Introduction
Type 2 diabetes mellitus (T2DM), a rapidly
increasing worldwide epidemic (1–3), is
a metabolic disease caused by the interaction of genetic and
environmental factors. Insulin resistance (IR) and β-cell
dysfunction are the main pathophysiological factors that contribute
to the development of T2DM.
Ghrelin, a 28-amino acid peptide predominantly
produced by the stomach, is the only known peripherally produced
hormone that stimulates food intake and decreases energy
expenditure (4,5). Leptin, a 167-amino acid peptide
mainly secreted from white adipocytes, is a key afferent signal
that suppresses food intake and increases energy expenditure
(6,7). Following release into the
circulation, both ghrelin and leptin cross the blood-brain barrier
and bind to their receptors in the arcuate nucleus (ARC) of the
hypothalamus (8,9). The two hormones play a role in the
regulation of energy balance and glucose metabolism (9–11).
The present study was conducted to investigate the
changes in the expression of ghrelin and leptin and their
correlation with IR in the development of T2DM in the rat, which
involves the production of ghrelin and leptin in the stomach and
adipose tissue, the blood levels of the two hormones and the
expression of their receptors in the ARC of the hypothalamus.
Materials and methods
Animals
Sixty male Sprague-Dawley rats (6 weeks of age, bred
at Hebei Medical University, China) were used for the experiments.
All procedures were performed in accordance with the Regulations
for the Administration of Affairs Concerning Experimental Animals.
The protocol was approved by the Hebei Medical University Animal
Care Research Committee. All rats were kept at 23–24°C on a 12/12-h
light/dark cycle. Animals were randomly divided into 4 groups: i)
the control group (n=15, fed standard laboratory chow); ii) the
HF-4W group [n=15, fed a high-fat (HF) diet for 4 weeks (HF diet
consisting of 5% fat, 55% carbohydrates, 22% protein, 7% ash and 5%
fiber); iii) the HF-8W group (n=15, fed a HF diet for 8 weeks); and
iv) the T2DM group (12–15) [n=12, after 8 weeks of the HF diet,
15 rats were administered a streptozotocin (STZ) injection (35
mg/kg); 10 days later, 12 rats with fasting blood glucose (FBG)
above 200 mg/dl were enrolled in the T2DM group].
The body weight of the rats was monitored once every
week. FBG, total cholesterol (TC) and triglycerides (TG) were
determined by an automatic biochemistry analyzer (Beckman ×20;
Beckman Coulter, Miami, FL, USA).
Hyperinsulinemic-euglycemic clamp
After an overnight fast for over 12 h, the rats were
anesthetized with pentobarbital sodium (50 mg/kg,
intraperitoneally), and the left jugular vein and left femoral
artery were catheterized for infusion and blood sampling,
respectively. The glucose and insulin solutions were stored in 2
digital syringe pumps and were joined by a ‘Y’ connector to the
jugular catheter. Human insulin (Novolin R, Novo Nordisk,
Bagsvaerd, Denmark) was continuously infused at 10 mU/kg/min for 2
h. The blood glucose concentration was clamped at the basal level
by estimating blood glucose concentration at 5 min intervals in
samples taken from the femoral artery and adjusting the rate of
infusion of a 20% glucose solution. Clamping was achieved by 60 min
and was maintained for 60 min. The glucose infusion rate (GIR)
during the second hour of the clamp was taken as a response
parameter, indicating insulin sensitivity.
Enzyme-linked immunosorbent assay
At 8:00 a.m. after overnight fasting for over 12 h,
blood was obtained from the rats and was transferred into chilled
tubes with 1 mg/ml EDTA-2Na and 500 U/ml aprotinin. Blood samples
were immediately centrifuged at 4°C (1,600 rpm for 15 min) and were
stored at −80°C until assay. After the rats were sacrificed, the
stomach was quickly removed, immediately frozen in liquid nitrogen
and stored at −80°C. To quantify plasma and stomach ghrelin levels,
a commercial ghrelin enzyme-linked immunosorbent assay (ELISA) kit
(Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) was used
according to the manufacturer’s instructions. Inter- and
intra-assay coefficients of variation were <14 and <5%,
respectively.
Radioimmunology assay
After overnight fasting for over 12 h, blood was
collected in tubes with gel and clot activator and centrifuged at
4°C (1,600 rpm for 15 min) to obtain serum. Blood serum was stored
at −80°C until assay. Epididymal fat pads were separated from the
left side of each rat during experiments, and they were immediately
frozen in liquid nitrogen and stored at −80°C. Cold normal saline
(1 ml) was added to each 200 mg epididymal fat pad sample; samples
were homogenized at 20,000 rpm for 30 sec at 4°C, repeated twice at
an interval of 10 sec. The homogenate was centrifuged at 12,000 rpm
for 20 min at 4°C; the supernatant was collected and stored at
−20°C. Serum and adipose leptin and serum insulin were determined
by radioimmunology assay (RIA) with leptin and insulin RIA kits
(Beijing Hi-Tech Atomic Technology, Inc., Beijing, China) according
to the manufacturer’s instructions.
RNA extraction and real-time reverse
transcription-polymerase chain reaction (real-time RT-PCR)
The stomach, left epididymal fat pad and
hypothalamus were rapidly excised, snap-frozen on dry ice and
immediately processed for RNA isolation. Total RNA was extracted
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
the manufacturer’s instructions. Single-stranded complementary DNA
(cDNA) was synthesized using Moloney murine leukemia virus (MMLV)
reverse transcriptase with random hexamer primers. Quantitative
real-time PCR was performed using SYBR® Premix Ex Taq™
(Takara, Shiga, Japan) on the ABI PRISM 7000 Sequence Detection
System (Applied Biosystems, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The mRNA level was normalized to the
housekeeping gene β-actin. The fold-change for an mRNA from control
to HF (or DM) was calculated as 2(−ΔΔCt), where ΔΔCt =
ΔCt control - ΔCt HF (or ΔCt DM) and ΔCt = Ct mRNA - CT-actin mRNA.
The primers and thermal cycle parameters are shown in Table I.
 | Table IGhrelin, leptin and their receptors as
well as actin primer sequences and PCR thermal cycle
parameters. |
Table I
Ghrelin, leptin and their receptors as
well as actin primer sequences and PCR thermal cycle
parameters.
| Target gene | Primer sequence | PCR thermal cycle
parameters |
|---|
| Ghrelin mRNA (160
bp) |
5′-CAGAGCACCAGAAAGCCCAGCAG-3′
5′-CCAACATCGAAGGGAGCATTGAA-3′ | 95°C 10 min, 95°C 5
sec, 57°C 10 sec, 72°C 31 sec (35 cycles), 72°C extended 5 min |
| Leptin mRNA (67
bp) |
5′-GGAAGCCTCGCTCTACTCCA-3′
5′-GAATGTCCTGCAGAGAGCCC-3′ | 95°C 10 min, 95°C 5
sec, 58°C 10 sec, 72°C 31 sec (40 cycles), 72°C extended 5 min |
| GHS-R1a mRNA (246
bp) |
5′-TTCTGCCTCACTGTGCTCTACAGT-3′
5′-AGCCAGTACTGCAACCTGGTGTCC-3′ | 94°C 5 min, 94°C 45
sec, 53°C 40 sec, 72°C 45 sec (35 cycles), 72°C extended 5 min |
| OB-Rb mRNA (116
bp) | 5′-GCA GCT ATG GTC
TCA CTT CTT TTG-3′
5′-GGT TCC CTG GGT GCT CTG A-3′ | 94°C 5 min, 94°C 45
sec, 52°C 40 sec, 72°C 45 sec (35 cycles), 72°C extended 5 min |
| β-actin (110 bp) |
5′-ATCCGTAAAGACCTCTATGCCAACA-3′
5′-GCTAGGAGCCAGGGCAGTAATCT-3′ | |
Immunohistochemistry
Animals were anesthetized and intracardially
perfused with 0.1 M PBS followed by freshly prepared 4%
paraformaldehyde in PBS. The stomach and brain were removed,
postfixed overnight, paraffin embedded and cut into 5-μM sections
with a microtome. After the paraffin had been removed, endogenous
peroxidase activity was blocked with 3% hydrogen peroxide for 10
min at room temperature (RT). The slides were microwaved for 20 min
in 0.01 M citrate buffer (pH 6.0). Slides were rinsed in PBS and
then blocked with 10% goat serum (Sigma, St. Louis, MO, USA) for 30
min at RT. Tissue slices were incubated with primary antibodies
(1:100) overnight at 4°C and were washed with PBS for 5 min 3 times
before a reaction with secondary antibody for 1 h at RT. The slides
were reacted with 3,30-diaminobenzidine tetrahydrochloride (DAB)
for 1–3 min at RT for a color reaction, and then they were
counterstained with Mayer’s hematoxylin and observed under a
microscope. Negative controls were reacted with PBS instead of
primary antibodies. The primary antibodies used were rabbit
anti-mouse ghrelin, rabbit anti-mouse GHS-R1a (Phoenix
Pharmaceuticals, Inc., Burlingame, CA, USA) and rabbit anti-mouse
OB-Rb (Beijing Biosynthesis Biotechnology Co., Ltd., Beijing,
China). The secondary antibody used was a biotinylated goat
anti-rabbit IgG. Image Pro Plus 6.0 software was used for imaging
analysis.
Statistical analysis
All data are expressed as the means ± SEM. A
normality and a homogeneity test for variance were conducted.
One-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK)
pairwise multiple comparisons were used to assess differences in
the means among groups. The Pearson correlation test was performed
between 2 parameters. All analyses were performed using SPSS for
Windows, version 10.0. P<0.01 was considered to indicate a
statistically significant difference.
Results
Body weight and serum concentrations
Comparing the HF-8W to the control group, the
average body weight increased significantly (P<0.01); however,
no significant difference was found between the HF-8W and T2DM
groups (P>0.05). The TC and TG serum concentrations increased
significantly (P<0.01) in the T2DM compared to the control
group. The average FBG levels of the control, HF-4W and HF-8W
groups were similar, but in the T2DM group, levels increased to
9.38±0.56 mmol/l (P<0.01, Table
II).
 | Table IIThe average body weight, TC, TG, FBG,
insulin levels and GIR in the control, HF-4W, HF-8W and T2DM
groups. |
Table II
The average body weight, TC, TG, FBG,
insulin levels and GIR in the control, HF-4W, HF-8W and T2DM
groups.
| Control | HF-4W | HF-8W | T2DM |
|---|
| Body weight (g) | 237.12±15.65 | 356.20±20.49a | 488.12±29.17a | 495.41±34.53a |
| TC (mmol/l) | 1.15±0.77 | 2.21±0.30a | 3.42±0.35a | 3.83±0.48a |
| TG (mmol/l) | 0.91±0.13 | 1.44±0.16a | 2.18±0.23a | 2.92±0.25a |
| FBG (mmol/l) | 5.34±0.33 | 5.40±0.32 | 5.60±0.38 | 9.38±0.56a |
| Insulin (IU/ml) | 16.98±1.75 | 22.57±2.01a | 30.48±2.08a | 27.41±1.58a,b |
| GIR
(mg/kg/min) | 12.70±0.54 | 10.16±0.49a | 7.72±0.36a | 6.25±0.32a,b |
Circulating insulin and GIR
The serum insulin levels increased from the control
to the HF-8W group (P<0.01); however, compared with the HF-8W
group, the levels decreased significantly in the T2DM group
(P<0.01). Compared with the control rats, GIR was gradually
reduced in the HF-4W, HF-8W and T2DM group rats (P<0.01,
Table II).
Gastric ghrelin
The gastric ghrelin levels were reduced
significantly in the HF-4W and HF-8W rats compared with the control
rats (P<0.01), whereas no significant differences were found
between the HF-8W and T2DM rats (P>0.05; 0.81±0.11 in the
control rats, 0.67±0.11 in the HF-4W rats, 0.57±0.11 in the HF-8W
rats and 0.59±0.09 in the T2DM group rats). The ghrelin mRNA levels
and ghrelin-expressing cells showed similar results (Fig. 1A and B).
Circulating concentrations of
ghrelin
Compared with the control rats (2.03±0.22 ng/ml),
the plasma ghrelin level was significantly suppressed in the HF-4W
and HF-8W rats (1.81±0.20 and 1.63±0.11 ng/ml; P<0.01); however,
there were no significant differences between the HF-8W and T2DM
group rats (1.64±0.13 ng/ml; P>0.05).
Expression of GHS-R1a
Compared with the control rats, a significant
reduction in the number of GHS-R1a-expressing cells was observed in
the ARC from the control to the HF-8W rats (P<0.01, Fig. 2A); however, no significant
differences were observed between the HF-8W and T2DM rats
(P>0.05, Fig. 2A). Accordingly,
total hypothalamic GHS-R1a mRNA showed similar results (P<0.01,
Fig. 2B)
Adipose leptin
Compared with the control rats, there were
significantly increased adipose leptin levels in the HF-4W, HF-8W
and T2DM rats (P<0.01, Fig.
3A). Accordingly, the leptin mRNA levels in the adipose tissue
showed similar results (P<0.01, Fig. 3B).
Circulating concentrations of leptin
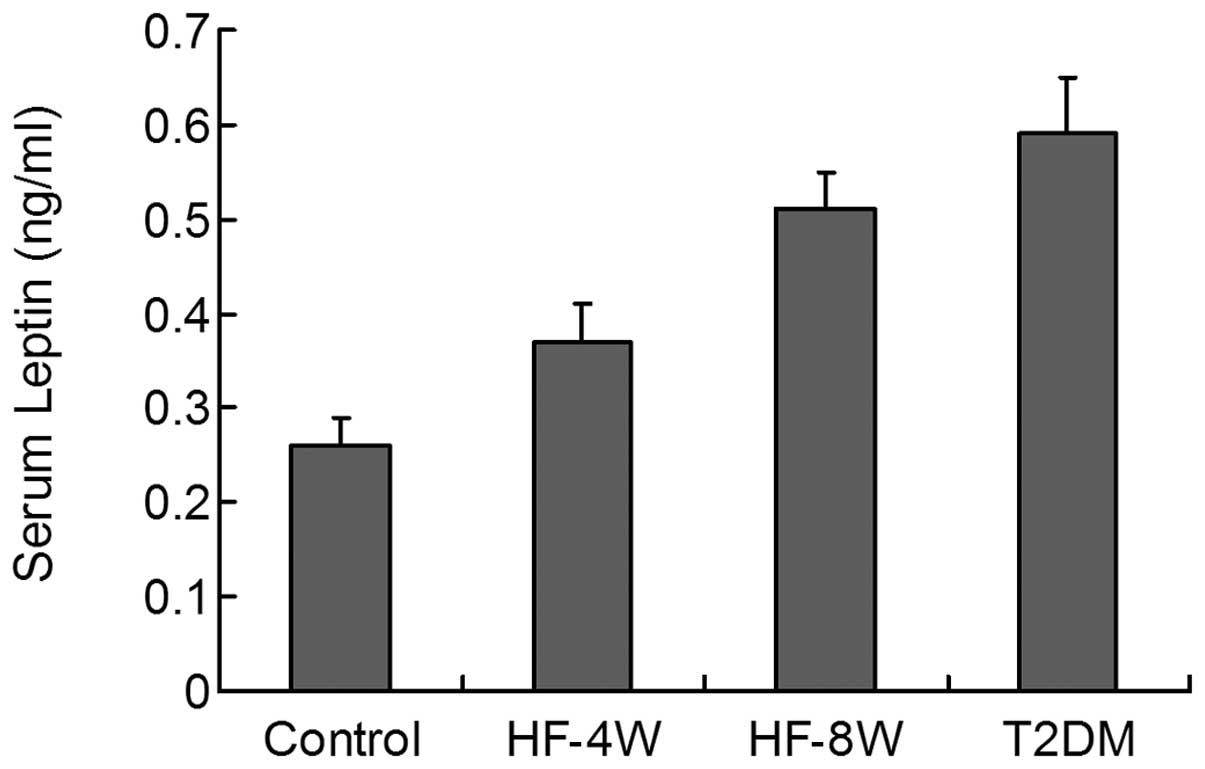
During the development of T2DM, the circulating
concentrations of leptin gradually, yet significantly increased in
the HF-4W, HF-8W and T2DM group rats (P<0.01, Fig. 4).
Expression of OB-Rb
With the development of T2DM, in the ARC of the
hypothalamus, the number of OB-Rb-expressing cells reduced
significantly (P<0.01, Fig.
5A). Similarly, total hypothalamic OB-Rb mRNA levels decreased
significantly when comparing the control to the T2DM group
(P<0.01, Fig. 5B).
Correlation between GIR and plasma
ghrelin, and between GIR and serum leptin
Pearson correlation analysis demonstrated a
significant negative correlation between the plasma ghrelin and
serum leptin levels (r=−0.813, r2=0.551, P=0.001). GIR
positively correlated with plasma ghrelin levels (r=0.945,
r2=0.893, P=0.0001) and negatively correlated with serum
leptin levels (r=−0.973, r2=0.946, P=0.001).
Discussion
The T2DM rat model in our research closely mimics
human T2DM. For the study of disease pathogenesis, prevention and
treatment, an effective T2DM model is essential. However, the basic
pathogenesis of the majority of T2DM models does not correspond
with what occurs in human T2DM. For instance, obesity and IR in the
db/db mouse and Zucker Diabetic Fatty (ZDF) rat result from
monogenic mutations that are rare in humans (16,17).
In the present study, we induced T2DM by injecting low-dose STZ
into HF-diet rats (HF feeding induced insulin resistance and
low-dose STZ injection led to hyperglycaemia). The HF-diet rat is a
useful model for human obesity and IR (18), which represents the interaction of
nature and nurture; whereas STZ is a diabetogenic agent, which
causes selective destruction of pancreatic β-cells. Injection of
low-dose STZ into HF-diet rats yielded an experimental model of
T2DM that replicates the natural history and metabolic
characteristics of the human syndrome.
A number of previous studies (19–21)
have indicated that low ghrelin levels are associated with IR,
obesity and T2DM; however, the systematic investigation of ghrelin
expression in the course of rat T2DM development has not been well
documented. The data presented in this study show that i) prior to
the injection of STZ (comparison of control to HF-8W group), with
the gain of body weight and aggravation of IR, the ghrelin levels
decreased significantly; ii) following the STZ injection, no
significant differences in ghrelin levels were found between the
HF-8W and T2DM group rats; iii) the ghrelin production in the
stomach and the GHS-R1a expression in the hypothalamus had the same
tendency to change as circulating ghrelin levels.
Ghrelin is currently considered the most potent
endogenous orexigenic peptide and plays a significant role in
glucose homeostasis (4,22–24).
A number of studies have shown that ghrelin decreases insulin
sensitivity and has diabetogenic effects: the deletion of the genes
encoding ghrelin and/or its receptor prevents a HF diet from
inducing obesity (25,26). Therefore, the reduction in ghrelin
expression is capable of decreasing food intake, preventing a
further increase in body weight, improving peripheral insulin
sensitivity, and counteracting diabetes and obesity. Above all, we
suggest that the decrease in ghrelin levels may be a compensatory
mechanism for obesity and T2DM.
Since T2DM is commonly associated with obesity in
humans, it is difficult to distinguish whether low ghrelin levels
correlate with diabetes alone or occur in conjunction with obesity.
Our results show for the first time that low ghrelin levels are
associated with obesity rather than diabetes, since with the onset
of T2DM, the ghrelin level no longer decreased. A possible
explanation for this is that as insulin is able to suppress
circulating ghrelin concentrations (27–29),
with the onset of T2DM, cells were impaired and insulin production
decreased and the suppression of ghrelin was reduced
accordingly.
As previous studies have reported, ghrelin and
leptin are involved in the regulation of GHS-R1a in the ARC, the
former upregulating and the latter downregulating gene expression
of GHS-R1a (30). In rats fed a
HF-diet, low levels of ghrelin and high levels of leptin contribute
to the reduction of GHS-R1a expression, whereas in T2DM rats, a
small reduction in ghrelin levels leads to no additional reduction
in GHS-R1a expression. The mechanism by which ghrelin and leptin
regulate GHS-R1a expression in the ARC remains unclear.
As numerous studies have demonstrated (31,32),
our study indicated that during the development of T2DM, with the
increase in rat body weight, serum leptin concentrations and
adipocyte leptin production were elevated significantly and were
found to be associated with the downregulation of the hypothalamic
leptin receptor.
The adipocyte-derived hormone leptin decreases food
intake and increases energy expenditure to maintain normal body
weight. The failure of elevated leptin levels to mediate weight
loss defines a state known as leptin resistance (33). The diminished leptin receptors that
lead to diminished physiological responses and defects in leptin
action in the ARC may play a role in the pathogenesis of
leptin-resistant obesity and T2DM.
Our data showed that ghrelin concentrations
negatively correlated with insulin levels and IR, whereas serum
leptin positively correlated with them. These data lend support to
the theory that leptin and ghrelin may play significant roles in
the development of hyperinsulinemia and insulin resistance. A
negative correlation between fasting plasma ghrelin levels and
serum leptin during the development of T2DM was also shown in our
study, supporting the opposing effects of ghrelin and leptin on
metabolic syndrome.
In conclusion, to the best of our knowledge, this is
the first study to systematically investigate both ghrelin and
leptin expression in the development of T2DM in the rat and to
discover the fact that low ghrelin levels are associated with
obesity rather than diabetes. However, further studies regarding
the effect of ghrelin on glucose homeostasis and the development of
T2DM should be conducted.
Acknowledgements
This study was supported by a grant from the Major
Medical Research Program of Hebei Health Department in the People’s
Republic of China (07067).
References
|
1
|
Yang W, Lu J, Weng J, et al: Prevalence of
diabetes among men and women in China. N Engl J Med. 362:1090–1101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mbanya JC, Motala AA and Sobngwi E:
Diabetes in sub-Saharan Africa. Lancet. 375:2254–2266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jermendy G, Nadas J, Szigethy E, et al:
Prevalence rate of diabetes mellitus and impaired fasting glycemia
in Hungary: cross-sectional study on nationally representative
sample of people aged 20–69 years. Croat Med J. 51:151–156.
2010.PubMed/NCBI
|
|
4
|
Dieguez C, da Boit K, Novelle MG, et al:
New insights in ghrelin orexigenic effect. Front Horm Res.
38:196–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Vriese C, Perret J and Delporte C:
Focus on the short- and long-term effects of ghrelin on energy
homeostasis. Nutrition. 26:579–584. 2010.PubMed/NCBI
|
|
6
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farooqi IS and O’Rahilly S: Leptin: a
pivotal regulator of human energy homeostasis. Am J Clin Nutr.
89:S980–S984. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coppari R, Ichinose M, Lee CE, et al: The
hypothalamic arcuate nucleus: a key site for mediating leptin’s
effects on glucose homeostasis and locomotor activity. Cell Metab.
1:63–72. 2005.
|
|
9
|
Cowley MA, Smith RG, Diano S, et al: The
distribution and mechanism of action of ghrelin in the CNS
demonstrates a novel hypothalamic circuit regulating energy
homeostasis. Neuron. 37:649–661. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klok MD, Jakobsdottir S and Drent ML: The
role of leptin and ghrelin in the regulation of food intake and
body weight in humans: a review. Obes Rev. 8:21–34. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klok K, Sone H and Yada T: Ghrelin is a
physiological regulator of insulin release in pancreatic islets and
glucose homeostasis. Pharmacol Ther. 118:239–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reed MJ, Meszaros K, Entes LJ, et al: A
new rat model of type 2 diabetes: the fat-fed,
streptozotocin-treated rat. Metabolism. 49:1390–1394. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang F, Ye C, Li G, et al: The rat model
of type 2 diabetic mellitus and its glycometabolism characters. Exp
Anim. 52:401–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: a model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Islam MS and Choi H: Nongenetic model of
type 2 diabetes: a comparative study. Pharmacology. 79:243–249.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peterson RG, Shaw WN and Neel MA: Zucker
diabetic fatty rat as a model for non-insulin-dependent diabetes
mellitus. ILAR News. 32:16–19. 1990. View Article : Google Scholar
|
|
17
|
Chang AY: Spontaneous diabetes in animals.
Gen Pharmacol. 9:447–450. 1978. View Article : Google Scholar
|
|
18
|
Woods SC, Seeley RJ, Rushing PA, D’Alessio
D and Tso P: A controlled high-fat diet induces an obese syndrome
in rats. J Nutr. 133:1081–1087. 2003.PubMed/NCBI
|
|
19
|
Ukkola O, Poykko S, Paivansalo M and
Kesäniemi YA: Interactions between ghrelin, leptin and IGF-I affect
metabolic syndrome and early atherosclerosis. Ann Med. 40:465–473.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pacifico L, Poggiogalle E, Costantino F,
et al: Acylated and nonacylated ghrelin levels and their
associations with insulin resistance in obese and normal weight
children with metabolic syndrome. Eur J Endocrinol. 161:861–870.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serra-Prat M, Alfaro SR, Palomera E, et
al: Relationship between ghrelin and the metabolic syndrome in the
elderly: a longitudinal population-based study. Clin Endocrinol
(Oxf). 70:227–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Asnicar M and Smith RG: Central and
peripheral roles of ghrelin on glucose homeostasis.
Neuroendocrinology. 86:215–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yada T, Dezaki K, Sone H, et al: Ghrelin
regulates insulin release and glycemia: physiological role and
therapeutic potential. Curr Diabetes Rev. 4:18–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dezaki K, Kakei M and Yada T: Ghrelin uses
Galphai2 and activates voltage-dependent K+ channels to
attenuate glucose-induced Ca2+ signaling and insulin
release in islet beta-cells: novel signal transduction of ghrelin.
Diabetes. 56:2319–2327. 2007.PubMed/NCBI
|
|
25
|
Wortley KE, del Rincon JP, Murray JD, et
al: Absence of ghrelin protects against early-onset obesity. J Clin
Invest. 115:3573–2578. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zigman JM, Nakano Y, Coppari R, et al:
Mice lacking ghrelin receptors resist the development of
diet-induced obesity. J Clin Invest. 115:3564–3572. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Flanagan DE, Evans ML, Monsod TP, et al:
The influence of insulin on circulating ghrelin. Am J Physiol
Endocrinol Metab. 284:E313–E316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueno M, Carvalheira JB, Oliveira RL,
Velloso LA and Saad MJ: Circulating ghrelin concentrations are
lowered by intracerebroventricular insulin. Diabetologia.
49:2449–2452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fick LJ, Cai F and Belsham DD:
Hypothalamic preproghrelin gene expression is repressed by insulin
via both PI3-K/Akt and ERK1/2 MAPK pathways in immortalized,
hypothalamic neurons. Neuroendocrinology. 89:267–275. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nogueiras R, Tovar S, Mitchell SE, et al:
Regulation of growth hormone secretagogue receptor gene expression
in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes.
53:2552–2558. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Shoumer KA, Al-Asousi AA, Doi SA and
Vasanthy BA: Serum leptin and its relationship with metabolic
variables in Arabs with type 2 diabetes mellitus. Ann Saudi Med.
28:367–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Levin BE, Dunn-Meynell AA, Ricci MR and
Cummings DE: Abnormalities of leptin and ghrelin regulation in
obesity-prone juvenile rats. Am J Physiol Endocrinol Metab.
285:E949–E957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Myers MG, Cowley MA and Munzberg H:
Mechanisms of leptin action and leptin resistance. Annu Rev
Physiol. 70:537–556. 2008. View Article : Google Scholar : PubMed/NCBI
|