Introduction
Previous studies have shown that bone morphogenetic
protein-2 (BMP-2), a transforming growth factor-β superfamily
member cytokine, participates significantly in vascular development
and pathophysiological processes (1,2).
Vascular endothelial and smooth muscle cells are a significant
source of BMPs (3,4). BMP-2 induction in blood vessels may
be related to oxidative stress, vascular inflammatory response,
hyperglycemia and hyperlipidemia (5,6).
Angiotensin II (AngII), the main effector of the renin-angiotensin
system, is essential for the regulation of blood pressure and is
also involved in arterial wall remodeling (7,8). The
inhibition of the renin-angiotensin-aldosterone system has
beneficial effects on endothelial functioning in animals and humans
(9–11). The aim of this study was to
investigate whether AngII induces BMP-2 expression. Furthermore, we
also examined the effects of probucol, a cholesterol-lowering drug
with potent antioxidative properties which attenuates
atherosclerosis in animals and humans (12,13),
on the AngII-induced BMP-2 expression.
Materials and methods
Human umbilical vein endothelial cell
(HUVEC) culture and treatment
HUVECs and supplements, purchased from Cascade
Biologics (Portland, OR, USA), were grown in Medium 200 with low
serum growth supplement (LSGS) on polystyrene plates until 90%
confluent. Non-adhering cells were poured off and the adhering
cells were incubated at 37°C under an atmosphere of 5%
CO2 and 95% air. After 3–5 days, the cultures formed a
confluent monolayer and were subcultured. The cells were used at
passages 5–7. The cells were cultured at 1×106 cells/25
cm2 flask and then were divided into 7 groups: a control
group (treated with medium only); a probucol group (treated with 10
μmol/l probucol); a pyrrolidine dithiocarbamate (PDTC) group
(treated with 15 μmol/l PDTC); 2 AngII groups (treated with 0.1 and
1.0 μmol/l AngII, respectively); an AngII + PDTC group (treated
with 1.0 μmol/l AngII plus 15 μmol/l PDTC); and an AngII + probucol
group (treated with 1.0 μmol/l AngII plus 10 μmol/l probucol). The
combination treatment groups were pretreated with 15 μmol/l PDTC or
10 μmol/l probucol for 6 h prior to the administration of
AngII.
BMP-2 mRNA expression by northern blot
analysis
Total cellular RNA was isolated from the HUVECs
using the TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA). Northern blot analysis was performed as previously
described (11). RNA blots were
hybridized overnight at 57°C in a hybridization oven with
106 cpm/ml of the [α-32P]dATP-labeled
oligonucleotide probes for BMP-2 or the cDNA for β-actin. The blots
were then washed, air dried and exposed to Hyperfilm X-ray films
(Amersham Biosciences, Piscataway, NJ, USA) at −80°C.
BMP-2 protein expression by western
blotting
The plated cells were washed 3 times with PBS and
lysed with 250 μl lysis buffer (50 mmol/l Tris-HCl, pH 8.0, 150
mmol/l NaCl, 0.02% sodium vanadate, 0.1% SDS, 0.5% deoxycholic
acid, 100 μg/ml PMSF, 0.2 μg/ml leupeptin and 1% NP-40). Samples
were separated on a 12% SDS-PAGE gel and transferred onto PVDF
filters by a wet transferring system (16). The membrane was blocked with
Blotto-Tween (5% non-fat milk and 0.05% Tween-20 in PBS) and
incubated with the primary antibodies against BMP-2 and β-actin for
2 h at room temperature. Horseradish peroxidase-conjugated
secondary IgG was then added and incubation was continued for 1 h
at room temperature. The blots were developed using an enhanced
chemiluminescence method (Zhongshan Biotechnology, Beijing,
China).
Measurement of malondialdehyde (MDA) and
BMP-2 concentrations in culture medium and total superoxide
dismutase (SOD) activity in cell lysates
The concentrations of MDA were determined as
thiobarbituric acid-reactive substances. The total activity of SOD
was determined using the SOD Assay kit-WST according to the
manufacturer’s instructions. BMP-2 protein levels were measured by
enzyme-linked immunosorbent assay (ELISA) according to the
manufacturer’s instructions. Each sample was assayed in duplicate.
The intra-assay and inter-assay precision variability was
<8%.
Effects of administering agents on
cytoplasmic and nuclear levels of NF-κB p65
The effects of various agents on the activation of
NF-κB in HUVECs were determined using an assay kit from Active
Motif (Carlsbad, CA, USA). This kit measures free p65, which is
generated when NF-κB is activated. To obtain assay material, the
cells were seeded at 1.0×106 cells/25cm2
flask. The cells were harvested 12 h after the addition of these
agents. Cytoplasmic and nuclear cell fractions were collected using
the reagents supplied in a nuclear extract kit (Active Motif). The
cells were harvested by scraping and pelleted by centrifugation at
2,000 × g at 4°C for 75 sec. The pelleted cells were resuspended in
200 μl hypotonic buffer and incubated on ice for 15 min. Nonidet
P-40 (Sigma, St. Louis, MO, USA), a solubilizing agent, was added
and the cells were vortexed for 10 sec. The material was
centrifuged for 45 s at 14,000 × g at 4°C. The supernatant fluids
(cytoplasmic fractions) were transferred to pre-chilled
microcentrifuge tubes and stored at −80°C. The resulting nuclear
pellets were resuspended in the lysis buffer supplied in the kit
and then vortexed for 10 sec. The suspension was incubated for 30
min on ice on a rocking platform, vortexed for 30 sec and then
centrifuged at 14,000 × g for 10 min at 4°C. The supernatant fluids
(nuclear fractions) were transferred to pre-chilled microcentrifuge
tubes and stored at −80°C. To assay the nuclear and cytoplasmic
samples for amounts of p65, 20 μl samples were added to the wells
of a 96-well plate and coated with an oligonucleotide containing
the NF-κB consensus binding site. The p65 subunit of the activated
NF-κB contained in the cell extracts specifically binds to this
oligonucleotide. The plate was incubated for 1 h at room
temperature on a rocking platform. After washing, an antibody to
p65 was added and the plate was incubated for 1 h at room
temperature. The wells were then washed, an HRP-conjugated antibody
was added and incubation was continued for 1 h at room temperature.
After washing, a substrate solution was added to the wells and the
plate was incubated at room temperature for 10 min, protected from
light. A stop solution (2N H2SO4) was added
and the absorbance at 450 nm was read. The amounts of protein in
the fractions were determined by a modification of the method of
Lowry et al(14,15). The results are expressed as
A450 nm/mg protein and expressed as a percentage (%) of
the control (value for cells exposed to control medium, set at
100%).
BMP-2 expression by
immunohistochemistry
We also examined the expression of BMP-2 by
immunohistochemical analysis. The treated HUVECs were grown on
6-well glass slides separately and fixed in acetone. After washing
with PBS, the cells were incubated in a 1%
H2O2 solution at room temperature for 10 min
to quench endogenous peroxidases. Non-specific binding was blocked
with 5% normal horse serum at room temperature for 5 min. The cells
then were incubated with anti-BMP-2 monoantibody (1:200 dilution)
at 4°C overnight. After washing with PBS, the secondary antibody,
biotinylated anti-rat, was added and the cells were incubated at
room temperature for 1 h. After washing with PBS, Vectastain
reagent was added and the cells were incubated at room temperature
for 10 min. 3,3′-Diaminobenzidine was used as the chromagen. After
10 min, the brown color signifying the presence of antigen bound to
antibodies was detected by light microscopy and images were
captured at ×100. Samples from each group were graded for
histopathological changes and immunohistochemistry staining. The
intensity of the immunostaining was graded from 0 (no staining) to
degree C (maximum staining).
Statistical analysis
All data are presented as the means ± SD. Multiple
comparisons among groups were performed by one-way ANOVA analysis.
P<0.05 was considered to indicate a statistically significant
result.
Results
Induction of BMP-2 expression in
HUVECs
The mRNA levels of BMP-2 in the HUVECs following the
various treatments are shown in Fig.
1. Compared with the control group, AngII treatment led to a
significant increase in the expression levels of BMP-2 mRNA;
however, administration of PDTC or probucol significantly
downregulated the AngII-induced BMP-2 expression (Fig. 1). BMP-2 and CuZnSOD protein
expression were also detected by western blot analysis. Our results
showed that CuZnSOD protein expression was downregulated by AngII
treatment, but this downregulation was partially reversed by
probucol or PDTC treatment (Fig. 2A
and B).
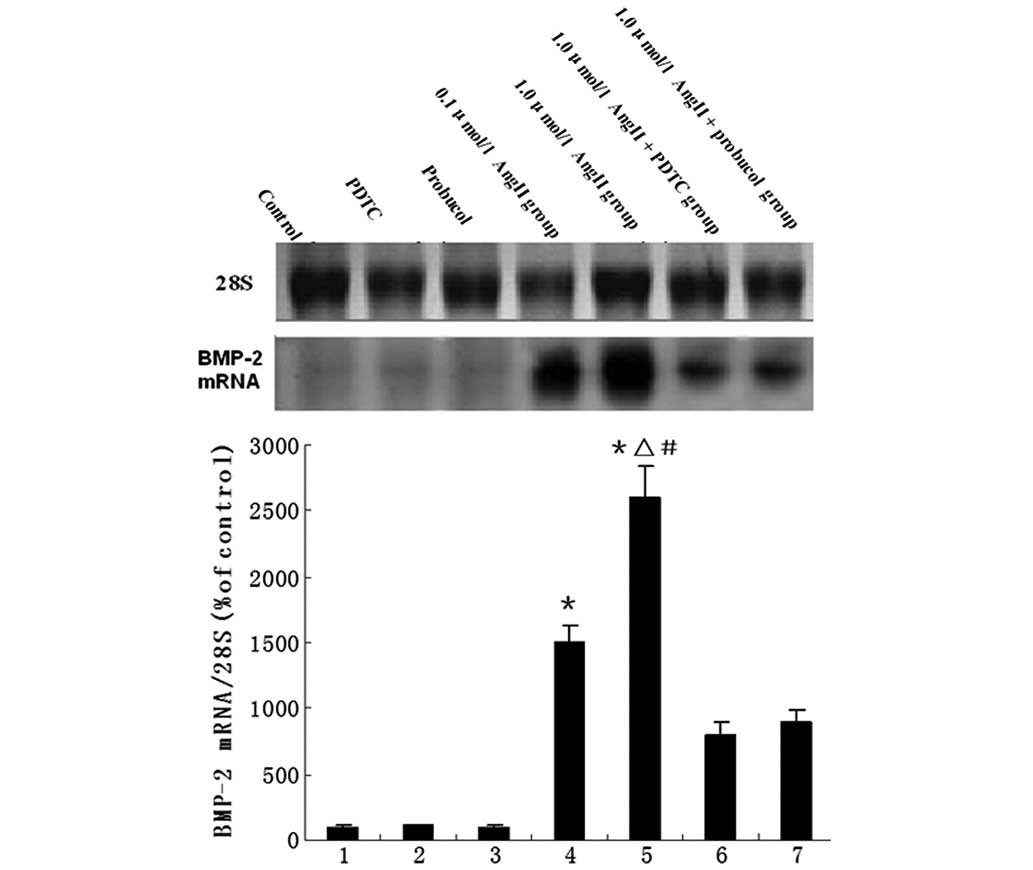 | Figure 1Northern blotting for evaluation of
BMP-2 mRNA. The bar graph shows mean values (± SEM) from the
densitometric analysis of 7 treatment groups, n=8 per group. The
groups are: 1, control; 2, PDTC; 3, probucol; 4, 0.1 μmol/l AngII;
5, 1.0 μmol/l AngII; 6, 1.0 μmol/l AngII + PDTC; and 7, 1.0 μmol/l
AngII + probucol. *P<0.05 vs. control group,
ΔP<0.05 vs. 1.0 μmol/l AngII + PDTC group,
#P<0.05 vs. 1.0 μmol/l AngII + probucol group. BMP-2,
bone morphogenetic protein-2; PDTC, pyrrolidine dithiocarbamate;
AngII, angiotensin II. |
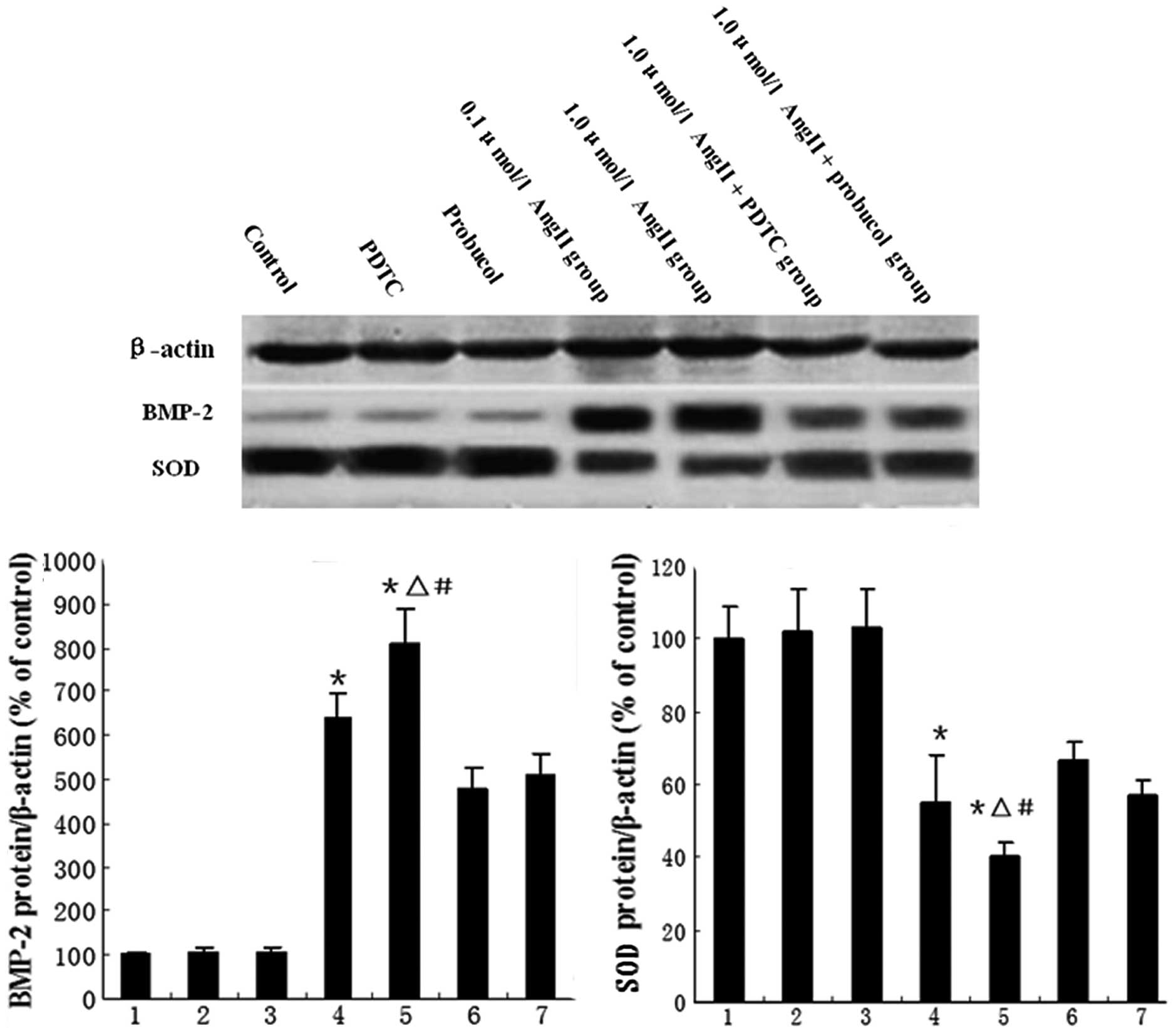 | Figure 2Western blotting for evaluation of
BMP-2 and SOD proteins. The bar graphs show mean values (± SEM)
from the densitometric analysis of (A) BMP-2 expression and (B) SOD
activity in the 7 treatment groups, n=8 per group. 1, control; 2,
PDTC; 3, probucol; 4, 0.1 μmol/l AngII; 5, 1.0 μmol/l AngII; 6, 1.0
μmol/l AngII + PDTC; and 7, 1.0 μmol/l AngII + probucol groups.
*P<0.05 vs. control group, ΔP<0.05 vs.
1.0 μmol/l AngII + PDTC group, #P<0.05 vs. 1.0 μmol/l
AngII + probucol group. BMP-2, bone morphogenetic protein-2; SOD,
superoxide dismutase; PDTC, pyrrolidine dithiocarbamate; AngII,
angiotensin II. |
Effects of agents on NF-κB p65 levels in
HUVECs
NF-κB p65 activation was detected using
immunohistochemistry and an assay kit from Active Motif. AngII
caused a significant increase in nuclear p65 levels compared with
those in the control group, reaching a maximum increase of ~5-fold
following 1.0 μmol/l AngII treatment, but this was decreased by the
administration of PDTC or probucol. In the cytoplasm of these
cells, we observed an increase in p65 levels following treatment
with AngII, which also was inhibited by PDTC or probucol treatment
(Table I).
 | Table IEffect of AngII on cytoplasmic and
nuclear levels of NF-κB p65. |
Table I
Effect of AngII on cytoplasmic and
nuclear levels of NF-κB p65.
| Groups | Nuclear NF-κB p65
levels | Cytoplasmic NF-κB p65
levels |
|---|
| Control | 205±22 | 107±15 |
| 15 μmol/l PDTC | 210±19 | 113±14 |
| 10 μmol/l
probucol | 207±25 | 108±13 |
| 0.1 μmol/l AngII | 529±49a | 213 ±21a |
| 1.0 μmol/l AngII | 1006±95a,b,c | 335 ±25a,b,c |
| AngII + PDTC | 308±27 | 190 ±20 |
| AngII + probucol | 312±28 | 204±18 |
Effect of AngII on total SOD activity and
MDA and BMP-2 protein levels in the HUVEC culture
The effects of AngII on SOD activity and BMP-2
expression are shown in Fig. 3.
Fig. 3A and B shows that AngII
significantly increased the BMP-2 protein concentration in the
supernatant and the MDA concentration in the HUVEC culture, but
these increases were diminished by the administration of PDTC or
probucol. Fig. 3C shows that AngII
reduced the total SOD activity in the cultured HUVECs; however,
this reduction was partially reversed by treatment with PDTC or
probucol.
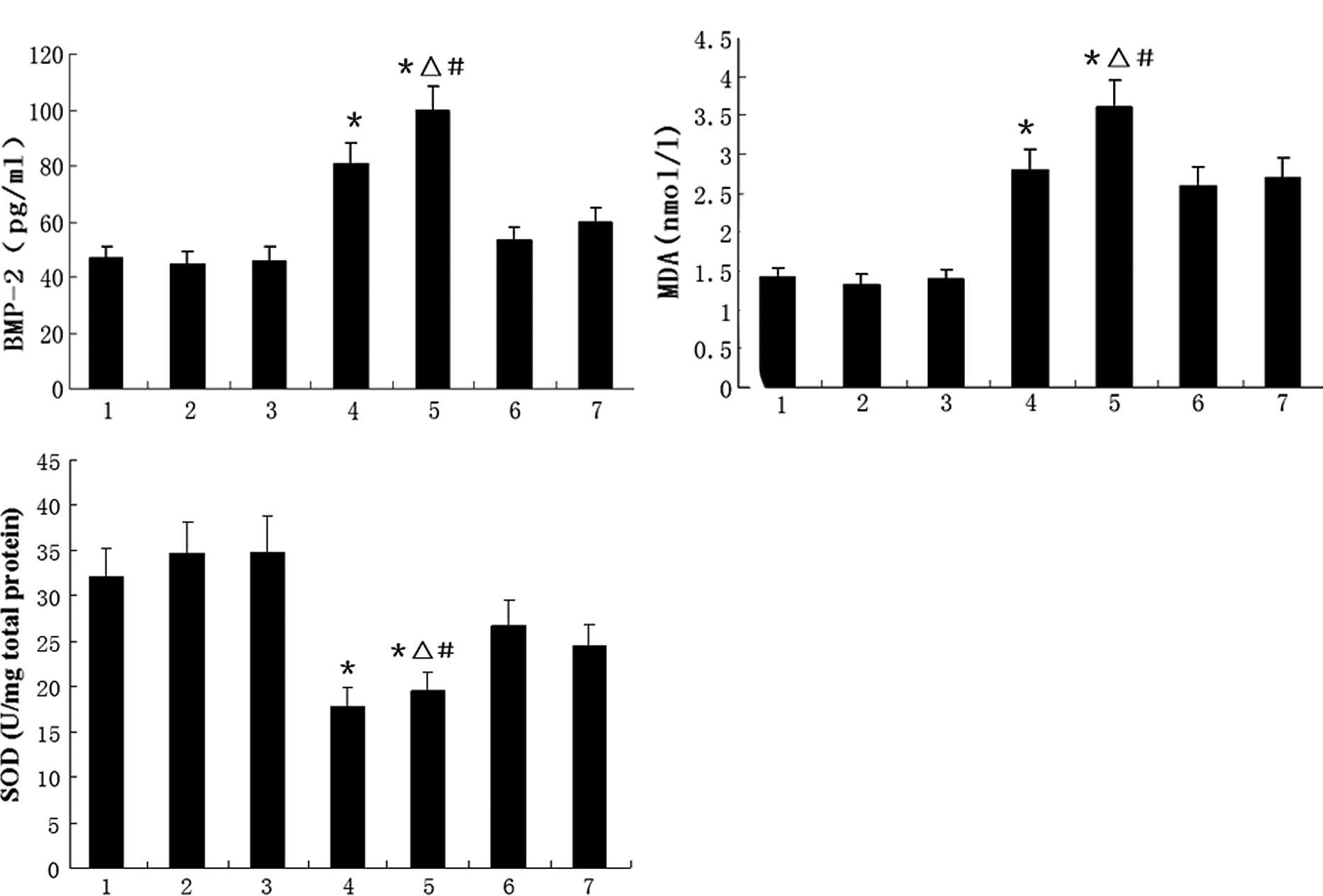 | Figure 3HUVECs were incubated with various
stimuli and the (A) BMP-2 and (B) MDA levels and (C) total SOD
activity of the cell cultures were analyzed. Data are the means ±
SD. 1, control; 2, PDTC; 3, probucol; 4, 0.1 μmol/l AngII; 5, 1.0
μmol/l; 6, 1.0 μmol/l AngII + PDTC; and 7, 1.0 μmol/l AngII +
probucol groups; n=8 per treatment group. *P<0.05 vs.
control group, ΔP<0.05 vs. 1.0 μmol/l AngII + PDTC
group, #P<0.05 vs. 1.0 μmol/l AngII + probucol group.
HUVECs, human umbilical vein endothelial cells; BMP-2, bone
morphogenetic protein-2; MDA, malondialdehyde; SOD, superoxide
dismutase; PDTC, pyrrolidine dithiocarbamate; AngII, angiotensin
II. |
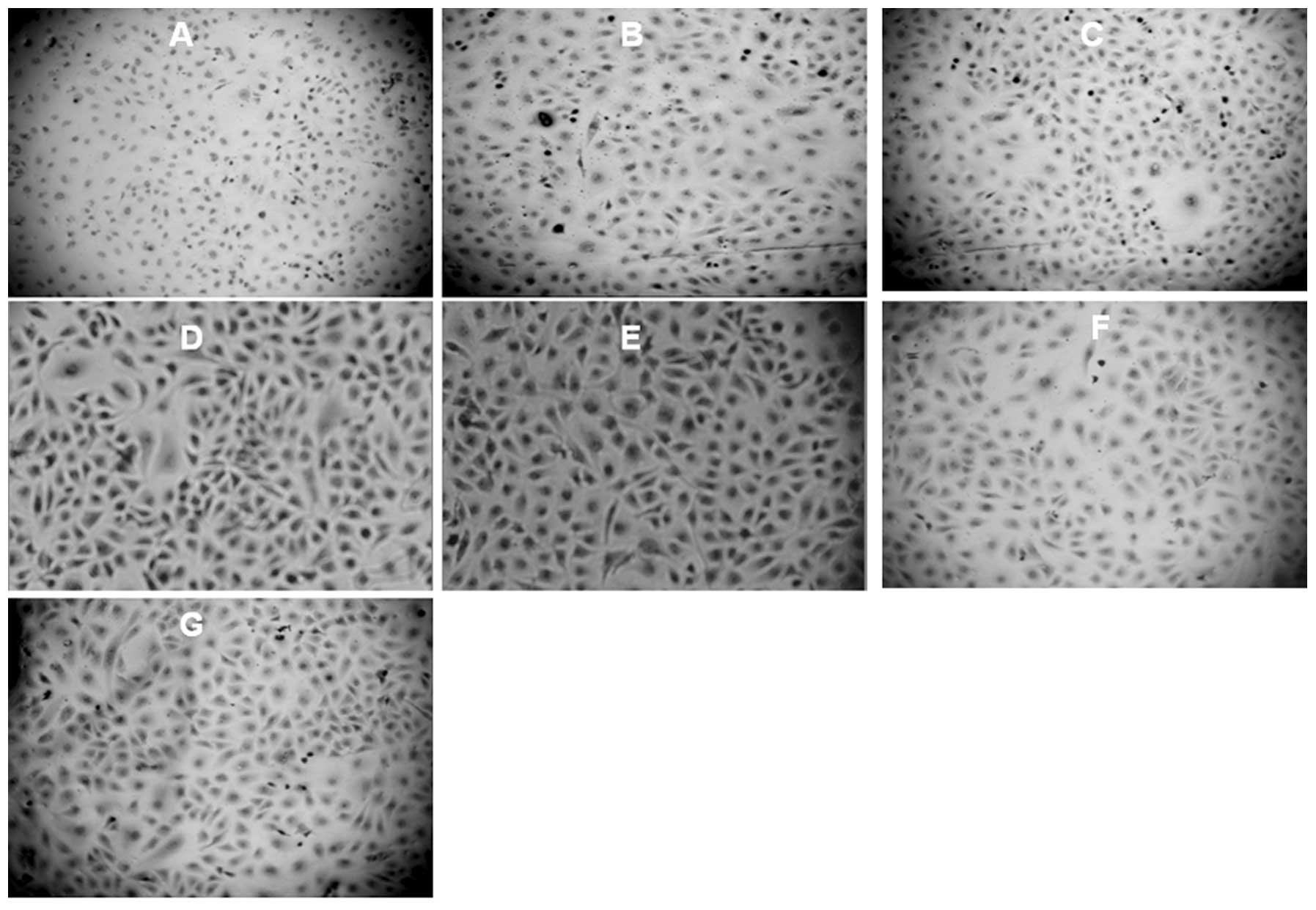
Immunohistochemistry for expression of
BMP-2 protein
Representative slides of the immunohistochemical
staining of BMP-2 protein in HUVECs (at ×100) are shown in Fig. 4. Our results demonstrate a
significantly higher staining of BMP-2 expression in the
endochylema of HUVECs treated with AngII (Fig. 4D and E); however PDTC or probucol
treatment inhibited the BMP-2 expression (Fig. 4F and G).
Discussion
BMP-2 is significantly involved in vascular
development and pathophysiological processes. Mice genetically
engineered to be deficient in BMP-2 die between days 7 and 10 of
gestation due to cardiac defects prior to bone formation, which
suggests the significance of BMP-2 in vascular development
(16). AngII has been demonstrated
to be critical in the initiation and progression of
atherosclerosis. It stimulates atherosclerosis through various
processes, including endothelial dysfunction, cellular
proliferation and inflammation. AngII elicits the production of
superoxide anion, a reactive oxygen species, from arterial
endothelial cells and SMCs (17,18).
Importantly, there is evidence that AngII elicits significant
increases in vascular H2O2 generation
(12,13). A study by Csiszar et al
indicated that vascular BMP-2 expression is regulated by the
H2O2-mediated activation of NF-κB evoked by
inflammatory stimuli or by high intravascular pressure (1); therefore, we hypothesized that AngII
activates BMP-2 expression via NF-κB activation. Indeed, our study
demonstrated that the administration of AngII significantly
increased BMP-2 expression. The hypothesis was also supported by
the detection of NF-κB activation. Our results revealed
significantly higher levels of NF-κB p65 protein expression in the
nuclei of the AngII-treated cells, which were reduced by treatment
with PDTC or probucol. These findings suggest that the suppression
of AngII-induced BMP-2 expression by probucol may involve the
inhibition of NF-κB activation. The activation of NF-κB may be an
important signal transduction pathway affecting the AngII-induced
increase in BMP-2 expression.
In the current study, we specially investigated
probucol, a cholesterol-lowering drug with potent antioxidative
properties and a clear radical-scavenging function (19). It was originally developed as a
hypolipidemic drug (20), but
interest has subsequently been focused on its potent antioxidant
properties. Probucol has been shown to reduce the extent of
atherosclerotic lesions in animal models and to inhibit
atherosclerosis and restenosis following percutaneous transluminal
coronary angioplasty (21,22). Our study demonstrated that probucol
inhibited the activation of NF-κB by AngII in HUVECs, which is the
likely mechanism responsible for the AngII-induced BMP-2
expression.
In order to evaluate the oxidative status of the
HUVECs, we detected the activity of SOD together with the level of
MDA, a well-known marker of oxidative stress. We observed that
AngII increased MDA levels and decreased the total SOD activity.
These findings indicated that excessive oxidative stress occurred
during the AngII stimulation process. However, probucol treatment
significantly reduced the MDA concentration and increased total SOD
activity, suggesting that probucol had potent antioxidative
properties, which is supported by a previous study (23). The protective effect of probucol
against atherosclerosis may partly be due to its ability to lower
MDA concentrations or increase antioxidant enzyme activities
(24).
These findings further suggest that oxidative stress
is a common mediator of such effects and indicate that the
activation of NF-κB is a significant signal transduction pathway
affecting the AngII-induced increase in BMP-2 expression. The
AngII-induced inhibition of BMP-2 expression may contribute to the
understanding of the initiation and progression of atherosclerosis
and may lead to a new therapeutic strategy.
Acknowledgements
This study was funded by the Beijing Science and
Technology New Star Program.
References
|
1
|
Csiszar A, Smith KE, Koller A, Kaley G,
Edwards JG and Ungvari Z: Regulation of bone morphogenetic
protein-2 expression in endothelial cells: role of nuclear
factor-kappaB activation by tumor necrosis factor-alpha,
H2O2, and high intravascular pressure.
Circulation. 111:2364–2372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abedin M, Tintut Y and Demer LL: Vascular
calcification: mechanisms and clinical ramifications. Arterioscler
Thromb Vasc Biol. 24:1161–1170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shanahan CM, Cary NR, Metcalfe JC and
Weissberg PL: High expression of genes for calcification-regulating
proteins in human atherosclerotic plaques. J Clin Invest.
93:2393–2402. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parhami F, Boström K, Watson K and Demer
LL: Role of molecular regulation in vascular calcification. J
Atheroscler Thromb. 3:90–94. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hruska KA, Mathew S and Saab G: Bone
morphogenetic proteins in vascular calcification. Circ Res.
97:105–114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boström KI, Jumabay M, Matveyenko A,
Nicholas SB and Yao Y: Activation of vascular bone morphogenetic
protein signaling in diabetes mellitus. Circ Res. 108:446–457.
2011.PubMed/NCBI
|
|
7
|
Libby P, Ridker PM and Maseri A:
Inflammation and Atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kranzhöfer R, Schmidt J, Pfeiffer CA, Hagl
S, Libby P and Kübler W: Angiotensin induces inflammatory
activation of human vascular smooth muscle cells. Arterioscler
Thromb Vasc Biol. 19:1623–1629. 1999.PubMed/NCBI
|
|
9
|
Tummala PE, Chen XL, Sundell CL, Laursen
JB, Hammes CP, Alexander RW, Harrison DG and Medford RM:
Angiotensin II induces vascular cell adhesion molecule-1 expression
in rat vasculature: A potential link between the renin-angiotensin
system and atherosclerosis. Circulation. 100:1223–1229. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yung LM, Wong WT, Tian XY, Leung FP, Yung
LH, Chen ZY, Yao X, Lau CW and Huang Y: Inhibition of
renin-angiotensin system reverses endothelial dysfunction and
oxidative stress in estrogen deficient rats. PLoS One.
29:e174372011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hernández-Presa M, Bustos C, Ortego M,
Tuñon J, Renedo G, Ruiz-Ortega M and Egido J:
Angiotensin-converting enzyme inhibition prevents arterial nuclear
factor-kappaB activation, monocyte chemoattractant protein-1
expression, and macrophage infiltration in a rabbit model of early
accelerated atherosclerosis. Circulation. 95:1532–1541. 1997.
|
|
12
|
Sawayama Y, Shimizu C, Maeda N, Tatsukawa
M, Kinukawa N, Koyanagi S, Kashiwagi S and Hayashi J: Effects of
probucol and pravastatin on common carotid atherosclerosis in
patients with asymptomatic hypercholesterolemia: Fukuoka
Atherosclerosis Trial (FAST). J Am Coll Cardiol. 39:610–616. 2002.
View Article : Google Scholar
|
|
13
|
Mackness B, Durrington PN and Mackness MI:
Lack of protection against oxidative modification of LDL by avian
HDL. Biochem Biophys Res Commun. 247:443–446. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the folin-phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
15
|
Oyama VI and Eagle H: Measurement of cell
growth in tissue culture with a phenol reagent (Folin-Ciocalteau).
Proceedings of the Society of Experimental Biology and Medicine.
91:305–307. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H and Bradley A: Mice deficient for
BMP2 are nonviable and have defects in amnion/chorion and cardiac
development. Development. 122:2977–2986. 1996.PubMed/NCBI
|
|
17
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O,
Ruperez M, Esteban V and Egido J: Inflammation and angiotensin II.
Int J Biochem Cell Biol. 35:881–900. 2003. View Article : Google Scholar
|
|
18
|
Durante A, Peretto G, Laricchia A, Ancona
F, Spartera M, Mangieri A and Cianflone D: Role of the
renin-angiotensin-aldosterone system in the pathogenesis of
atherosclerosis. Curr Pharm Des. 18:981–1004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tardif JC, Grégoire J and L’Allier PL:
Prevention of restenosis with antioxidants: mechanisms and
implications. Am J Cardiovasc Drugs. 2:323–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buckley MMT, Goa KL, Price AH and Brogden
RN: Probucol: a reappraisal of its pharmacological properties and
therapeutic use in hypercholestecmia. Drugs. 37:761–800. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tardif JC, Grégoire J, Schwartz L, Title
L, Laramée L, Reeves F, Lespérance J, Bourassa MG, L’Allier PL,
Glass M, Lambert J and Guertin MC; Canadian Antioxidant Restenosis
Trial (CART-1) Investigators. Effects of AGI-1067 and probucol
after percutaneous coronary interventions. Circulation.
107:552–558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kasai T, Miyauchi K, Kubota N, Kajimoto K,
Amano A and Daida H: Probucol therapy improves long-term
(>10-year) survival after complete revascularization: a
propensity analysis. Atherosclerosis. 220:463–469. 2012.
|
|
23
|
Traverso N, Menini S, Maineri EP,
Patriarca S, Odetti P, Cottalasso D, Marinari UM and Pronzato MA:
Malondialdehyde, a lipoperoxidation-derived aldehyde, can bring
about secondary oxidative damage to proteins. J Gerontol A Biol Sci
Med Sci. 59:B890–B895. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Memişoğulari R and Bakan E: Levels of
ceruloplasmin, transferring, and lipid peroxidation in serum of
patients with Type 2 diabetes mellitus. J Diabetes Complications.
18:193–197. 2004.PubMed/NCBI
|