Introduction
Pulmonary fibrosis has an aggressive course and is
usually fatal an average of 3 to 6 years after the onset of
symptoms. Pulmonary fibrosis refers to a group of lung diseases
characterized by inflammation, fibroblast proliferation and
excessive collagen deposition. Although the mechanisms underlying
pulmonary fibrosis are not clearly understood, current evidence
suggests that it is associated with the deposition of extracellular
matrix (ECM) components in the lung interstitium (1).
Matrix metalloproteinases (MMPs) are a major group
of proteinases known to regulate ECM remodeling and so they are
hypothesized to be important in the process of lung fibrosis
(2). MMPs and its specific tissue
inhibitors of metalloproteinases (TIMPs) have been shown to
participate in the parenchymal destruction and repair processes
that result in ECM remodeling (3–5). The
balance between MMPs and TIMPs is critical in tissue repair and
remodeling, and its homeostasis is important in the breakdown and
deposition of ECM in the lung (6).
Increasing TIMP expression or activation may strengthen the
inhibitory effect on MMPs and induce excessive ECM deposition.
Conversely, through decreasing lung TIMP expression or activation,
excessive ECM may be degraded by increased MMPs, which may relieve
pulmonary fibrosis.
Among the MMPs, MMP-2, preferentially secreted by
fibroblasts and epithelial cells and mainly suppressed by TIMP-1,
has been reported to possess substrate specificity for type IV
collagen and is able to degrade basement membrane structures via
collagenolytic actions (7).
Thus, after using the antisense TIMP-1 gene to
transfect rats with pulmonary fibrosis induced by bleomycin (BLM),
we observed the degree of lung fibrosis and lung tissue expression
of MMP-2 to investigate the protective role of antisense TIMP-1
gene therapy.
Materials and methods
Materials
Healthy male Sprague-Dawley (SD) rats weighing
180–220 g and aged 4–8 weeks were supplied by the specific
pathogen-free (SPF) Laboratory Animal Center of Dalian Medical
University, Dalian, China. Sense and antisense TIMP-1 cDNA
retroviral vectors and empty vector were supplied by the Central
Laboratory of the First Affiliated Hospital of Dalian Medical
University. BLM was purchased from Nippon Shinyaku Co., Ltd.
(Kyoto, Japan). TIMP-1 and MMP-2 polyclonal antibodies were
supplied by NeoMarkers Inc. (Fremont, CA, USA). The HYC test kit
was from Nanjing JianCheng Bioengineering Institute (Nanjing,
China). The MaxPoly Plus anti-mouse/rabbit horseradish peroxidase
IHC kit was supplied by Fujian Maixin Biological Technology Co.
(Fuzhou, China). Primers, DNA markers (DL2000) and the Takara RNA
polymerase chain reaction (PCR) 3.0 (AMV) kit were from Takara
Biotechnology (Dalian) Co., Ltd. (Dalian, China).
Animal treatment
A total of 180 SD rats were randomly divided into 5
groups: the control, pulmonary fibrosis model, sense TIMP-1
transfection, antisense TIMP-1 transfection and empty vector
transfection groups. The SD rat model of pulmonary fibrosis was
established by administering a 0.4 mg% BLM saline injection (5
mg/kg) by intratracheal injection. For the transfection groups,
following the intratracheal injection of BLM on days 1, 3, 7, 14,
28 and 60, the rats were treated once with retroviral vectors. The
control group was subjected to intratracheal injection of normal
saline. The pulmonary fibrosis model group was subjected to
intratracheal injections of BLM and normal saline at the same
time-points as the transfection groups. Rats from all groups, 6
rats per group, were sacrificed on day 28 after the first
intratracheal injection. Right lung tissue was collected for
reverse transcription-polymerase chain reaction (RT-PCR) and
hydroxyproline (HYP) concentration assays and left lung tissue was
collected for immunohistochemical analysis. The rats were kept in a
normally controlled breeding room with standard laboratory food and
water for one week prior to the experiments. The rats were
maintained in accordance with internationally accepted principles
for laboratory animal use.
HYP concentration test
A 1-g sample of right lung tissue was added to 9 ml
0.86% saline to prepare a lung tissue homogenate and its supernate
was collected for a HYP concentration assay according to the
manufacturer’s instructions.
TIMP-1 and MMP-2 mRNA expression
mRNA was extracted from the lung tissue samples
using TRIzol according to the instructions of the manufacturer
(Invitrogen, Carlsbad, CA, USA) and RT-PCR was performed according
to the instructions of the RNA PCR 3.0 (AMV) kit. An equal amount
of cDNA from each sample was amplified using primers specific to
each gene (Table I). DNA
amplification was performed using a thermocycler under the
following conditions: for TIMP-1, 30 cycles of denaturation at 94°C
for 60 sec, annealing at 51°C for 60 sec and extension at 72°C for
90 sec; for MMP-2, 30 cycles of denaturation at 94°C for 45 sec,
annealing at 55°C for 45 sec and extension at 72°C for 60 sec; and
for β-actin, 30 cycles of denaturation at 94°C for 30 sec,
annealing at 55°C for 30 sec and extension at 72°C for 60 sec. The
RT-PCR products were measured by photodensitometry using a gel
image analysis system following agarose gel electrophoresis and
ethidium bromide staining.
 | Table IOligonucleotide primers of target
genes. |
Table I
Oligonucleotide primers of target
genes.
| mRNA species | Primer sequence
(5′-3′) | PCR product |
|---|
| TIMP-1 | S
TTTGCATCTCTGGCCTCTG
A AATGACTGTCACTCTCCAG | 495 bp |
| MMP-2 | S
CCACATTCTGGCCTGAGCTCCC
A GATTTGATGCTTCCAAACTTCAC | 436 bp |
| β-actin | S
CCTTCCTGGGCATGGAGTCCTG
A GGAGCAATGATCTTGATCTTC | 205 bp |
Measurement of TIMP-1 and MMP-2 protein
expression
Immunohistochemical analysis was carried out
according to the Max Vision kit instructions. Image analysis
software (Image-pro plus 6.0) was used to measure the light density
of the positive control cells in which the cytoplasms were
tan-yellow or brown following 3,3′-diaminobenzidine (DAB) staining.
For each section, the positive integrated optical density (IOD) and
total area of 5 representative visual fields without overlap were
observed under a high-powered microscope (x400). The ratio of IOD
and total area represents the mean value of optical density, with a
higher ratio indicating a higher level of protein expression.
Statistical analysis
All data were analyzed using the SPSS statistical
package version 11.5. The data showed a normal distribution and are
expressed as the means ± standard deviation. The responses of the
different experimental groups were analyzed using one-way ANOVA.
P<0.05 was considered to indicate a statistically significant
result.
Results
Results of lung tissue HYP concentration
assays
The HYP concentrations in the lung tissue of the
antisense TIMP-1 group decreased significantly on days 1 and 3
compared with those of the empty vector and pulmonary fibrosis
groups at the same time-points (P<0.01), but increased
significantly in the sense TIMP-1 group (P<0.01). No significant
difference was observed in the HYP concentrations of the antisense
TIMP-1, sense TIMP-1, empty vector and pulmonary fibrosis groups on
days 7, 14, 28 and 60 (Table
II).
 | Table IILung tissue HYP concentration (mg;
mean ± SD). |
Table II
Lung tissue HYP concentration (mg;
mean ± SD).
| Group | 1 day | 3 days | 7 days | 14 days | 28 days | 60 days |
|---|
| Control | 0.431±0.460 | 0.450±0.054 | 0.481±0.060 | 0.502±0.086 | 0.422±0.058 | 0.432±0.048 |
| Pulmonary
fibrosis | 1.152±0.157 | 1.221±0.172 | 1.083±0.156 | 1.182±0.129 | 0.973±0.148 | 1.012±0.170 |
| Empty vector | 1.224±0.197 | 1.182±0.162 | 1.110±0.149 | 1.243±0.139 | 1.110±0.135 | 1.173±0.847 |
| Antisense TIMP-1 | 0.723±0.153a | 0.642±0.107a | 0.961±0.188 | 1.104±0.243 | 1.140±0.129 | 1.204±0.200 |
| Sense TIMP-1 | 1.712±0.231a | 1.830±0.210a | 1.224±0.193 | 1.202±0.177 | 1.134±0.177 | 1.184±0.216 |
Results of RT-PCR analysis
The lung TIMP-1 mRNA expression levels of the
antisense TIMP-1 group were significantly lower on days 1 and 3
than those of the empty vector and pulmonary fibrosis groups
(P<0.01), but in the sense TIMP-1 group, the TIMP-1 mRNA
expression levels were significantly increased (P<0.01). No
significant differences were observed between the TIMP-1 mRNA
expression levels in the antisense TIMP-1, sense TIMP-1, empty
vector and pulmonary fibrosis groups on days 7, 14, 28 and 60, or
between the lung mRNA expression level of MMP-2 in all groups, with
the exception of the control group (P>0.05; Table III).
 | Table IIILung TIMP-1 mRNA expression at
different time-points (mean ± SD). |
Table III
Lung TIMP-1 mRNA expression at
different time-points (mean ± SD).
| Day | Control group | Fibrosis group | Empty vector
group | Antisense group | Sense group |
|---|
| 1 | 0.4005±0.0157 | 0.7639±0.0167 | 0.7589±0.0187 | 0.6384±0.0152a | 0.9032±0.0208a |
| 3 | 0.4035±0.0147 | 0.7666±0.0158 | 0.7596±0.0164 | 0.5760±0.0015a | 1.4948±0.0355a |
| 7 | 0.4036±0.0156 | 0.7687±0.0473 | 0.7645±0.0363 | 0.7574±0.0140 | 0.7560±0.0195 |
| 14 | 0.4030±0.0154 | 0.7665±0.0101 | 0.7575±0.0101 | 0.7545±0.0134 | 0.7599±0.0129 |
| 28 | 0.4035±0.0157 | 0.7436±0.0114 | 0.7476±0.0254 | 0.7470±0.0450 | 0.7446±0.0185 |
| 60 | 0.4025±0.0160 | 0.7440±0.0113 | 0.7465±0.0215 | 0.7335±0.02189 | 0.7364±0.0941 |
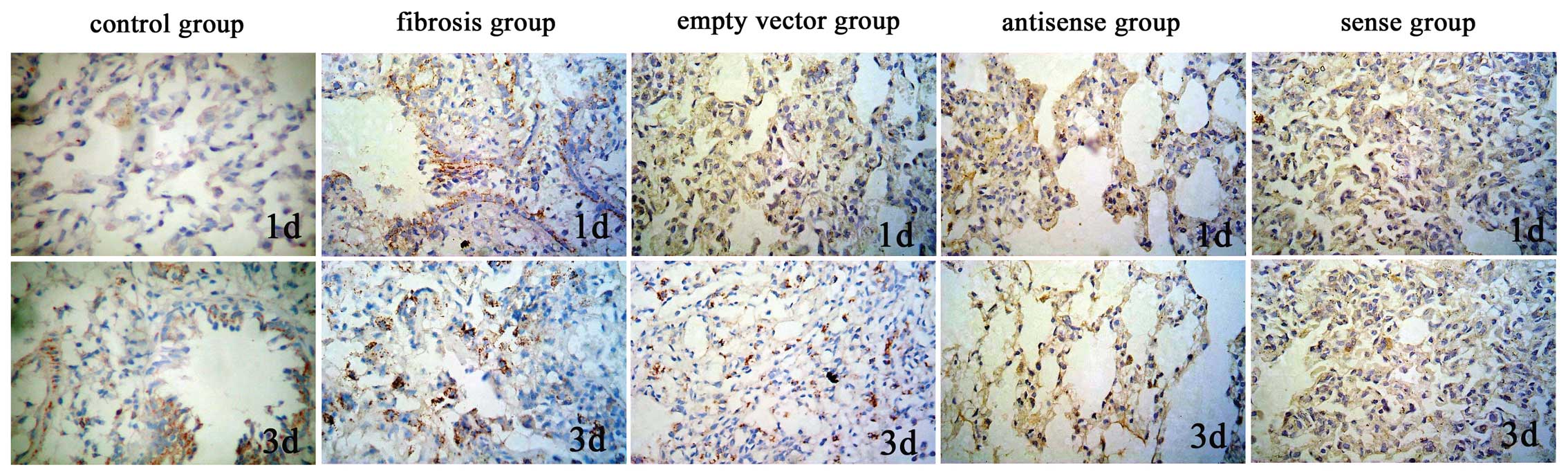
Results of immunohistochemical
analysis
The lung TIMP-1 protein expression levels of the
antisense TIMP-1 group were significantly lower on days 1 and 3
than those of the empty vector and pulmonary fibrosis groups
(P<0.01), but in the sense TIMP-1 group, the protein expression
levels of TIMP-1 were significantly increased (P<0.01). No
significant differences were observed between the TIMP-1 protein
expression levels in the antisense TIMP-1, sense TIMP-1, empty
vector and pulmonary fibrosis groups on days 7, 14, 28 and 60, or
between the lung protein expression levels of MMP-2 in all groups,
with the exception of the control group (P>0.05; Table IV; Figs. 1 and 2).
 | Table IVLung TIMP-1 protein expression on
different time-points (mean ± SD). |
Table IV
Lung TIMP-1 protein expression on
different time-points (mean ± SD).
| Day | Control group | Fibrosis group | Empty vector
group | Antisense group | Sense group |
|---|
| 1 | 0.0702±0.0002 | 0.0783±0.0008 | 0.0781±0.0018 | 0.0749±0.0014a |
0.0812±0.0017b |
| 3 | 0.0700±0.0018 | 0.0782±0.0008 | 0.0780±0.0006 | 0.0736±0.0010a |
0.0853±0.0013b |
| 7 | 0.0707±0.0002 | 0.0782±0.0007 | 0.0779±0.0005 | 0.0773±0.0010 | 0.0772±0.0008 |
| 14 | 0.0768±0.0002 | 0.0768±0.0006 | 0.0767±0.0004 | 0.0762±0.0002 | 0.0763±0.0010 |
| 28 | 0.0708±0.0019 | 0.0772±0.0003 | 0.0771±0.0009 | 0.0768±0.0003 | 0.0769±0.0005 |
| 60 | 0.0707±0.0002 | 0.0772±0.0004 | 0.0773±0.0005 | 0.0766±0.0005 | 0.0768±0.0005 |
Discussion
Tissue fibrosis is the result of abnormal responses
to organ injury or to irritation and is typically characterized by
the hyperproliferation of fibroblasts and excessive ECM synthesis
and secretion. In pulmonary fibrosis, evidence suggests that the
constitutive activation of collagen-secreting myofibroblast-like
cells is ultimately responsible for increasing the quantity and
concentration of collagen. These cells are able to synthesize and
secrete excessive amounts of ECM components, particularly
collagens, leading to increased tissue stiffness and progressive
organ dysfunction (8).
MMPs are a major group of proteinases that are known
to regulate ECM remodeling and are hypothesized to be important in
the process of lung fibrosis. TIMPs control MMP activities and,
therefore, minimize matrix degradation (9). The balance between MMPs and TIMPs is
critical in tissue repair and remodeling, and its homeostasis is
important in the breakdown and deposition of ECM in the lung. Among
the MMPs, MMP-2, preferentially secreted by fibroblasts and
epithelial cells and mainly suppressed by TIMP-1, has been reported
to possess substrate specificity to type IV collagen and is able to
degrade basement membrane structures via collagenolytic actions
(7).
In our preliminary study, in vitro, sense and
antisense TIMP-1 cDNA retroviral vectors were used to transfect
mouse fibroblast cells in order to investigate the relationship
between pulmonary fibrosis and TIMP-1. The results demonstrated
that antisense TIMP-1 cDNA was able to decrease endogenic TIMP-1
expression, increase MMPs activation and then restrain the
proliferation of fibroblast cells and reduce the ECM components
(10). Based on the above
research, in the current study, we investigated the role of
antisense TIMP-1 cDNA in pulmonary fibrosis in vivo by
observing the effect of antisense TIMP-1 cDNA on the concentration
of HYP and the expression of TIMP-1 and MMP-2 in the lung tissue of
rats with pulmonary fibrosis induced by BLM.
HYP is a type of collagen having an unusual amino
acid composition, the level of which indicates the total content of
collagen and the degree of tissue or organ fibrosis. In our study,
the HYP concentrations of the antisense TIMP-1 group decreased
significantly on days 1 and 3 compared with those of the empty
vector and pulmonary fibrosis groups at the same time-points, but
increased significantly in the sense TIMP-1 group. This indicates
that the use of antisense TIMP-1 cDNA to transfect rats with
BLM-induced pulmonary fibrosis relieves lung tissue fibrosis on
days 1 and 3, while sense cDNA aggravates fibrosis at the same
time-points, in accordance with the pathological changes in the
lung tissue.
Pulmonary fibrosis is characterized by the excessive
deposition of ECM in the interstitium resulting in respiratory
failure. The turnover of ECM is partially regulated by proteases,
including MMPs and their inhibitors. Previous studies have
demonstrated that the high expression levels of TIMP-1 and low
expression levels or weakened activity of MMPs in lung tissue
result in pulmonary fibrosis (11–14).
The expression levels of TIMP-1 and MMP-2 in the
lung tissue were detected at the mRNA and protein levels in order
to investigate the mechanism of the ECM changes in pulmonary
fibrosis following transfection by sense and antisense TIMP-1.
In the present study, no significant differences
were observed between the lung mRNA and protein expression levels
of MMP-2 in the sense-and antisense-transfected groups at all
time-points. The results demonstrated that TIMP-1 preferentially
reduced MMP-2 activity rather than expression to degrade ECM
following transfection with antisense TIMP-1 cDNA.
We found that the TIMP-1 mRNA and protein expression
levels in the lungs of the antisense TIMP-1 group were
significantly reduced on days 1 and 3 compared with those of the
pulmonary fibrosis group, and in the sense TIMP-1 group, the TIMP-1
expression level increased significantly at these time-points.
However, no significant differences were observed in the TIMP-1
expression levels in the antisense and sense TIMP-1 groups on days
7, 14, 28 and 60. It is well known that the cell division of
fibroblast cells increases in the early stages of pulmonary
fibrosis. Gene carriers, including retroviruses, only transfect
cells at the mitotic phase (15),
therefore the expression of TIMP-1 in the lung tissue of the
antisense TIMP-1 group decreased on days 1 and 3. In the later
stage of fibrosis, no significant therapeutic effect of the
antisense TIMP-1 cDNA was observed due to low or no transfection
efficiency, resulting from a decrease in the number of cells at the
mitotic phase, which means that antisense TIMP-1 cDNA retroviral
vector transfection has no ability to reverse the formation of
pulmonary fibrosis. The results showed that TIMP-1 expression
levels increased following transfection with antisense TIMP-1
compared with those of the controls, which suggests that the
progress of lung fibrosis is regulated by several factors; growth
factors, cytokines, chemokines and regulators of apoptosis have all
been implicated in its progression (16).
In conclusion, lung fibrosis induced by BLM may be
suppressed by transfection with antisense TIMP-1 cDNA retroviral
vectors following the intratracheal injection of BLM on days 1 and
3. Moreover, the observation emphasizes that effective therapies
for pulmonary fibrosis must be given in the early stages of the
disease, prior to the development of expansive lung destruction and
fibrosis.
Acknowledgements
The authors thank the Central Laboratory of The
First Affiliated Hospital of Dalian Medical University. We also
thank the SPF Laboratory Animal Center of Dalian Medical
University. The study was supported by grants from the National
Natural Science Foundation of China (NSFC; 30370620).
References
|
1
|
Suga M, Iyonaga K, Okamoto T, Gushima Y,
Miyakawa H, Akaike T and Ando M: Characteristic elevation of matrix
metalloproteinase activity in idiopathic interstitial pneumonias.
Am J Respir Crit Care Med. 62:1949–1956. 2000. View Article : Google Scholar
|
|
2
|
Lagente V, Manoury B, Nénan S, Le Quément
C, Martin-Chouly C and Boichot E: Role of matrix metalloproteinases
in the development of airway inflammation and remodeling. Braz J
Med Biol Res. 38:1521–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hayashi T, Stetler-Stevenson WG, Fleming
MV, Fishback N, Koss MN, Liotta LA, Ferrans VJ and Travis WD:
Immunohistochemical study of metalloproteinases and their tissue
inhibitors in the lungs of patients with diffuse alveolar damage
and idiopathic pulmonary fibrosis. Am J Pathol. 149:1241–1256.
1996.
|
|
4
|
Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M
and Yamanaka N: Localization of matrix metalloproteinases-1, -2,
and -9 and tissue inhibitor of metalloproteinase-2 in interstitial
lung diseases. Lab Invest. 78:687–698. 1998.PubMed/NCBI
|
|
5
|
Finlay GA, O’Driscoll LR, Russell KJ,
D’Arcy EM, Masterson JB, FitzGerald MX and O’Connor CM: Matrix
metalloproteinase expression and production by alveolar macrophages
in emphysema. Am J Respir Crit Care Med. 156:240–247. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki R, Miyazaki Y, Takagi K, Torii K
and Taniguchi H: Matrix metalloproteinases in the pathogenesis of
asthma and COPD: implications for therapy. Treat Respir Med.
3:17–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cataldo DD, Gueders MM, Rocks N, Sounni
NE, Evrard B, Bartsch P, Louis R, Noel A and Foidart JM: Pathogenic
role of matrix metalloproteases and their inhibitors in asthma and
chronic obstructive pulmonary disease and therapeutic relevance of
matrix metalloproteases inhibitors. Cell Mol Biol (Noisy-le-grand).
49:875–884. 2003.
|
|
8
|
Cox TR and Erler JT: Remodeling and
homeostasis of the extracellular matrix: implications for fibrotic
diseases and cancer. Dis Model Mech. 4:165–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu TH, Ling HL and Zhu ZM: Effect of sense
and antisense TIMP-1 gene to ECM expression of mouse fibroblast
cells. Zhong Guo Man Xing Bing Yu Fang Yu Kong Zhi. 12:20–23.
2004.(In Chinese).
|
|
11
|
McKeown S, Richter AG, O’Kane C, McAuley
DF and Thickett DR: MMP expression and abnormal lung permeability
are important determinants of outcome in IPF. Eur Respir J.
33:77–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manoury B, Nénan S, Guénon I, Lagente V
and Boichot E: Influence of early neutrophil depletion on
MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int
Immunopharmacol. 7:900–911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manoury B, Caulet-Maugendre S, Guénon I,
Lagente V and Boichot E: TIMP-1 is a key factor of fibrogenic
response to bleomycin in mouse lung. Int J Immunopathol Pharmacol.
19:471–487. 2006.PubMed/NCBI
|
|
14
|
Tian XL, Yao W, Guo ZJ, Gu L and Zhu YJ:
Low dose pirfenidone suppresses transforming growth factor beta-1
and tissue inhibitor of metalloproteinase-1, and protects rats from
lung fibrosis induced by bleomycina. Chin Med Sci J. 21:145–151.
2006.PubMed/NCBI
|
|
15
|
Robbins PD and Ghivizzani SC: Viral
vectors for gene therapy. Pharmacol Ther. 80:35–47. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cook DN, Brass DM and Schwartz DA: A
matrix for new ideas in pulmonary fibrosis. Am J Respir Cell Mol
Biol. 27:122–124. 2002. View Article : Google Scholar : PubMed/NCBI
|