Introduction
A new era commenced when metallic stents were
firstly introduced into clinical practice for the management of
coronary and peripheral artery disease following transluminal
angioplasty. This development brought about a significant reduction
in restenosis compared to balloon angioplasty (1). Stent implantation has improved
outcomes and become the preferred revascularization treatment for
occlusive blood vessel disease. However, during surgical
intervention, the unavoidable vascular trauma may initiate
undesirable responses such as the excessive proliferation of
vascular smooth muscle cells (SMCs), extracellular matrix
synthesis, thrombosis and a chronic inflammatory reaction, leading
to complications resulting in the re-narrowing of the treated
artery, known clinically as in-stent restenosis (ISR) (2). ISR is defined as diameter stenosis of
≥50% in the stented area of the vessel mainly due to excessive
neointimal proliferation within the stented segment. This reduces
the long-term efficacy of metal stent implantation to 20–40%.
ISR represents an abnormal vascular response and
repair to injury that results in excessive tissue growth.
Angioscopic and pathological evidence suggests that endothelial
cell (EC) dysfunction contributes to such development (3). A healthy endothelium sustains an
antithrombotic milieu by secretion of various factors that exert
antiaggregatory effects on platelets or have anticoagulatory or
fibrinolytic properties (4). When
in a confluent monolayer, ECs cease replication. Following stent
implantation, the attendant endothelial denudation allows thrombus
formation in response to exposure of highly thrombogenic substances
from ruptured or erosive plaques, and in the meantime, the SMCs
proliferate and migrate to the deendothelialized vessel surface,
where they continue to proliferate and secrete extracellular matrix
proteins, leading to neointimal tissue formation.
A possible future approach to overcome ISR is to
promote and accelerate reendothelialization in the injured vessel,
which is more natural and consequently safer. Among them,
EC-capture stent, a type of stent used to attract ECs, would
ultimately promote the elution of biologically active substances
through a functioning endothelium monolayer, revealing correlation
with a decrease in thrombosis and intimal thickening (5). Endothelium cell seeding techniques
could be of high value for the establishment of a confluent
monolayer on the stent in vitro. However, in vitro
experiments showed that limited ECs were retained after stent
expansion at the time of implantation and the subsequent pulsatile
flow exposure (6,7).
Prior studies have suggested that coating the stent
with a matrix such as fibronectin or collagen would facilitate the
attachment of ECs. These studies have also demonstrated that
fibronectin-coated stents can be seeded in vitro with
vascular ECs (8,9) and that a number of the cells remain
adherent to the stent following balloon expansion of the stent
(10). However, these matrices are
thrombogenic and could increase stent thrombogenicity. In addition,
their poor mechanical property and limited ability to sustain stent
expansion and deployment are also substantial challenges in
clinical application (10,11).
A number of other different compounds have been
utilized as scaffold for the growth of ECs in the last decade.
Among them, silk fibroin, the material that remains after the
removal of sericin from silk, has demonstrated unique mechanical
properties as well as excellent biocompatibility, controlled
degradability and versatile processability in different material
formats, and could be used to coat the stent in an attempt to
increase attachment to ECs (12,13).
Silk has been used for centuries primarily as suture material. Its
fibers are composed of two major types of protein: a) sericin, the
antigenic gum-like protein surrounding the fibers; and b) fibroin,
the core filaments of silk responsible for silk’s elasticity
(14). If sericin is removed,
purified silk fibroin exhibits few immunological reactions,
retaining the strength and many other desirable features of silk
just as mentioned above.
Recent studies have revealed that physical
modifications of biomaterials may influence cell reaction (15), and that nanostructured materials
have been shown to support endothelial cellular attachment and
growth (16). Thus, to validate
silk fibroin’s ability for coating the stent, it is our belief that
a deeper understanding of cell-material interactions is essential
for controlling endothelial cellular function by the physical
parameters of the material. In the present study, we focused on
employing a micromolding technique to produce silk fibroin with
different microstructural features at micron scale, and at the same
time, to evaluate the effect of these microtopographical features
on cell behavior, such as attachment and growth, using human
umbilical vein ECs (HUVECs). Furthermore, we analyzed the cell
cycle and investigated relevant molecules involved in the cell
growth and adhesive molecules such as integrin α5β1 and α5β3 for
the study of the biological performance of cells on different
patterned surfaces.
Materials and methods
Fabrication of silk fibroin
Silk fibroins with different microtopographical
features were kindly supplied by the Laboratory of Advanced
Materials, Fudan University. Polydimethylsiloxane (PDMS) molds were
surface modified by carving various combinations of grooves and
ridges into the surface using a computer controlled excimer laser
beam through a metal photomask. The mask was not in close contact
with the surface and the desired pattern was projected via the
laser beam onto the area of interest. The silk fibroin solutions
prepared earlier were pipetted on top of the pattern present on the
PDMS mold. As the silk fibroin solution evaporated and transformed
to membrane, the micropatterns of the PDMS mold were transferred to
the membrane. In this way, 4 types of silk fibroin were obtained
having microtopographical structures at micron scale, as well as
one control, having a non-patterned structure.
Cell culture on the silk fibroin
surfaces
HUVECs were isolated according to a previously
published method (17). Cells were
cultured in medium M199 (Sigma, St. Louis, MO, USA) supplemented
with 20% fetal bovine serum (Thermo Scientific Hyclone, South
Logan, UT, USA). Cells were seeded at a density of 1×104
cells/cm2 in a 6-well plate containing 4 types of silk
fibroin with different microtopographic structures and one with a
non-patterned structure. The plate was incubated at 37<C in 5%
carbon dioxide. Cells were cultured for 3 days and were imaged at
×160 magnification using an IX-71 microscope (Olympus, Tokyo,
Japan) equipped with a high resolution digital camera.
Cell proliferation assay
Cell proliferation was evaluated during culture time
(24, 48 and 72 h) to detect the effect of microtopographic
structures on the proliferation of HUVECs by Sulforhodamine B
(SRB). Cells (5×103 cells/well in 100 μl medium) were
seeded in 96-well plates containing the 4 types of silk fibroin
with different microtopographic structures and one with a
non-patterned structure. After each time point, 50 μl of 30%
trichloroacetic acid was added for 60 min at 4°C. After washing and
drying the plate, 100 μl of 0.4% SRB was added for 30 min. The
plates were rinsed with 0.1% acetic acid and air-dried, after which
100 μl of Tris base (10 mmol/l) was added, and the plates were
agitated for 5 min. The SRB value was measured at a wavelength of
490 nm by iMark™ Microplate Reader (Bio-Rad, Hercules, CA, USA).
The experiment was performed in quintuplicate and repeated three
times.
Real-time PCR analysis
HUVECs were seeded in 60 mm plates
(1×106/plate) containing the 4 types of silk fibroin
with different microtopographic structures and one with a
non-pattened structure. After incubation for 72 h, cells were
removed from plates with trypsin and then centrifuged. The total
cellular RNA was extracted from the cell pellets using TRIzol
(Invitrogen, Carlsbad, CA, USA) and then transcribed into cDNA with
a RevertAid™ first strand cDNA synthesis kit (Fermentas, Vilnius,
Lithuania) using 2 μg total RNA and oligo(dT) primers. The
quantitative PCR included 2 μl cDNA and 10 μl SYBR-Green Master mix
(Takara, China) with a pair of primers. The reactions were
monitored on the ABI PRISM 7500 sequence system (Applied
Biosystems, Carlsbad, CA, USA). mRNA levels of VEGF, VCAM-1,
Eselectin, α5β3 and E-cadherin were calculated using the equation
2−ΔΔCt and normalized to human GAPDH mRNA levels. The
primer sequences for specific mRNA are shown in Table I.
 | Table IPrimer sequences for specific
genes. |
Table I
Primer sequences for specific
genes.
| Gene | Primer sequences
(5′-3′) |
|---|
| VEGF | F:
AATCATCACGAAGTGGTGAAG |
| R:
AATCTGCATGGTGATGTTGGA |
| VCAM-1 | F:
TTAGATAATGGGAATCTACA |
| R:
TCAACATGACTGAGTCTCCAA |
| Eselectin | F:
TGAGTGTGATGCTGTGACAAA |
| R:
TTCTGAGGCTGGCGGACGG |
| α5β3 | F:
TTAAGAATGCTTACAATAA |
| R:
AACGACTGCTCCTGGATGCAC |
| E-cadherin | F:
TTGCTCACATTTCCCAACTCC |
| R:
TTTCAATAATAAAGACACCAA |
| GAPDH | F:
TTCACCACCATGGAGAAGGCTG |
| R:
TTCCACGATACCAAAGTTGTCA |
Western blot analysis for adhesive
molecules
HUVECs were seeded in 60-mm plates as described
above. Following incubation for 72 h, cells were removed from the
plates with trypsin and then centrifuged. Cell pellets were
resuspended in lysis buffer (1% Triton X100, 50 mM Hepes, pH 7.4,
150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM
NaPPi and 10% glycerol to which 1 mM PMSF, 1 mM
Na3VO4, and 1X protease inhibitor were added
before use) to harvest cell lysates. Proteins from total cell
lysates were separated using a 10–15% SDS-PAGE gel and then
transferred to PVDF membranes (Millipore, Billerica, MA, USA). The
membranes were blocked, washed, and incubated with specific primary
antibodies. The primary antibody incubation was followed by
incubation with HRP-conjugated secondary antibodies. The bands were
detected with an enhanced chemiluminescence assay (PerkinElmer,
Waltham, MA, USA). The protein expression level of α5β1 and α5β3
was calculated using Quantity One software (Bio-Rad).
Enzyme-linked immunosorbent assay (ELISA)
test
HUVECs were seeded in 60-mm plates as described
above. Supernatants were harvested after 72 h and centrifuged at
2,000 × g to measure the concentration of protein factor by an
ELISA according to the manufacturer’s instructions for the ELISA
kit (R&D Systems, Minneapolis, MN, USA).
Cell cycle analysis
HUVECs were seeded in 60-mm plates as described
above. After incubation for 72 h, cells were trypsinized, collected
in phosphate-buffered saline (PBS) and fixed on ice followed by 70%
cold ethanol. After treatment with 10 μg/ml RNase, cells were
stained with 50 μg/ml propidium iodide (Sigma) for 15 min at room
temperature to prepare for cell cycle analysis. Stained cells were
analyzed by flow cytometry (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA). The cell cycle information was analyzed using
ModFit 3.0 software.
Statistical analysis
All data were subjected to statistical analysis and
were reported as the mean ± standard deviation. ANOVA was used to
assess the statistical differences in means among these groups. The
criterion for statistical significance was taken as P<0.05 using
a two-tailed t-test. The analyses were performed using SPSS 15.0
software.
Results
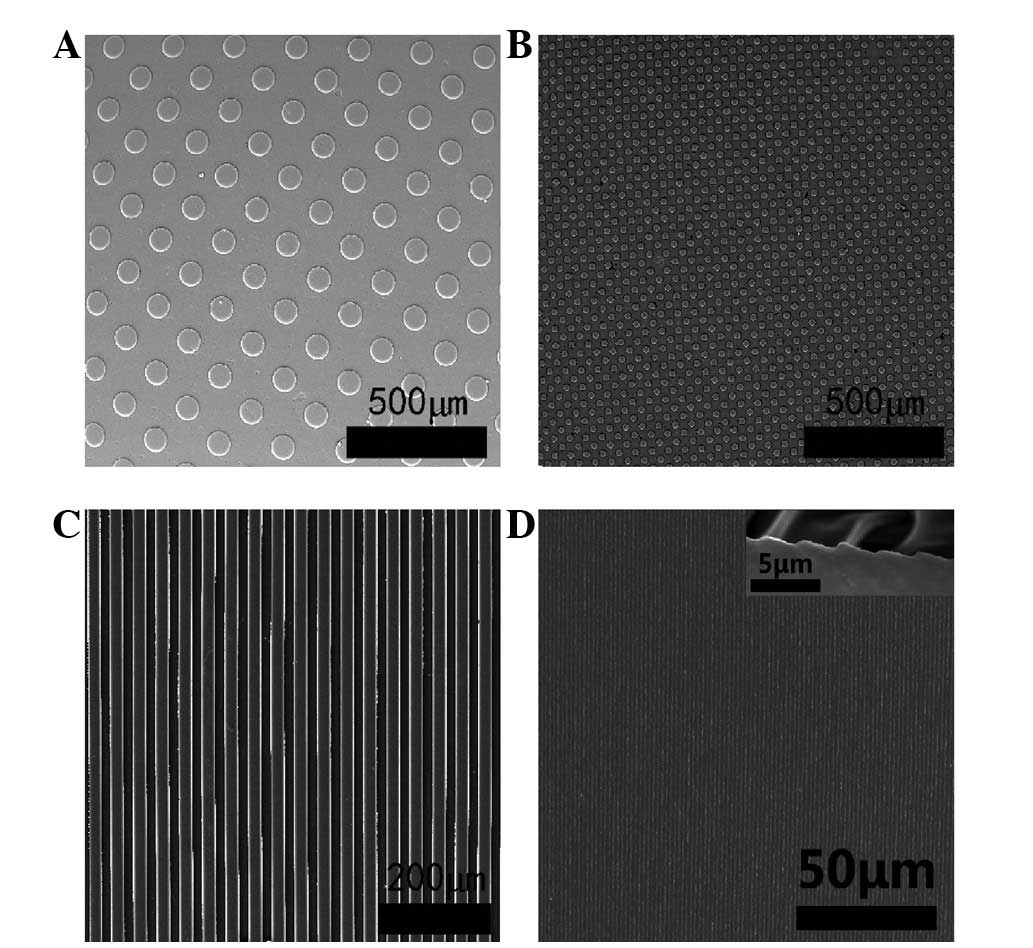
Fabrication of micropatterned silk
fibroin
In this study, micromolding was used to develop
microtopographic structures of silk fibroin. Four defined types of
silk fibroin demonstrated features of microtopographical structure
and another showed a non-patterned structure as a control (Fig. 1).
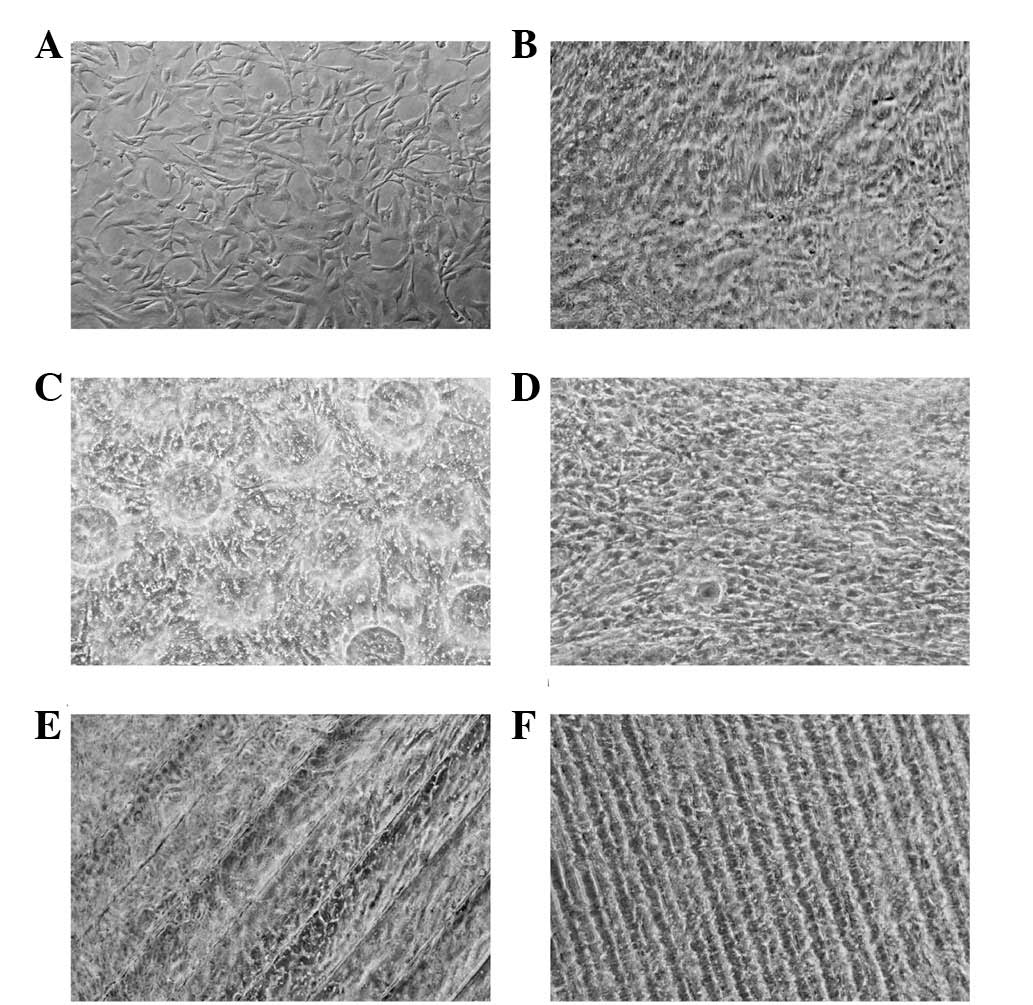
Cell behavior on micropatterned silk
fibroin surfaces
Fig. 2 shows
micrographs of HUVEC imaged at ×160 magnification on silk fibroin
with different microtopographic structures or non-patterned
structure after 72 h in culture. Notably, most of the ECs had
preferential spreading along the ridges and grooves of the
microtopographic structure. The cells clearly show a different
adhesion and proliferation behavior depending on the underlying
microtopographic structure as described in Table II. Cells in the non-patterned
structure group showed a changed morphology, such as fusiform or
spherical, compared to the cells of the microtopographic structure
groups, which were well attached and stretched, particularly in
Structure 1. Meanwhile, the cell debris in the non-patterned
structure group was far greater than in the other groups.
 | Table IIMicrographs of HUVECs on different
types of silk fibroin. |
Table II
Micrographs of HUVECs on different
types of silk fibroin.
| Group | Cell debris | Morphology |
|---|
| Non-patterned
structure | More | Changed |
| Structure 1 | Less | Very good |
| Structure 2 | Less | Not bad |
| Structure 3 | Less | Good |
| Structure 4 | Less | Good |
Effect of microtopographic structure of
silk fibroin on cell growth
Cell proliferation evaluated by SRB suggested that
cultured HUVECs on silk fibroin with different microtopographic
structures or non-patterned structure proliferated from day 1 to 3
of culture and the cell number in Structure 1 increased the most.
Compared to the non-patterned structure group, the optical density
(OD) values were statistically significant for the microtopographic
structure groups (P<0.01, P<0.05), with the exception of
Structure 4 (P>0.05), although for the first 2 days no
statistically significant difference was observed, suggesting that
HUVECs would proliferate better on silk fibroin with different
microtopographic structures (Fig.
3).
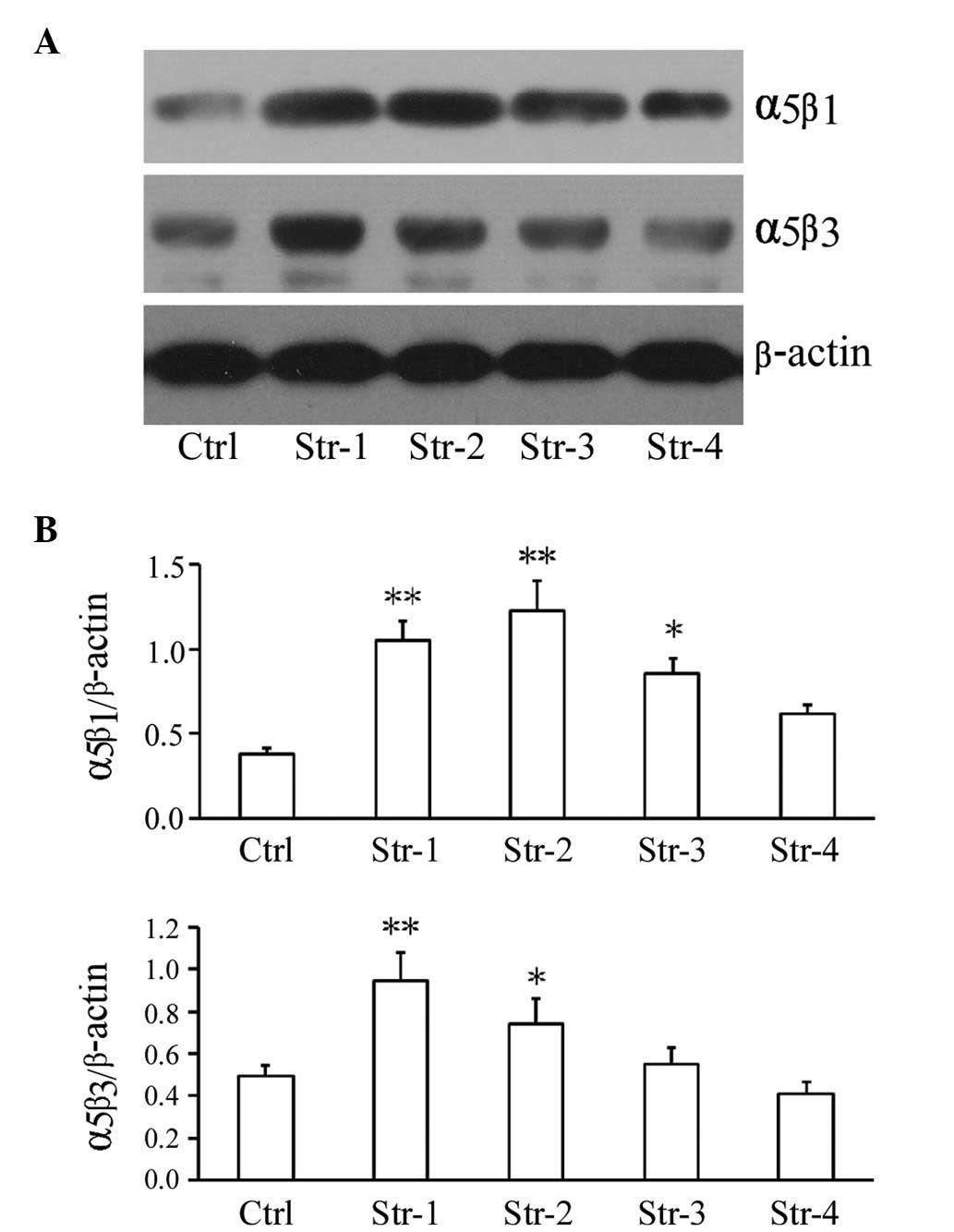
Effect of microtopographic structure of
silk fibroin on mRNA level and protein expression of growth factor
and adhesive molecules
To understand the mechanisms of how most ECs adhere
and grow more effectively on silk fibroin with microtopographic
structures compared to that of non-patterned structure, we
performed molecular studies on the adhesive molecules and growth
factor using real-time PCR and western blot analysis. We found that
after 72 h, the mRNA level of adherent molecule Eselectin was
upregulated in Structure 1, and VCAM-1 was upregulated in Structure
1 and 3; moreover, α5β3, E-cadherin and VEGF were upregulated in
all microtopographic structure groups except Structure 4, compared
to the non-patterned structure group, particularly in Structure 1
group (P<0.01, P<0.05; Fig.
4). In addition, the protein expression of adhesive molecule
α5β1 was upregulated in the microtopographic structure groups
except Structure 4, and α5β3 was upregulated in Structure 1 and 2,
compared to the non-patterned structure group (P<0.01,
P<0.05; Fig. 5).
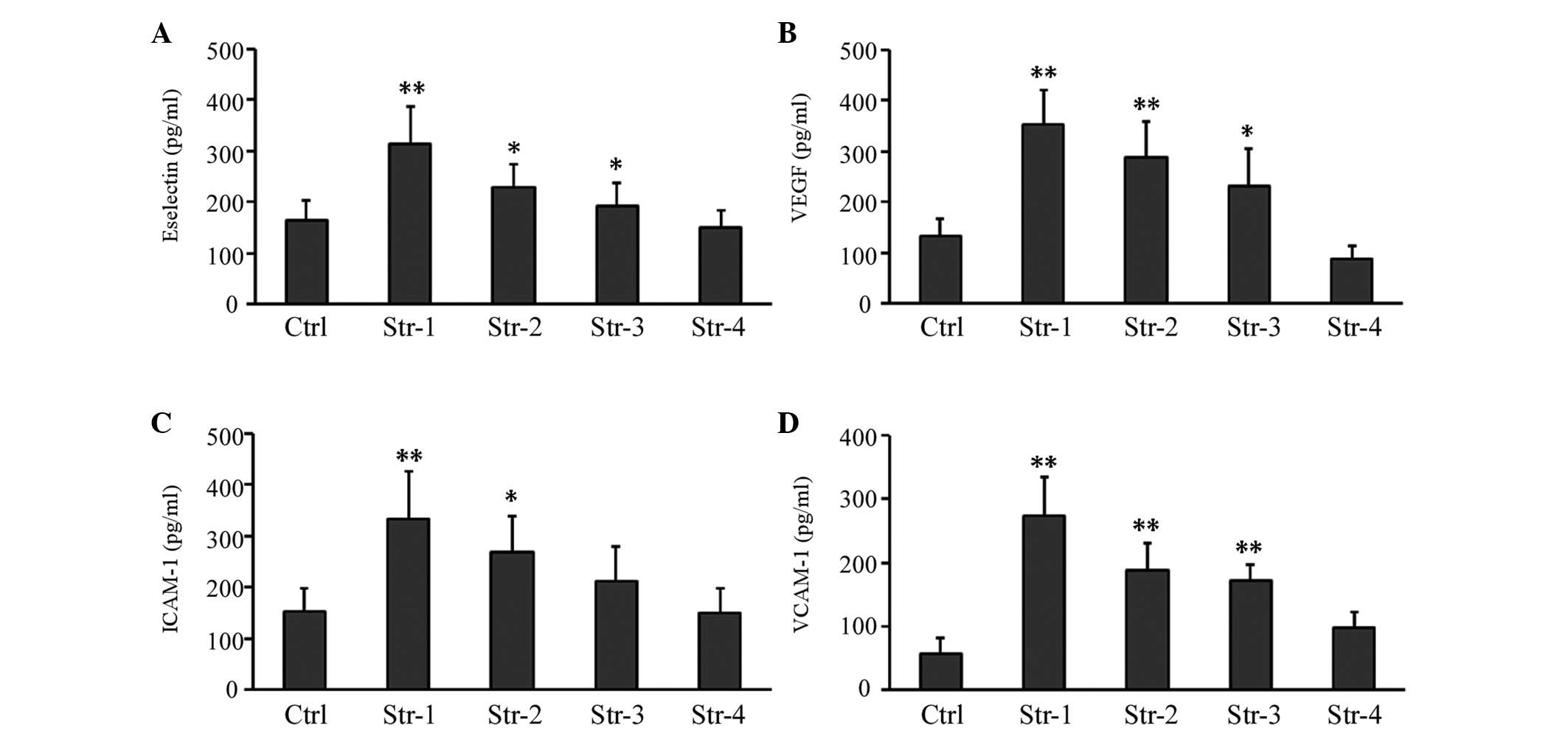
Effect of microtopographic structure of
silk fibroin on secretion of protein factor
The influence of microtopographic structure on
protein factor secreted in the HUVEC culture supernatant was
further investigated using ELISA. We found that after 72 h in
culture, the secretion of growth factor VEGF and adhesive molecules
Eselectin and VCAM-1 increased significantly in microtopographic
structure groups except Structure 4. Moreover, the secretion of
adhesive molecule ICAM-1 increased significantly in Structure 1 and
2 groups (particularly in the former), compared with the
non-patterned structure group (P<0.01, P<0.05; Fig. 6).
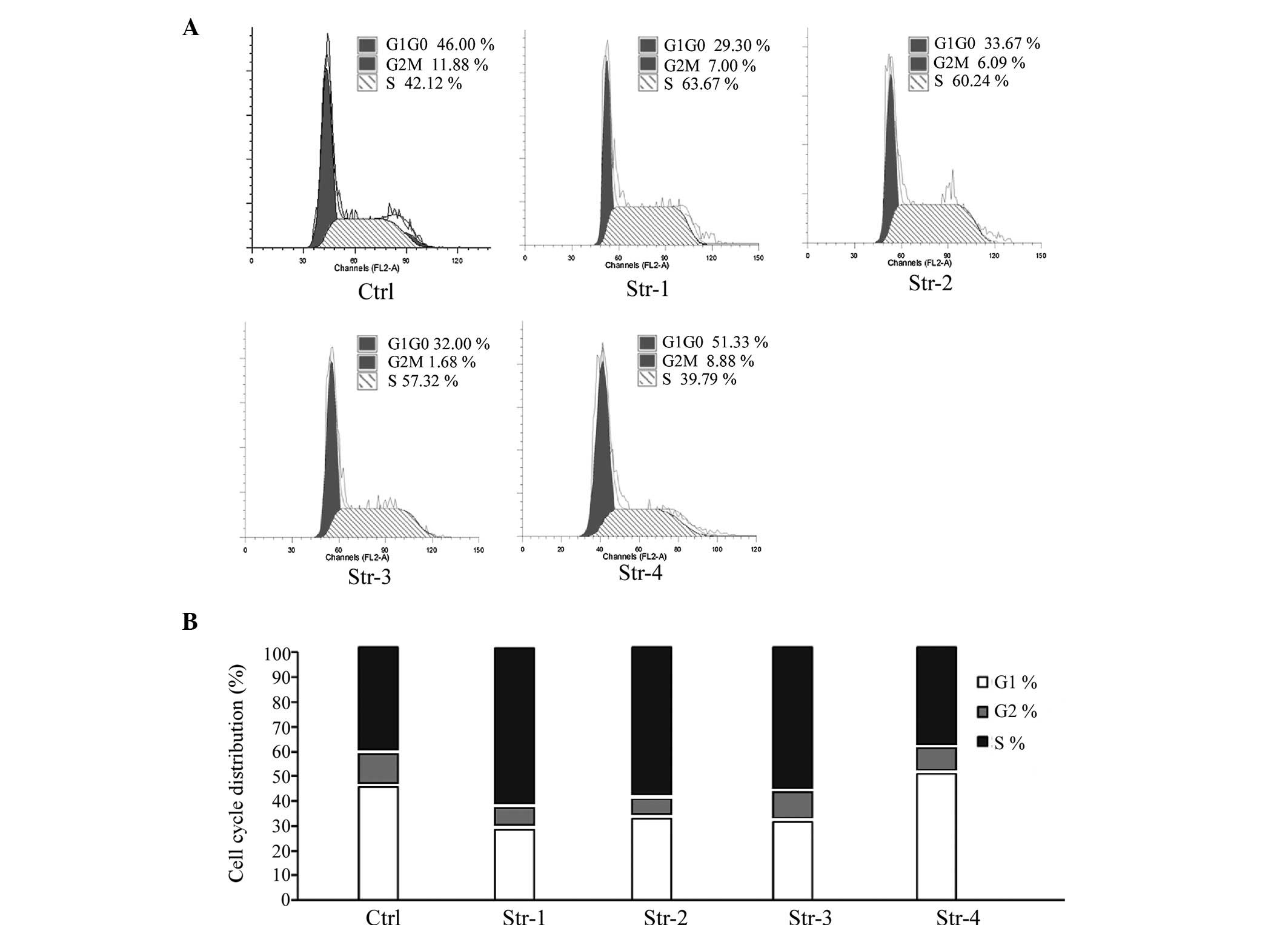
Effect of microtopographic structure of
silk fibroin on cell cycle
In line with the cell proliferation results,
Fig. 7 shows that, with the
exception of Structure 4, the frequency of cells at the S or G1
phase was markedly increased or decreased in the microtopographic
structure groups compared to the non-patterned structure group
(P<0.01, P<0.05; Table
III).
 | Table IIICell cycle analysis of HUVECs. |
Table III
Cell cycle analysis of HUVECs.
| Group | G0/G1 (%) | S (%) | G2/M (%) |
|---|
| Non-patterned
structure | 46.00±4.58 | 11.88±2.37 | 42.12±2.78 |
| Structure 1 | 29.33±8.08 | 7.00±1.76 | 63.67±6.36 |
| Structure 2 | 33.67±8.02 | 6.09±2.07 | 60.24±7.08 |
| Structure 3 | 32.00±3.61 | 10.68±3.35 | 57.32±5.58 |
| Structure 4 | 51.33±3.51 | 8.88±0.73 | 39.79±4.25 |
Discussion
One of the currently discussed concepts to improve
the vascularization of metallic stents is the prevascularization by
including vascular ECs. A wide variety of coatings have been
explored for stents, targeting the objective to promote
endothelialization. The success of such biomaterial is dependent on
its ability to support the growth and functioning of the cells
growing on it. Prior studies (12,13)
showed that silk fibroin had unique properties that fulfill many of
the requirements for biomaterial scaffolds and could be used as a
coating applied to stent for the culture of human ECs. It was
demonstrated that the biophysical environment consisting of
microstructures could influence the cellular behavior; however,
whether the silk fibroin of different microstructures could support
the growth of human ECs and exert effects on endothelial phenotypes
or functions have never been discussed.
In this study, silk fibroin of microtopographic
structure with four different patterns developed by micromolding
were synthesized to evaluate the response of vascular ECs to
topographic cues. Laser and UV-based patterning are two prevalent
alternative methodologies for engineering and micromolding the
surface with specific patterns (18), allowing enhanced resolution despite
the higher costs. Of these methods, we adopted the former. We used
HUVECs in our experiment since this endothelial cell type is of
embryonic origin and of the macrovascular type, whereas ECs
involved in inflammation, healing and vascularization are primarily
of microvascular origin.
In the present study, we provided quantitative data
in order to gain more insight into the effect of the microstructure
of silk fibroin on cell growth and further investigated the
mechanism of it. The response of HUVECs growing on non-patterned
and microtopographic structure silk fibroin scaffolds was compared,
with emphasis on cell morphology, proliferation, cell cycle,
expression of adhesive molecules and secretion of relevant protein
factors.
Our results suggested that the morphology of the
HUVECs could be influenced by the microstructure of silk fibroin.
It has been reported that cells align along microfeatures in a
process resulting from the rearrangement of the cellular
cytoskeleton (18,19) and that the cells form cytoplasm
extensions and cellular associations over different ridges while
populating the groove of the pattern. Cells may have different
morphologies on sensing the distinct surface topography and in
aggregate, a microtopographic structure would be more helpful to
the morphology of the cells than a non-patterned structure.
We also found that silk fibroin of microtopographic
structure had positive effects on endothelial cell attachment, with
respect to the adhesive molecules expressed intracytoplasm and
secreted in the medium, suggesting the potential ability of the
cells induced by the microtopographic structure to synthesize,
adapt and produce adhesive proteins, to ensure effective adhesion
on the surface. In light of the fact that the microstructure
influenced the morphology of cells, it is obvious that there is an
initial correlation between the attachment and the morphology of
cells, which is supported by our observation that cells on silk
fibroin of non-patterned structure were round in shape and the
substrate demonstrated a low ability to stimulate cell adhesion.
However, the mechanism of this requires further investigation.
It is reported that changes in cell shape impacted
by the microstructure of the biomaterial have been implicated in
alterations in the cell cycle which would directly influence the
proliferative state of ECs (20).
In our studies, HUVECs demonstrated a significant change in
proliferation and cell cycle on the silk fibroin of microstructures
compared with that of the non-patterned structure, which was
consistent with the morphology of the cells.
Of all the microtopographic structures, the
behaviors and inherent phenomena of the cells were particularly
influenced by Structure 1, containing arrays of large holes within
a flat surface. As most of the cells were inclined to spread along
the ridges and grooves of the microtopographic structure, we
supposed that an architecture like Structure 1, possessing a great
number of large holes, may afford more space for cells to adhere
and thus obtain the best advantage. However, the mechanism has to
be further investigated.
Acknowledgements
This study was supported by the Fundamental Research
Projects fund (no. 09JC1403200) sponsored by the Shanghai Science
and Technology Committee.
References
|
1
|
Hinohara T: Percutaneous coronary
intervention: current perspective. Keio J Med. 50:152–160. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alfonso F, Pérez-Vizcayno MJ, Cruz A,
García J, Jimenez-Quevedo P, Escaned J and Hernandez R: Treatment
of patients with in-stent restenosis. EuroIntervention. 5(Suppl D):
D70–D78. 2009.PubMed/NCBI
|
|
3
|
Thansayari P, Kathir K, Celemajer DS and
Adams MR: Endothelial dysfunction and restenosis following
percutaneous coronary intervention. Int J Cardiol. 119:362–367.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonetti PO, Lerman LO and Lerman A:
Endothelial dysfunction: a marker of atherosclerotic risk.
Arterioscler Thromb Vasc Biol. 23:168–175. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kipshidze N, Baker J and Nikolaychik N:
Fibrin coated stents as an improved vehicle for endothelial cell
seeding. Circulation. 90:I-5971994.
|
|
6
|
Flugelman MY, Virmani R, Leon MB, Bowman
RL and Dichek DA: Genetically engineered endothelial cells remain
adherent and viable after stent deployment and exposure to flow in
vitro. Circ Res. 70:348–354. 1992. View Article : Google Scholar
|
|
7
|
Scott NA, Candal FJ, Robinson KA and Ades
EW: Seeding of intracoronary stents with immortalized human
microvascular endothelial cells. Am Heart J. 129:860–866. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van der Giessen WJ, Serruys PW, Visser WJ,
Verdouw PD, van Schalkwijk WP and Jongkind JF: Endothelialization
of intravascular stents. J Intervent Cardiol. 1:109–120. 1988.
|
|
9
|
Dichek DA, Neville RF, Zwiebel JA, Freeman
SM, Leon MB and Anderson WF: Seeding of intravascular stents with
genetically engineered endothelial cells. Circulation.
80:1347–1353. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen MC, Liang HF, Chiu YL, Chang Y, Wei
HJ and Sung HW: A novel drug-eluting stent spray-coated with
multi-layers of collagen and sirolimus. J Control Release.
108:178–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thierry B, Winnik FM, Merhi Y, Silver J
and Tabrizian M: Bioactive coatings of endovascular stents based on
polyelectrolyte multilayers. Biomacromolecules. 4:1564–1571. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Baughman CB and Kaplan DL: In
vitro evaluation of electrospun silk fibroin scaffolds for vascular
cell growth. Biomaterials. 29:2217–2227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Zhang X, Castellot J, Herman I,
Iafrati M and Kaplan DL: Controlled release from multilayer silk
biomaterial coatings to modulate vascular cell responses.
Biomaterials. 29:894–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Altman GH, Diaz F, Jakuba C, Calabro T,
Horan RL, Chen J, Lu H, Richmond J and Kaplan DL: Silk-based
biomaterials. Biomaterials. 24:401–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diener A, Nebe B, Lüthen F, Becker P, Beck
U, Neumann HG and Rychly J: Control of focal adhesion dynamics by
material surface characteristics. Biomaterials. 26:383–392. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Karuri NW, Porri TJ, Albrecht RM, Murphy
CJ and Nealey PF: Nano- and microscale holes modulate
cell-substrate adhesion, cytoskeletal organization, and -beta1
integrin localization in SV40 human corneal epithelial cells. IEEE
Trans Nanobioscience. 5:273–280. 2006. View Article : Google Scholar
|
|
17
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teixeira AI, Nealey PF and Murphy CJ:
Responses of human keratocytes to micro- and nano structured
substrates. J Biomed Mater Res A. 71:369–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rebollar E, Frischauf I, Olbrich M,
Peterbauer T, Hering S, Preiner J, Hinterdorfer P, Romanin C and
Heitz J: Proliferation of aligned mammalian cells on
laser-nanostructured polystyrene. Biomaterials. 29:1796–1806. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roca-Cusachs P, Alcaraz J, Sunyer R,
Samitier J, Farré R and Navajas D: Micropatterning of single
endothelial cell shape reveals a tight coupling between nuclear
volume in G1 and proliferation. Biophys J. 94:4984–4995.
2008.PubMed/NCBI
|