Introduction
Local anesthetics (LAs) have been used for relieving
acute, intraoperative, postoperative and chronic pain for several
years. Generally LAs are thought to be safe, but the neurotoxicity
of LAs remains a considerable concern in certain cases. Ropivacaine
is thought to be one of the safest LAs for pain relief, however,
its efficiency is low, resulting in the utilization of high
concentrations of LAs to relieve pain completely (1,2).
Previous studies have reported considerable neurotoxicities
associated with ropivacaine, particularly at high concentrations
(3,4). However, the molecular mechanisms
through which LAs induce neurotoxicity remain poorly understood.
Apoptosis, necrotic cell death and protein kinase B (Akt) signaling
pathways may be involved (5–7).
It has been demonstrated that the activation of Akt
is critical for cell anti-apoptosis (8). Survival factors suppress apoptosis by
activating the serine/threonine kinase Akt, which then
phosphorylates and inactivates components of the apoptotic process.
Therefore, cells destined for apoptosis exhibit lower levels of
Akt. Measuring Akt concentrations, under experimental conditions,
may allow for an improved understanding of one of the possible
mechanisms for cell death under specific conditions.
It previously has been reported (9) that high concentrations of ropivacaine
result in neurotoxicity in specific cell lines. The current study
was designed to determine the molecular neurotoxic mechanism of 1%
ropivacaine in in vivo and in vitro models. In the
present study, spinal cord in vivo and PC12 cell line in
vitro models were utilized, with the aim to assess the possible
neurotoxic cellular mechanistic effects of ropivacaine.
Materials and methods
Ethics and animals
The in vivo study was approved by the Ethics
Committee for Animal Research of XiangYa Hospital in Central South
University, China. Animal care and handling were in accordance with
the Guide for the Care and Use of Laboratory Animals.
Chemicals
Commercially available ropivacaine (Naropin 10
mg/ml, Astra-Zeneca, Rueil-Malmaison, Sweden) was used and diluted
with NaCl 0.9%.
Animal selection and housing
The experiment was performed using male
Sprague-Dawley rats weighing 280–320 g. Rats were housed two/cage
in the animal facility for one week, under 12:12 h light-dark
cycle. Food and water were available at all times. Animal studies
were performed following approval from the Institutional Animal
Care and Use Committee in Xiangya Hospital, Central South
University, China.
Surgical procedure for intrathecal
catheterization
Rats were anesthetized with 10% chloral hydrate. As
described previously (10), a
sterile PE-10 tube filled with saline was inserted through the
L5/L6 intervertebral space, placing the tip of the tube at the
spinal lumbar enlargement level. The cannulated rats were observed
for five days following intrathecal catheterization. Rats
demonstrating symptoms of traumatic nerve injury or signs of
neurological impairment during catheterization were excluded from
further experiments.
Intrathecal administration of drugs
Following successful intrathecal catheterization, 72
rats were randomly divided into two groups (2×36). In group R, rats
received 8 injections of 1% ropivacaine (0.12 ml/kg), at 90-min
intervals, for a duration of 12 h. Ropivacaine concentration was
determined by a pilot study (11).
A dosing interval of 1.5 h was based on a study by Rose et
al(12). An identical amount
of saline was administered by the same method in the group NS
(control group). The catheter was flushed with 10 μl of saline to
account for the dead-space of the catheter. At each injection, the
solution was administered over seconds and then capped with a pin.
Each group was observed on days 1, 3, 5, 7, 14 and 28 (n=6 at each
time point).
Spinal cord section specimen
The animals in each group were sacrificed at each
time point under terminal anesthesia. Transverse sections of the
spinal lumbar enlargement region (L2–L6) in each animal were
removed en bloc and each was dissected into two gross sections (A
and B). The transverse specimen with spinal cord L3 (sample A) was
used for TUNEL staining (see below) and embedded in paraffin. The
posterior section specimen (sample B) was used for immunostaining
examination.
TUNEL staining for apoptosis
For the detection of apoptosis, we used a
Fluorescein FragEL™ DNA fragmentation detection kit QIA39
(Calbiochem, Darmstadt, Germany) to identify apoptotic nuclei in
paraffin-embedded tissue sections. TUNEL was performed on 5-μm
thick transverse sections of sample A. We used fluorescence
microscopy and a ×40 oil lens. Fields under ×40 lenses within one
section were randomly selected for quantification of TUNEL-positive
cells by counting. Data were expressed as average
count/field/animal. Images were captured under high-power
magnification (x200).
Western blot analysis
Sample B specimens from each group were homogenized
in a lysis buffer containing protease inhibitors and phosphatase
inhibitors. Supernatants were obtained following centrifugation at
10,000 × g (4°C, 5 min). Protein concentration was estimated using
the Bradford reagent (Sigma, St. Louis, MO, USA). The protein was
mixed with laemmli sample buffer and heated at 99°C for 5 min. For
western blot analysis, an equal amount of protein (50–80 μg) was
loaded into each well and subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The
separated proteins were then transferred from the gel to
polyvinylidene fluoride (Millipore, Bedford, MA, USA) membranes and
blocked in 5% skimmed milk prepared in 1X TBST. Membranes were
incubated with the following primary antibodies overnight at 4°C:
anti-Akt (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
anti-phospho-Akt (Stressgene, San Diego, CA, USA) or anti-β-actin
(Santa Cruz Biotechnology, Inc.; each at 1:1,000). Blots were
developed using Beyo ECL plus detection system (Beyotime Institute
of Biotechnology, Shanghai, China) and relative band density was
measured using FluroChem FC2 System (NatureGene Corp., Medford, NJ,
USA).
Cell culture and treatments
PC12 cells (ProMab Bitechnologines Inc., Hunan,
China) were routinely cultured in growth medium (GM) composed of
DMEM and 10% fetal bovine serum (FBS; Hyclone SH30084.03,
Haikelong, China) at 37°C and 5% CO2. The cells were
pre-seeded at appropriate densities. Ropivacaine was prepared as
stocks of 1% (15 mmol/l) in GM with the pH adjusted to 7.4. The
concentrations of ropivacaine used in the present study were based
on dose-dependent neurotoxicity effects and on previous in
vitro studies (13,14). The concentrations of ropivacaine
were 1, 2.5, 5, 10, 15 and 25 mM. A high concentration of
ropivacaine was included since it most likely causes cell death in
PC12 cells. Each assay was observed at 48 h.
MTT cell counting assay
The cell toxicity of ropivacaine was assessed using
the 3-(4,5-dimethyl-thiszol-2-yl)-2, 5-diphenyl tetrazolium bromide
(MTT) assay (Cayman Chemical Co., Ann Arbor, MI, USA). Cells were
plated at 4,000 cells/well in 96-well plates and grown in GM in the
absence (control group NS) or presence of ropivacaine (group R) for
48 h. MTT assay was performed using an enzyme-linked immunosorbent
assay, measuring optical density values at 570 nm (630 nm
calibration).
Measurement of cell viability
Trypan blue exclusion assay was used to measure cell
viability. Cells were cultured in GM in group NS or group R and
plated at 20,000 cells/well in 24-well plates. Time and
concentrations of ropivacaine were as described for the MTT assay.
The medium was changed and images of the cells were captured daily
with an inverted microscope (Olympus, Tokyo, Japan). Following
image capture, cells were trypsinized and stained with trypan blue
(Mediatech, Manassas, VA, USA). Viable (non-stained) and non-viable
(blue) cells were counted using a hemocytometer (Beckman, Brea, CA,
USA).
Cell western blot analysis
PC12 cells were grown in GM in group NS or group R
for 48 h. Cells were harvested and lysed in a lysis buffer
containing protease and phosphatase inhibitors. Protein
quantification, SDS-PAGE and immunoblotting were performed as
described previously (15), using
the following primary antibodies: anti-Akt and anti-phospho-Akt
(Thr308; Cell Signaling, Danvers, MA, USA; each at 1:1,000) and
anti-β-actin (Abcam, Cambridge, MA, USA; 1:5,000). Blots were
developed and the relative band density was measured as described
for sample B specimens.
Statistical analysis
Statistical data were from multiple experiments or
measurements and were presented as the mean ± SEM (n=6). The
significance of differences (P<0.05) was evaluated by one-way
ANOVA followed by the Bonferroni/Dunnett post hoc test, where
appropriate. SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was utilized
to perform the tests.
Results
Surgical procedure
No visual evidence of CSF leakage was recorded
following catheter placement or during the experiments. The rats
demonstrated no signs of clinical complications of the central
nervous system. Postmortem examination confirmed that all catheters
were sited correctly.
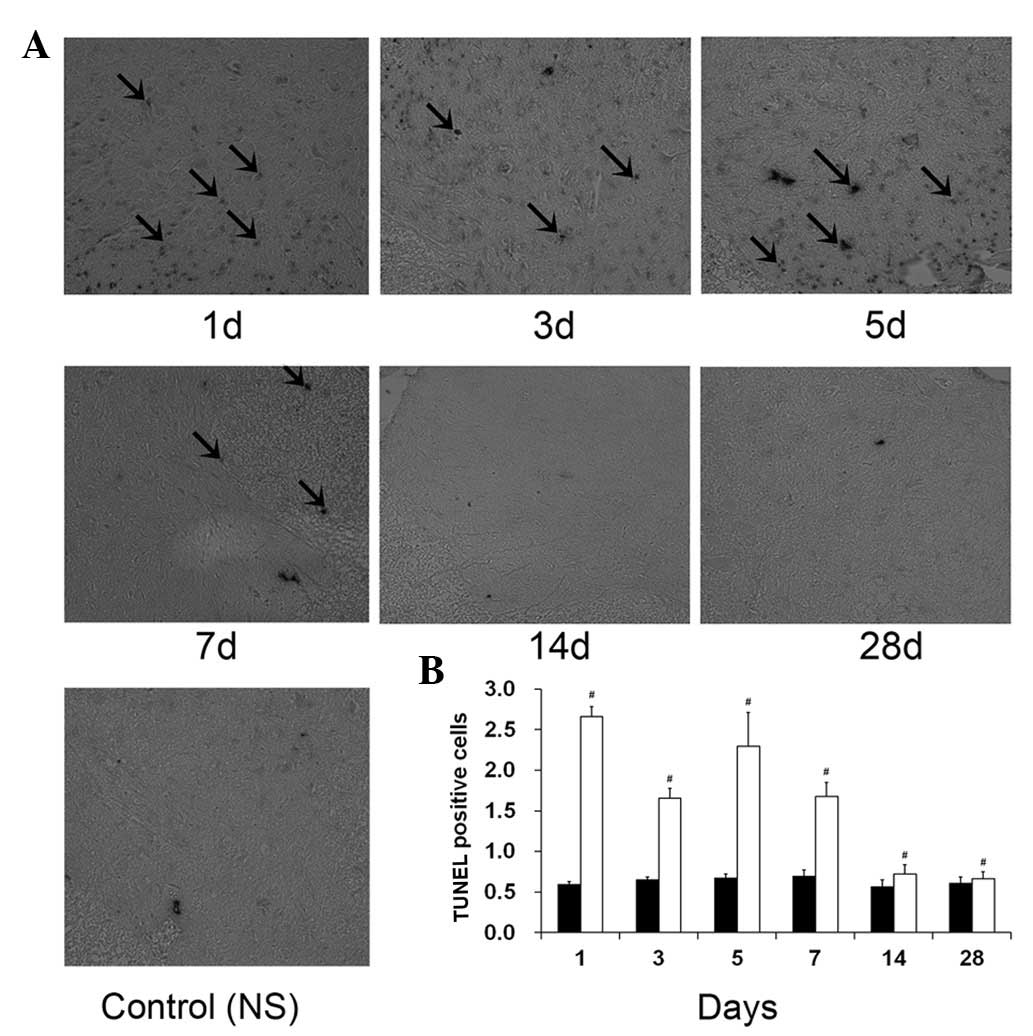
Measurement of apoptosis - TUNEL
staining
TUNEL staining was performed to determine whether
exposure to 1% ropivacaine on days 1, 3, 5, 7, 14 and 28 triggers
apoptosis-mediated neurotoxicity (Fig.
1). The percentages of TUNEL-positive cells in group NS were
0.030±0.005, 0.032±0.008, 0.034±0.007 and 0.035±0.003% on days 1,
3, 5 and 7, respectively. The percentages of TUNEL-positive cells
in group R were 1.33±0.26, 0.89±0.15, 1.15±0.18 and 1.34±0.21% at
the same time points. The mean number of TUNEL-positive cells in
sample A of group R was significantly higher than that in group NS
on days 1, 3, 5 and 7 (P<0.05).
Ropivacaine and the Akt pathway
Akt activation was assessed by immunoblotting with
an anti-activated Akt antibody (pAkt) in sample B, as described in
Fig. 2A. Ropivacaine significantly
suppressed Akt activation (Fig.
2A, lanes 1–6). Multiple experiments (n=6, Fig. 2B) demonstrated that the expression
levels of pAkt in group R were significantly less than those in
group NS at all time points (P<0.05). Total Akt levels were also
examined by immunoblotting using an anti-total Akt antibody. We
observed no significant difference of total Akt proteins in group R
compared with group NS (Fig. 2C;
P>0.05). This correlated with the degree of apoptosis under the
same conditions (compare Fig. 1B
with Fig. 2A). Akt was activated
in group NS cells (Fig. 3A, lane
1). By contrast, ropivacaine suppressed Akt activation (Fig. 3A, lane 2). Ropivacaine was
associated with greater apoptosis in spinal cord and suppressed Akt
activation.
Measurement of cell death - MTT
assay
An MTT assay was performed to investigate the
molecular mechanism(s) underlying this in vitro toxicity. To
evaluate the toxic effects of ropivacaine, we performed cell counts
using the culture model system PC12 (16,17).
As shown in Fig. 4 by MTT assay,
group NS cells grew vigorously and reached minimum confluence at 48
h; the cell survival rate was 99%. By contrast, the cell density in
the presence of ropivacaine significantly declined; the cell
survival rate was 46.1±7.3%. Notably, group NS cells grew to a
significantly higher density than those of group R (P<0.05).
These data demonstrate that ropivacaine leads to cell death.
Apoptosis and necrosis have been suggested to be two
of the mechanisms by which LAs induce cell death (18). In the present study, we used trypan
blue exclusion to assess the viability of PC12 cells. As shown in
Fig. 5A, dead cells appear stained
due to nuclear condensation. Viable cells were not stained. PC12
cells were grown in the absence or presence of ropivacaine. As
already observed in Fig. 4, the
total cell number in group R was significantly less than that of
group NS (Fig. 5B). In group NS,
dead cells (stained apoptotic and necrotic) were rare (2±1%). In
group R, the percentage of stained cells was 79.2±13.4% at 48 h.
Cell death percentages were calculated by normalizing non-viable
cells to total cells and statistical results were exhibited
(Fig. 5C). PC12 cells treated with
ropivacaine had a had greater number of stained death
identifiers.
Discussion
Ropivacaine is available for spinal or intrathecal
use and its use in clinical practice is common. The active duration
of ropivacaine is 1.6–6 h and is considered a long-acting LA
(19). Ropivacaine has numerous
advantages for epidural analgesia compared with traditional
medicines, including bupivacaine (20–22).
However, in vitro and in vivo studies suggest that
ropivacaine exhibits neurotoxicity (11,14).
In a previous study, we reported that following 6- or 48-h exposure
during continuous spinal anesthesia, ropivacaine induced mild
neuronal injury to the spinal cord and nerve roots (11). However, detailed molecular
mechanisms, particularly the signaling pathways, by which the LAs
exert neurotoxicity remain to be explored. The aim of the present
study was to uncover the possible signaling pathways by which
ropivacaine causes neurotoxicity. Numerous neurotoxicity studies
have been conducted under various conditions, with exposure times
ranging between 10 min and 48 h. In this experiment, we used a rat
model of repeated intrathecal administration of 1% ropivacaine for
12 h and examined the long-term (for 28 days) effects on lumbar
enlargement spinal cord apoptosis following drug administration.
The dosing interval, of 1.5 h, was based on a study by Rose et
al(12). We utilized the PC12
cell line and examined the toxic effects of 1% ropivacaine.
Analysis of dose- and time-dependent effects in PC12 cells was
based on a study conducted by Lirk et al(14). The present study focused on Akt,
due to its critical signaling mode within cells under physiological
and pathological cell survival mechanisms (8). In previous studies, Yuan et al
suggested signaling pathways mediated by Akt are correlated to
neuronal survival (23). In
addition, Nakajima et al reported that persistent activation
of Akt is important in neuronal cells (24).
In the present study, we demonstrated that
ropivacaine exposure in vivo caused significant lumbar
enlargement spinal cord apoptosis on days 1, 3, 5 and 7. In
addition, we demonstrated that ropivacaine significantly inhibits
cell growth and caused cell death following 48 h exposure in PC12
cells. Apoptosis is an essential process for cell integrity in
development and survival. It is also triggered by physiological and
non-physiological stimulation or insult, leading to pathological
conditions. Although apoptosis is considered a major mechanism of
LA-induced cell death, particularly in the case of neurotoxicity,
LAs also induce necrotic cell death (25,26).
Our results indicate that apoptosis and cell death occur when
ropivacaine is administrated intrathecally and exposed to PC12
cells. Our data suggest that therapeutic concentrations of the LA
ropivacaine may be sufficient to cause local anesthetic-induced
long term neurotoxicity. We also identified that Akt activation was
decreased in the presence of intrathecal ropivacaine and in
cultured PC12 cells exposed to ropivacaine. Inhibition of the Akt
pathway may explain the resultant apoptosis upon exposure to
ropivacaine. An inverse correlation between the levels of Akt
activation and degree of apoptosis was noted in the current study.
This supports the hypothesis that LAs, in particular ropivacaine,
induce apoptosis via regulation of the Akt pathway. In the first
part of this study, we assessed Akt activation by immunoblotting in
repeated intrathecal ropivacaine. In the present study, we assessed
Akt activation by immunoblotting with an anti-activated Akt
antibody (pAkt) in PC12 cells (Fig.
2). When we examined total Akt levels by immunoblotting with an
anti-total Akt antibody, we observed a significantly decreased
level of total Akt proteins in group R cells compared with group NS
(Fig. 3A). Compared with group NS,
group R decreased Akt activity to 44.7±6.8%. Total Akt was also
decreased in group R to 33.2±8.5% compared with group NS. This
largely correlated with the degree of dead cells under the same
conditions (compare Fig. 3B with
Fig. 4 or Fig. 5B/5C). Taken together, our data
suggest that ropivacaine caused PC12 cell death, at least in part,
via the inhibition of Akt activation. However, we cannot completely
rule out other signaling pathway(s) as significant contributors to
LA-induced toxicity. p38 MAPK and c-Jun N-terminal kinase (JNK)
pathways are known to be involved in ropivacaine-induced
neurotoxicity (11,27). These findings raise the possibility
that certain, if not all, of these pathways may modulate specific
LA-induced neurotoxicity independently or collectively. Further
investigation is required to address these issues. Limited clinical
studies have suggested that ropivacaine may lead to transient
neurological syndromes (28,29).
The present study provides direct supportive evidence that in
vitro and in vivo ropivacaine exposure results in
neurotoxicity. The neurotoxicity of ropivacaine demonstrated in
this study contributes to an emerging body of data supporting the
detrimental neurotoxic effects of ropivacaine when used as an
intrathecal anesthetic.
In conclusion, ropivacaine induces neurotoxicity
in vivo and in vitro, and the Akt pathway is involved
in the neurotoxicity of ropivacaine.
Acknowledgements
This study was supported by the Academician’s Grant
of Changsha (no. K0803154-31).
References
|
1
|
Malhotra N, Chanana C, Roy KK, Kumar S,
Rewari V and Sharma JB: To compare the efficacy of two doses of
intraperitoneal bupivacaine for pain relief after operative
laparoscopy in gynecology. Arch Gynecol Obstet. 276:323–326. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iida H, Watanabe Y, Dohi S and Ishiyama T:
Direct effects of ropivacaine and bupivacaine on spinal pial
vessels in canine. Assessment with closed spinal window technique.
Anesthesiology. 87:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee SJ, Shin TJ, Kang IS, Ha JH, Lee SC
and Kim HJ: AMPK attenuates bupivacaine-induced neurotoxicity. J
Dent Res. 89:797–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Radwan IA, Saito S and Goto F: The
neurotoxicity of local anesthetics on growing neurons: a
comparative study of lidocaine, bupivacaine, mepivacaine, and
ropivacaine. Anesth Analg. 94:319–324. 2002.PubMed/NCBI
|
|
5
|
Maurice JM, Gan Y, Ma FX, Chang YC, Hibner
M and Huang Y: Bupivacaine causes cytotoxicity in mouse C2C12
myoblast cells: involvement of ERK and Akt signaling pathways. Acta
Pharmacol Sin. 31:493–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zink W and Graf BM: Local anesthetic
myotoxicity. Reg Anesth Pain Med. 29:333–340. 2004. View Article : Google Scholar
|
|
7
|
Zink W and Graf BM: The toxicity of local
anesthetics: the place of ropivacaine and levobupivacaine. Curr
Opin Anaesthesiol. 21:645–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Werdehausen R, Fazeli S, Braun S, et al:
Apoptosis induction by different local anaesthetics in a
neuroblastoma cell line. Br J Anaesth. 103:711–718. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu F, Li T and Zhang B: An improved method
for protecting and fixing the lumbar catheters placed in the spinal
subarachnoid space of rats. J Neurosci Methods. 183:114–118. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong Z, Qulian G, Yuan Z, Wangyuan Z and
Zhihua S: Repeated intrathecal administration of ropivacaine causes
neurotoxicity in rats. Anaesth Intensive Care. 37:929–936.
2009.PubMed/NCBI
|
|
12
|
Rose FX, Estebe JP, Ratajczak M, et al:
Epidural, intrathecal pharmacokinetics, and intrathecal
bioavailability of ropivacaine. Anesth Analg. 105:859–867. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan Z, Dohi S, Chen J, Banno Y and Nozawa
Y: Involvement of the mitogen-activated protein kinase family in
tetracaine-induced PC12 cell death. Anesthesiology. 96:1191–1201.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lirk P, Haller I, Colvin HP, et al: In
vitro, inhibition of mitogen-activated protein kinase pathways
protects against bupivacaine- and ropivacaine-induced
neurotoxicity. Anesth Analg. 106:1456–1464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y, Li J, Zhang Y and Wu C: The roles
of integrin-linked kinase in the regulation of myogenic
differentiation. J Cell Biol. 150:861–872. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greene LA and Tischler AS: Establishment
of a noradrenergic clonal line of rat adrenal pheochromocytoma
cells which respond to nerve growth factor. Proc Natl Acad Sci USA.
73:2424–2428. 1976. View Article : Google Scholar
|
|
17
|
Fasolato C, Zottini M, Clementi E,
Zacchetti D, Meldolesi J and Pozzan T: Intracellular
Ca2+ pools in PC12 cells. Three intracellular pools are
distinguished by their turnover and mechanisms of Ca2+
accumulation, storage, and release. J Biol Chem. 266:20159–20167.
1991.
|
|
18
|
Boselli E, Duflo F, Debon R, et al: The
induction of apoptosis by local anesthetics: a comparison between
lidocaine and ropivacaine. Anesth Analg. 96:755–756. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McLure HA and Rubin AP: Review of local
anaesthetic agents. Minerva Anestesiol. 71:59–74. 2005.PubMed/NCBI
|
|
20
|
Wildsmith JA and Selander DE: Measuring
the relative potencies of bupivacaine and ropivacaine in spinal
anesthesia. Reg Anesth Pain Med. 34:73–74; author reply 734–735.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guryay D, Karaege GT, Katircioglu K,
Ozkalkanli MY, Ozgurbuz U and Savaci S: The effects of an epidural
infusion of ropivacaine versus saline on sensory block after spinal
anesthesia. Reg Anesth Pain Med. 33:217–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michalek-Sauberer A, Kozek-Langenecker SA,
Heinzl H, Deusch E and Chiari A: Median effective local anesthetic
doses of plain bupivacaine and ropivacaine for spinal anesthesia
administered via a spinal catheter for brachytherapy of the lower
abdomen. Reg Anesth Pain Med. 33:4–9. 2008. View Article : Google Scholar
|
|
23
|
Yuan Y, Guo Q, Ye Z, Pingping X, Wang N
and Song Z: Ischemic postconditioning protects brain from
ischemia/reperfusion injury by attenuating endoplasmic reticulum
stress-induced apoptosis through PI3K-Akt pathway. Brain Res.
1367:85–93. 2011. View Article : Google Scholar
|
|
24
|
Nakajima T, Iwabuchi S, Miyazaki H, et al:
Preconditioning prevents ischemia-induced neuronal death through
persistent Akt activation in the penumbra region of the rat brain.
J Vet Med Sci. 66:521–527. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koike T, Tanaka S, Oda T and Ninomiya T:
Sodium overload through voltage-dependent Na(+) channels induces
necrosis and apoptosis of rat superior cervical ganglion cells in
vitro. Brain Res Bull. 51:345–355. 2000.
|
|
26
|
Mukherjee PK, Marcheselli VL, Serhan CN
and Bazan NG: Neuroprotectin D1: a docosahexaenoic acid-derived
docosatriene protects human retinal pigment epithelial cells from
oxidative stress. Proc Natl Acad Sci USA. 101:8491–8496. 2004.
View Article : Google Scholar
|
|
27
|
Haller I, Hausott B, Tomaselli B, et al:
Neurotoxicity of lidocaine involves specific activation of the p38
mitogen-activated protein kinase, but not extracellular
signal-regulated or c-jun N-terminal kinases, and is mediated by
arachidonic acid metabolites. Anesthesiology. 105:1024–1033. 2006.
View Article : Google Scholar
|
|
28
|
Capdevila X, Pirat P, Bringuier S, et al:
Continuous peripheral nerve blocks in hospital wards after
orthopedic surgery: a multicenter prospective analysis of the
quality of postoperative analgesia and complications in 1,416
patients. Anesthesiology. 103:1035–1045. 2005. View Article : Google Scholar
|
|
29
|
Kitagawa N, Oda M and Totoki T: Possible
mechanism of irreversible nerve injury caused by local anesthetics:
detergent properties of local anesthetics and membrane disruption.
Anesthesiology. 100:962–967. 2004. View Article : Google Scholar
|