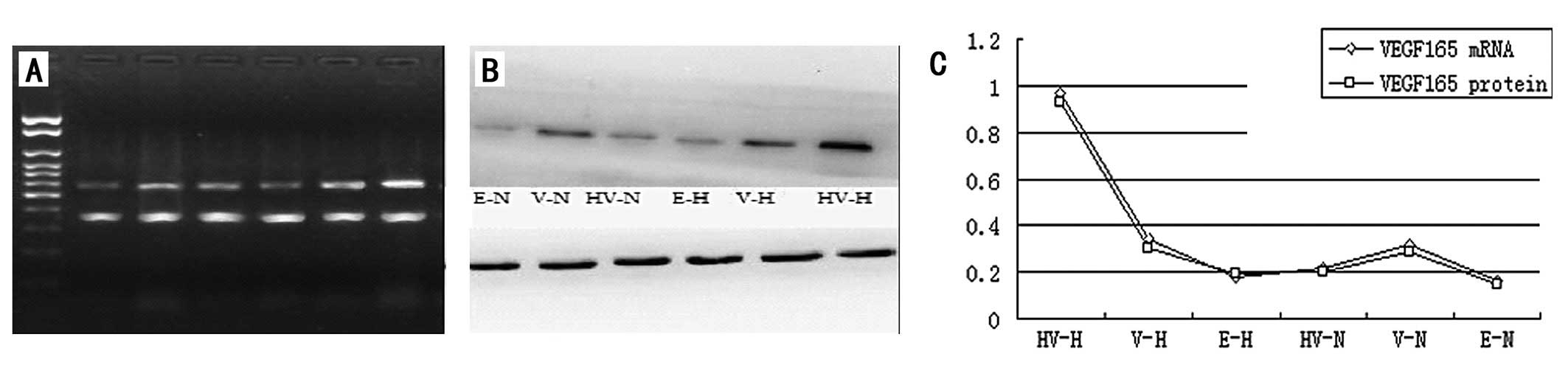
The levels of VEGF165 mRNA and protein
expression in the EPCs transfected with the recombinant plasmid
p6HRE-CMV-VEGF165 were significantly higher than those
in the EPCs transfected with the recombinant plasmid
pCMV-VEGF165 and those not transfected with any
recombinant plasmid (P<0.05) under hypoxic induction (Fig. 3). Nonetheless, the levels of
VEGF165 gene expression in the
p6HRE-CMV-VEGF165-transfected and untransfected EPCs
were comparatively equivalent but significantly lower than those in
the EPCs transfected with pCMV-VEGF165 (Fig. 3) when cultivated in normal oxygen
conditions. The level of VEGF165 gene expression by the
pCMV-VEGF165-transfected EPCs appeared higher under
hypoxic conditions than in normal oxygen conditions, but the
difference was not statistically significant (P>0.05; Fig. 3).
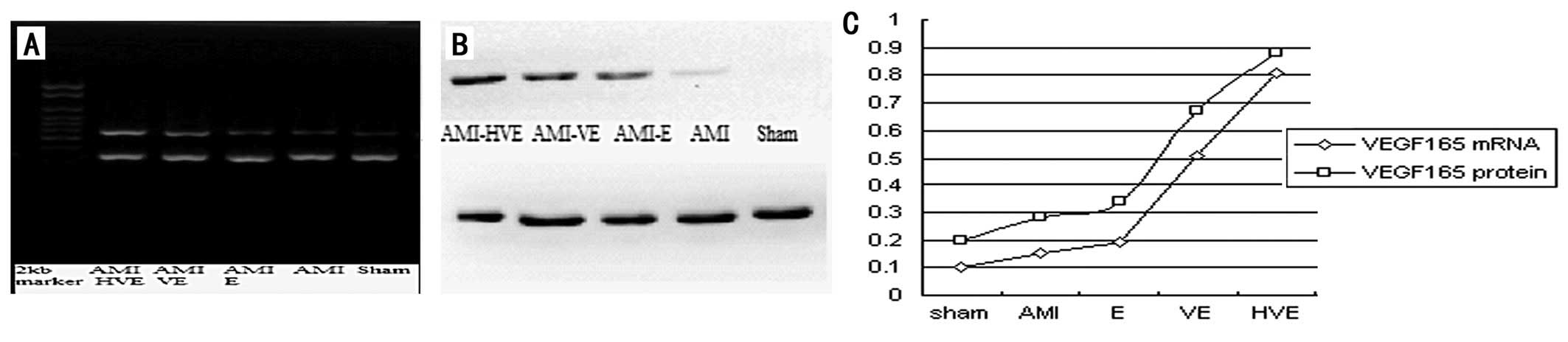
Detection results of
VEGF165 gene expression under in vivo regulation of the
hypoxia promoter
The present results revealed that the levels of
VEGF165 mRNA and protein expression in the EPCs
transplanted near the infarction area of the rats were
significantly higher in the
p6HRE-CMV-VEGF165-transfected EPCs than those in the
other groups (P<0.05), and that VEGF165 gene
expression levels in the normal EPC transplantation group were
higher than those in the AMI group (P<0.05) but lower than those
in the pCMV-VEGF165-transfected EPC transplant group
(P<0.05; Fig. 4).
H&E staining and
immunohistochemical results of the myocardium near the infarction
area
Rats of different experimental groups were
sacrificed at week 4 after cell transplantation. Their cardiac
tissues were removed, embedded in paraffin and cut into sections.
H&E staining revealed that the myocardium of normal cardiac
tissues was arranged in order without manifestations of muscle
bundle fracture or fiber hyperplasia, but that myocardial
disarrangement and muscle bundle fracture was observed following
myocardial infarction with the proliferation of a large number of
fiber cells in the disarranged myocardium (data not shown).
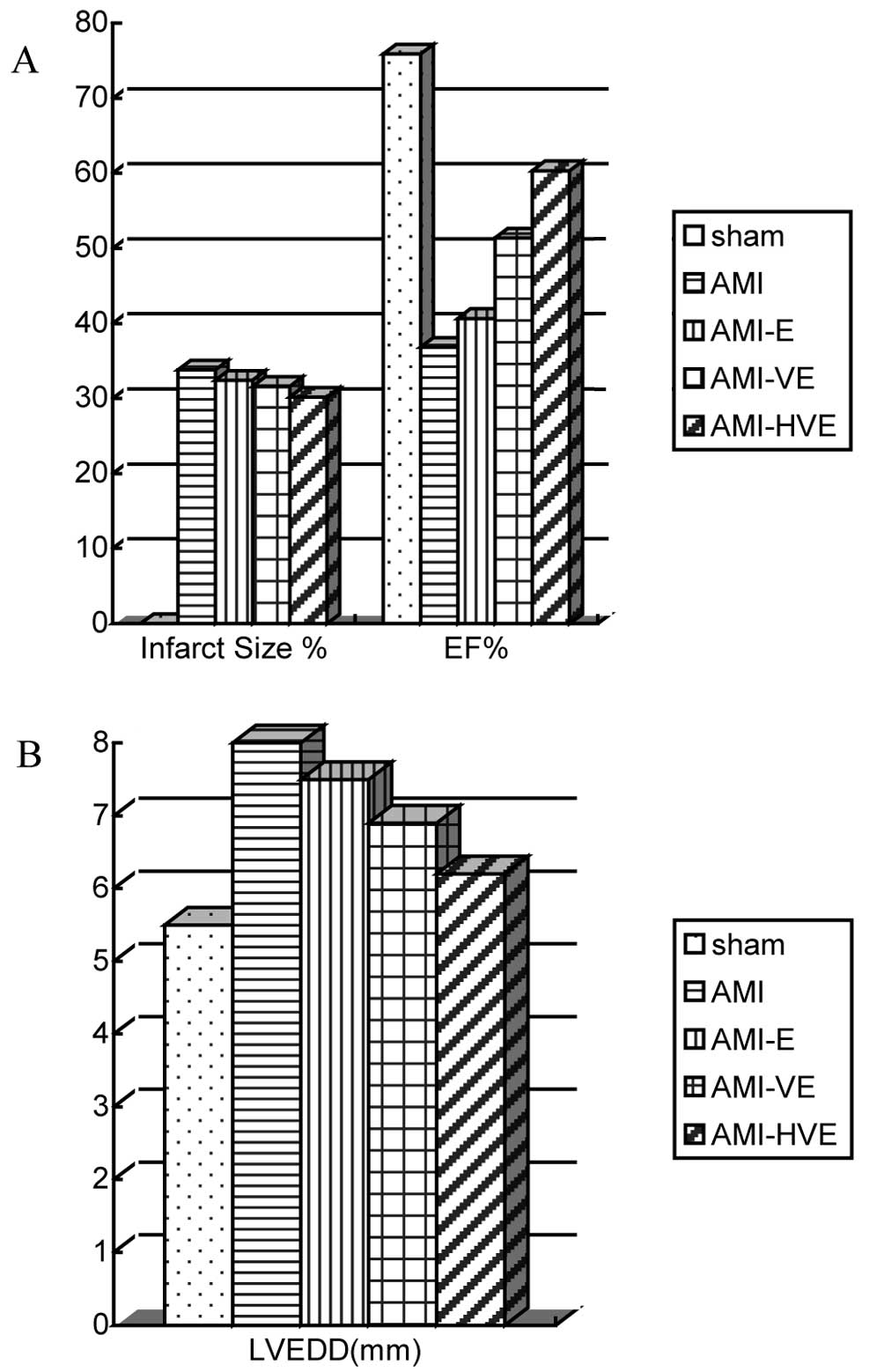
The infarct area size in the
pCMV-VEGF165-transfected EPC transplantation group was
smaller than those in the untransfected EPC transplantation and AMI
groups but larger than that in the
p6HRE-CMV-VEGF165-transfected EPC transplantation group
by H&E staining (P<0.05). The infarct area size in the
untransfected EPC transplantation group was not observed to be
significantly different from that in the AMI group (P>0.05;
Fig. 6A).
The results of capillary area calculation following
the factor VIII staining of the vascular endothelial cells in the
myocardium near the infarction area into brown or brownish yellow
particles using the SABC immunocytochemical detection kit are
presented in Fig. 5. The capillary
density determination indicated that the numbers of
VIII+ vessels in the pCMV-VEGF165- and
p6HRE-CMV-VEGF165-transfected EPC transplantation groups
were higher than those in the sham surgery and AMI groups 4 weeks
post-MI; those in the p6HRE-CMV-VEGF165-transfected EPC
group were significantly higher (P<0.05). The capillary density
in the normal EPC transplantation group was lower than that in the
sham group (P<0.05) and not significantly different from that in
the AMI group (P>0.05).
Results of cardiac function testing of
the rats by echocardiography
Evalution of the cardiac functions of the rats by
echocardiography at week 4 following cell transplantation revealed
the formation of false ventricular aneurysms on the left
ventricular wall in the infarction group. The rats in the
p6HRE-CMV-VEGF165-transfected EPC transplantation group
had a significantly lower left ventricular end-diastolic diameter
but higher ejection fraction than did the other experimental groups
(Fig. 6; P<0.05).
Discussion
The present study found that: i) hypoxia-specific
expression of the VEGF165 gene carried by EPCs and
regulated by 6HRE could be achieved in vivo and in
vitro; and ii) when the transplantation of EPCs was modified by
the VEGF165 gene and regulated by 6HRE, it produced a
stronger therapeutic effect in promoting angiogenesis. It radically
improved cardiac function following myocardial infarction, reducing
the myocardial infarct size.
The VEGF gene is the most classic angiogenic growth
factor that has been demonstrated to have an effect on angiogenesis
by basic and clinical studies. In combination with VEGFR-1
(9), it promotes the vascular
regeneration of ischemic limbs, heart and brain (10–12),
and increases the collateral circulation and improves the function
of ischemic tissue. Studies have shown that its overexpression and
expression in non-target organs may cause inflammation, edema, the
formation of vascular tumors and other side effects, and may even
trigger other grave consequences. Nevertheless, the primary focus
of the present study was to ensure safe VEGF gene transfer. This is
likely to be effective in the treatment of ischemic diseases. The
present study primarily focused on determining the specificity of
VEGF gene expression regulated by hypoxia.
Previous studies have identified a short gene
sequence upstream of the VEGF gene promoter, namely the HRE, which
binds with hypoxia inducible factor-1 (HIF-1) specifically under
hypoxic conditions. This initiates the translation and expression
of target genes, indicating that hypoxia is a natural VEGF
gene-regulating factor. However, studies by Tang and other
researchers have found no upregulatory role for the single-copy HRE
promoter under hypoxic conditions, whereas subsequent studies have
confirmed that series of multi-copy HRE promoters are able to
enhance the transcriptional activity of the target gene under
hypoxic conditions (13). The
regulation capacity of HRE is proportional to the copy number
(14), while there is no
expression of target genes under normal oxygen concentrations
(15). Thus, multiple copies of
HRE induce a switch effect in regulating target genes in hypoxic
conditions (16), enhancing the
intensity of target gene expression and specificity for
hypoxia.
Su et al (17) have regulated VEGF with MLC-2 and
9HRE, and identified that the gene expression level of VEGF in the
regulated myocytes after 16 h of hypoxia inducement in vitro
was approximately twice that in the control group and approximately
8-fold higher than that in the non-hypoxic group. However, there
was almost no expression of the target gene in the regulated group
under non-hypoxic conditions. It was also significantly lower than
that in the control group. A gene expression study using 2
regulators (9HRE and ODD), as reported by Fomicheva et al
(18), revealed that the
expression levels of target genes increased 1,000-fold when induced
by 0.5% oxygen after 48 min. Recently, Wei et al reported
the construction of a CD151 gene vector regulated by HRE using an
adeno-associated virus which was transfected into an ischemic
heart. The results revealed enhanced VEGF gene expression of CD151
and vascularization in the ischemic myocardium (19).
In the current study, the VEGF165 gene
expression plasmid regulated by 6HRE was constructed and
transfected into EPCs cultured in vitro. These were then
placed in a 1% O2 hypoxic incubator. After 12-h
incubation, the VEGF165 mRNA and protein expression
levels were approximately 4-fold higher than those in the normoxic
control group and were approximately 3-fold higher than those in
the group without 6HRE regulation under hypoxic conditions.
However, VEGF mRNA and protein expression were at low levels in the
6HRE-regulated group cultured in normoxic conditions. Thereafter,
gene transfected EPCs were transplanted into the myocardium and the
expression level of VEGF in the 6HRE-regulated group was determined
to be significantly greater than that in the non-HRE group. This
further indicated that 6HRE exerted a switch effect in regulating
the expression of the VEGF165 gene under hypoxic
conditions and may improve the angiogenic effect of
VEGF165 gene therapy, enhancing the targeting of anoxic
tissues with gene therapy.
EPCs with the remedial angiogenesis gene
have improved targeting, transportation and therapeutic
effects
EPCs are able to specifically heal damaged vessels,
differentiate endothelial cells, secrete various angiogenic growth
factors, and promote angiogenesis and neovascularization. It has
been widely verified that they have a role in therapeutic
angiogenesis and are without the potential risk of arrhythmia
promotion (19,20).
A study conducted by Iwaguro et al involving
the transplantion of VEGF gene-modified EPCs identified for the
first time that the ability of EPCs to promote angiogenesis was
enhanced in the ischemic extremities of animals (21). Since then, a number of studies have
found that the transplantation of gene-modified EPCs promotes
restoration of the bloodstream in ischemic extremities, improves
the function of ischemic tissue and reduces the frequency of EPC
transplantation (21,22). In addition, it increases the number
of EPCs in local ischemic tissue (23), enhancing the migration of EPCs,
tubular formation and survival. It also relieves the extent of
myocardial perfusion deficiency (24). Hence, gene-modified EPCs have a
strong angiogenetic effect, with reduced ectopic expression of
genes. It is not yet clear whether EPCs modified by the
VEGF165 gene under the regulation of hypoxia play an
important role in increasing angiogenesis, improving cardiac
function following AMI and reducing myocardial infarct size.
This study identified that capillary density was
significantly higher in the 6HRE-VEGF165 gene-modified
EPC transplantation group than in the non-6HRE-VEGF165
gene-modified EPC transplantation, normal EPC transplantation and
normal saline control groups. This significant contrast in
capillary density was observed at the ischemic myocardium near the
myocardial infarction area at week 4. This was in accordance with
the aforementioned results. Investigation of the cardiac function
of the rats by echocardiography revealed that the left ventricular
end diastolic volume and myocardial infarction areas in the
6HRE-VEGF165 gene-modified EPC transplantation group
were significantly reduced compared with those in the other three
groups. However, the left ventricular ejection fraction was
significantly higher than that in the other three groups. In the
6HRE-VEGF165 gene-modified EPC transplantation group,
due to the homing of EPCs to the highly ischemic and hypoxic
ventricle near the infarction area, the hypoxia promoter rapidly
and powerfully initiated and induced the expression of
VEGF165 mRNA and protein. It increased the regional and
circulating amount of VEGF, further promoting the release, homing
and differentiation of EPCs in the rats and also intensified the
secretion of regional growth factors (23), speeding up the formation and
maturation of new blood vessels, rescuing the dying myocardium and
reducing the area of myocardial infarction and deficiency in
myocardial perfusion (23,24). This ameliorated the cardiac
function of the rats compared with that in the
non-6HRE-VEGF165 gene-modified EPC transplantation
group. Dong et al (25)
reported that following transfection of the AAV-9HRE-VEGF virus
into ischemic myocardium, the 9HRE-VEGF gene was able to further
ameliorate the function of ischemic rat hearts and upregulate the
level of CD31 cells, supporting the speculation in the current
study. 6HRE hypoxia regulation, the angiogenic growth factor gene
(VEGF165) and progenitor cell transplantation were
effectively combined in the present experiment. The results
verified that the outcome of therapeutic angiogenesis and heart
function improved with the combination of these three methods. It
was superior to single EPC transplantation and EPC transplantation
combined with the VEGF gene.
In conclusion, the results of this study confirmed
that the transplantation of VEGF165 gene-modified EPCs
under 6HRE regulation significantly reduced the left ventricular
end diastolic volume of rats. It also significantly increased the
left ventricular ejection fraction and capillary density in the
ischemic zone. Therefore, a method that combines the expression of
the 6HRE hypoxia regulation gene with progenitor cell
transplantation is safe and effective for the treatment of ischemic
heart diseases and other ischemic diseases.
Acknowledgements
The authors wish to thank Yang Wang, PhD for
offering important suggestions regarding this manuscript. This
study was supported by the National Natural Science Foundation of
China (81100088/H0203), the Important Project of Chongqing Health
Administration (20090113) and the Chongqing Medical University
Science Foundation.
Abbreviations:
|
HRE
|
hypoxic response element
|
|
EPCs
|
endothelial progenitor cells
|
|
AMI
|
acute myocardial infarction
|
|
VEGF165
|
vascular endothelial growth factor
165
|
|
Dil-ac-LDL
|
Dil-acetylated low density
lipoproteins
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
EF
|
left ventricular ejection fraction
|
References
|
1
|
Korpisalo P, Rissanen TT, Bengtsson T, et
al: Therapeutic angiogenesis with placental growth factor improves
exercise tolerance of ischaemic rabbit hindlimbs. Cardiovasc Res.
80:263–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leong-Poi H, Kuliszewski MA, Lekas M, et
al: Therapeutic arteriogenesis by ultrasound-mediated VEGF165
plasmid gene delivery to chronically ischemic skeletal muscle. Circ
Res. 101:295–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lähteenvuo JE, Lähteenvuo MT, Kivelä A, et
al: Vascular endothelial growth factor-B induces
myocardium-specific angiogenesis and arteriogenesis via vascular
endothelial growth factor receptor-1 and neuropilin
receptor-1-dependent mechanisms. Circulation. 119:845–856.
2009.
|
|
4
|
Fortuin FD, Vale P, Losordo DW, et al:
One-year follow-up of direct myocardial gene transfer of vascular
endothelial growth factor-2 using naked plasmid deoxyribonucleic
acid by way of thoracotomy in no-option patients. Am J Cardiol.
92:436–439. 2003.
|
|
5
|
Losordo DW, Vale PR, Hendel RC, et al:
Phase 1/2 placebo-controlled, double-blind, dose-escalating trial
of myocardial vascular endothelial growth factor 2 gene transfer by
catheter delivery in patients with chronic myocardial ischemia.
Circulation. 105:2012–2018. 2002. View Article : Google Scholar
|
|
6
|
Sato K, Wu T, Laham RJ, et al: Efficacy of
intracoronary or intravenous VEGF165 in a pig model of chronic
myocardial ischemia. J Am Coll Cardiol. 37:616–623. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zemani F, Silvestre JS, Fauvel-Lafeve F,
et al: Ex vivo priming of endothelial progenitor cells with SDF-1
before transplantation could increase their proangiogenic
potential. Arterioscler Thromb Vasc Biol. 28:644–650. 2008.
View Article : Google Scholar
|
|
8
|
Xiao J, She Q, Wang Y, et al: Effect of
allopurinol on cardiomyocyte apoptosis in rats after myocardial
infarction. Eur J Heart Fail. 11:20–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clayton JA, Chalothorn D and Faber JE:
Vascular endothelial growth factor-A specifies formation of native
collaterals and regulates collateral growth in ischemia. Cir Res.
103:1027–1036. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie D, Li Y, Reed EA, et al: An engineered
vascular endothelial growth factor-activating transcription factor
induces therapeutic angiogenesis in ApoE knockout mice with
hindlimb ischemia. J Vasc Surg. 44:166–175. 2006. View Article : Google Scholar
|
|
11
|
Toyota E, Warltier DC, Brock T, et al:
Vascular endothelial growth factor is required for coronary
collateral growth in the rat. Circulation. 112:2108–2113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Jin K, Xie L, et al: VEGF-induced
neuroprotection, neurogenesis, and angiogenesis after focal
cerebral ischemia. J Clin Invest. 111:1843–1851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Y: Gene therapy for myocardial
ischemia using the hypoxia-inducible double plasmid system. Methods
Mol Med. 112:37–47. 2005.PubMed/NCBI
|
|
14
|
Harada H, Kizaka-Kondoh S, Itasaka S,
Shibuya K, et al: The combination of hypoxia-response enhancers and
an oxygen-dependent proteolytic motif enables real-time imaging of
absolute HIF-1 activity in tumor xenografts. Biochem Biophys Res
Commum. 360:791–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dulak J, Zagorska A, Wegiel B, et al: New
strategies for cardiovascular gene therapy: regulatable pre-emptive
expression of pro-angiogenic and antioxidant genes. Cell Biochem
Biophys. 44:31–42. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su H and Kan YW: Adeno-associated viral
vector-delivered hypoxia-inducible gene expression in ischemic
hearts. Methods Mol Biol. 366:331–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su H, Joho S, Huang Y, et al:
Adeno-associated viral vector delivers cardiac-specific and
hypoxia-inducible VEGF expression in ischemic mouse hearts. Proc
Natl Acad Sci USA. 101:16280–16285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fomicheva EV, Turner II, Edwards TG, et
al: Double oxygen-sensing vector system for robust
hypoxia/ischemia-regulated gene induction in cardiac muscle in
vitro and in vivo. Mol Ther. 16:1594–1601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei Q, Huang XL, Lin JY, et al: Adeno
associated viral vector-delivered and hypoxia response
element-regulated CD151 expression in ischemic rat heart. Acta
Pharmacol Sin. 32:201–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Losordo DW, Schatz RA, White CJ, et al:
Intramyocardial transplantation of autologous CD34+ stem
cells for intractable angina: a phase I/IIa double-blind,
randomized controlled trial. Circulation. 115:3165–3172.
2007.PubMed/NCBI
|
|
21
|
Iwaguro H, Yamaguchi J, Kalka C, et al:
Endothelial progenitor cell vascular endothelial growth factor gene
transfer for vascular regeneration. Circulation. 105:732–738. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murasawa S, Llevadot J, Silver M, et al:
Constitutive human telomerase reverse transcriptase expression
enhances regenerative properties of endothelial progenitor cells.
Circulation. 106:1133–1139. 2002. View Article : Google Scholar
|
|
23
|
Yu JX, Huang XF, Lv WM, et al: Combination
of stromal-derived factor-1alpha and vascular endothelial growth
factor gene-modified endothelial progenitor cells is more effective
for ischemic neovascularization. J Vasc Surg. 50:608–616. 2009.
View Article : Google Scholar
|
|
24
|
Chen SY, Wang F, Yan XY, et al: Autologous
transplantation of EPCs encoding FGF1 gene promotes
neovascularization in porcine model of chronic myocardial ischemia.
Int J Cardiol. 135:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong HY, Wang Q, Zhang Y, et al:
Angiogenesis induced by hVEGF165 gene controlled by hypoxic
response elements in rabbit ischemic myocardium. Exp Biol Med
(Maywood). 234:1417–1424. 2009. View Article : Google Scholar : PubMed/NCBI
|