Introduction
Growth hormone secretagogue receptor 1a (ghrelin
receptor, GHS-R1a) is a specific G protein-coupled receptor
(1). Ghrelin is an endogenous
ligand for GHS-R1a (1) that has
been identified in tissues of the central nervous system, including
the hypothalamus and anterior pituitary gland (2,3), as
well as in multiple peripheral organs and tissues (4,5),
including the stomach and intestine (6), pancreas (7) and kidney (8).
Ghrelin was initially identified due to its
stimulatory effect on the release of growth hormone (9). Following this discovery, a wide
variety of biological functions of ghrelin were found. Ghrelin is
known to stimulate appetite and acid secretion (10,11)
and a positive energy balance (12), has cardiovascular actions (13) and controls digestive motility
(14,15).
The effect of ghrelin on gastrointestinal tract
motility has been of increasing interest. The central and
peripheral administration of ghrelin increases the gastric emptying
rate (16,17). However, the majority of studies on
the effect of ghrelin on gastrointestinal tract motility have been
performed in normal animals. Although some studies have been
carried out on GHS-R gene-knockout mice (18,19),
the changes in gastrointestinal tract motility in
Ghsr−/− mice have not yet been reported. The aim of this
study was to investigate the effects and possible mechanisms of
GHS-R deficiency on gastric motility in Ghsr−/−
mice.
Materials and methods
Chemicals
Ghrelin and carbachol were obtained from Tocris
Cookson (Bristol, UK). GHS-R1a (F-16; goat anti-mouse) and
neurofilament heavy polypeptide (NF-H; H-5; mouse anti-mouse)
antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Phenol red solution (0.5%) and methylcellulose (400
cp, 2%) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
FITC-conjugated secondary antibody (goat anti-mouse) and
TRITC-conjugated secondary antibody (rabbit anti-goat) were
obtained from Jackson ImmunoResearch, Inc. (Baltimore Pike, PA,
USA).
Animals
Male and female Ghsr+/− mice (mixed
129S3/SvImJ and C57BL/6J background) were obtained from the
Shanghai Research Center for Model Organisms (Shanghai, China). The
offspring of heterozygous parents underwent genotype identification
for subsequent experiments. GHS-R-knockout mice (Ghsr−/−
mice) and their normal littermates (Ghsr+/+ mice) were
used (20). Animals (10 weeks old,
20–24 g) were kept under specific pathogen-free conditions with a
normal 12/12 h light/dark cycle (21) for at least 7 days prior to the
start of experimentation. Animal procedures were conducted
according to the ethical guidelines of Shanghai Jiao Tong
University. Measurements were performed in conscious animals
following an 18-h fasting period.
Generation and identification of
Ghsr−/− mice
A previously reported approach (19) was used to generate the
Ghsr−/− mice. This procedure was performed in the
Shanghai Research Center for Model Organisms.
The genotypes of the Ghsr+/+ and
Ghsr−/− mice were identified by PCR. Genomic DNA was
extracted from mice tails. The primers were: P1
(5′-GTGCGCACTGTCTCCTCTGAT TTG-3′); P2
(5′-GTGCTTTGGGGTGCGTGTGATGGA-3′) and P3
(5′-CACGCCCACCAGCACGAAGA-3′). The PCR process consisted of 35
amplification cycles (95°C for 5 min, 94°C for 50 sec, 61°C for 50
sec and 72°C for 3 min), with a final elongation period of 10 min
at 72°C. The expected PCR product sizes were 1.9 kbp for the
Ghsr−/− mice and 1.2 kbp for the Ghsr+/+
mice, while two PCR products were expected for Ghsr+/−
mice. The PCR products were separated by electrophoresis on a 1.4%
agarose gel, after which images were captured.
Gastric emptying
All the Ghsr+/+ and Ghsr−/−
mice received gavage feeding of 0.4 ml prewarmed (35°C) phenol red
meal (50 mg/100 ml in distilled H2O with 1.5%
methylcellulose; viscosity 400 centipoise). The mice were
sacrificed by cervical dislocation 20 min after gavage feeding.
Four animals were sacrificed immediately after the gavage feeding
to serve as internal controls. The entire stomach was carefully
isolated, ligated just above the cardia and below the pylorus, and
removed. Gastric emptying measurements were performed as described
previously (22,23). The stomach and its contents (phenol
red meal plus possible gastric secretions) were homogenized with 10
ml NaOH (0.1 N). The mixture was kept at room temperature for 1 h.
The supernatant (5 ml) was added to 0.5 ml trichloroacetic acid
solution (20%, w/v) to precipitate any proteins. After centrifuging
(2500 × g, 20 min), 5 ml supernatant was added to 4 ml NaOH (0.5 N)
to develop the maximum intensity of color. The solutions were read
at a wavelength of 560 nm with a spectrophotometer (Shanghai Yixian
Co., Shanghai, China). The percentage of gastric emptying (GE%) was
determined as: GE% = (1-X/Y) × 100, where X and Y are the
absorbances of phenol red collected from the stomachs of animals
sacrificed 20 min after gavage and immediately after gavage
feeding, respectively.
The gastric emptying rates were studied following
the intraperitoneal administration of 0, 20, 40 or 80 μg/kg
ghrelin. Ghrelin was dissolved in 0.9% NaCl. The intraperitoneal
administration volume (ghrelin plus saline solution) was 0.2
ml.
Organ bath
Contractility measurements of smooth muscle
strips
Ghsr+/+ and Ghsr−/− mice were
fasted for 18 h and sacrificed by cervical dislocation. Circular
muscle strips, freed from mucosa (length 10 mm, width 1 mm) were
cut from the gastric antrum and suspended vertically in an organ
bath filled with Krebs solution (121.5 mM NaCl, 4.7 mM KCl, 2.5 mM
CaCl2, 1.2 mM MgSO4, 1.2 mM
KH2PO4, 25.0 mM NaHCO3 and 11.0 mM
glucose). The organ bath chamber was gassed with 95%
O2/5% CO2 and warmed to 37°C. Figures
obtained from the isometric force transducer (Harvard Apparatus,
South Natick, MA, USA) were continuously recorded and stored on a
computer and analyzed using the SMUP-E biological signal processing
system (Chengdu Equipment Factory, Chengdu, China). The initial
load was set at 0.5 g for each strip. The Krebs solution was
changed every 15 min and the organ bath was allowed to equilibrate
for 1 h. Electrical field stimulation (EFS) was applied to the
preparation through a pair of platinum ring electrodes fixed on the
top and bottom of the bath. A frequency spectrum (4 Hz) was
obtained using pulse trains (duration 1 msec, train 10 sec, 2-min
intervals) (24,25).
The contractile responses of the smooth muscle
strips to EFS (4 Hz) and ghrelin (0.01, 0.1, 0.5 and 1.0 μM) plus
EFS (4 Hz) were observed in the control and model mice. The
amplitude of contraction or relaxation of the strips was normalized
using the responses evoked by EFS in the absence of ghrelin to
evaluate the ghrelin-induced action. The EFS-induced action was
normalized using the amplitude of spontaneous contraction or
relaxation.
Contractility measurements of isolated
stomach
Intragastric pressure levels were used to evaluate
the reactive ability of all the gastric muscle layers to
experimental substances as described in previous studies (26). The entire stomach was carefully
isolated, removed and placed in Krebs solution. The stomach content
(possible meal and gastric secretions) was flushed with Krebs
solution. A soft polyethylene catheter (inner diameter, 1.7 mm;
outer diameter, 2.2 mm) was implanted through the pylorus into the
gastric cavity and connected to an external pressure transducer
(Harvard Apparatus). Figures were recorded and stored on a computer
for analysis using the SMUP-E biological signal processing system.
The intragastric pressure was initially kept at 5 cm H2O
and allowed to equilibrate for 1 h. The buffer was changed every 15
min. Measurements were taken when spontaneous pressure fluctuations
were relatively stable.
The intragastric pressure responses to carbachol
(50, 100, 200, 400 and 600 nM) were observed in the organ bath. The
effect of carbachol on intragastric pressure was normalized by the
mean of three maximal spontaneous pressure wave peak values.
Immunofluorescence staining
GHS-R staining in gastric muscle layers
Fluorescent staining of GHS-Rs in the gastric antrum
muscle layers was studied in Ghsr+/+ and
Ghsr−/− mice. Gastric muscle layers, freed from mucous
layers, were fixed on a platform and stretched to ~150%. The
samples were subsequently fixed with 4°C acetone for 15 min, after
which the acetone was washed off with PBS. The samples were then
incubated with 0.5% Triton X-100 for 30 min and flushed, after
which they were incubated with 10% fetal bovine serum for 60 min.
The primary antibody to GHS-R1a (F-16; goat anti-mouse) was diluted
in PBS and added to the muscle tissues at a ratio of 1:100. The
samples were incubated at 4°C for 2 days. The secondary antibody
(rabbit anti-goat) coupled with rhodamine (TRITC, red fluorescence)
was diluted in PBS and added to the fixed samples at a ratio of
1:200. The tissues were then incubated for half a day in a dark
room. DAPI was used as a counterstain for the cell nuclei. The
samples were then coverslipped with 50% glycerol. Negative controls
were prepared in the same manner as the samples, but without
application of the primary antibody.
Double fluorescent staining of the nerve cells in
the gastric antrum muscle layers was studied in the
Ghsr+/+ and Ghsr−/− mice. The GHS-R1a
antibody (F-16) was used to label the GHS-Rs and the NF-H antibody
(H-5), a neuron-specific marker, was used to label the nerve cells.
The procedures of staining were as described above. The dilution
rates of the primary antibodies GHS-R1a (F-16; goat anti-mouse) and
NF-H (H-5; mouse anti-mouse) were 1:100. The dilution rates of the
secondary antibodies, coupled with rhodamine (TRITC, rabbit
anti-goat) or fluorescein (FITC, goat anti-mouse), were 1:200. The
samples were examined and scanned under a laser confocal microscope
(Olympus, FV-1000, Tokyo, Japan).
Statistical analysis
Results were expressed as the mean ± SEM. Data were
analyzed with Origin 8.0 software. Photoshop 8.0.1 and CorelDRAW X4
software were used to produce the figures. Data recordings were
evaluated by one way analysis of variance (ANOVA) followed by
Dunnett’s test. P<0.05 was considered to indicate a
statistically significant result.
Results
Gastric emptying in Ghsr+/+
and Ghsr−/− mice
The GE% values were significantly reduced in the
Ghsr−/− mice when no drug was injected (Fig. 1A). Ghrelin increased the GE% in a
dose-dependent manner in the Ghsr+/+ mice when
intraperitoneally administered, but had no effect on the GE% in the
Ghsr−/− mice (Fig.
1B).
 | Figure 1Gastric emptying rates (GE%) in
Ghsr+/+ and Ghsr−/− mice. (A) GE% for 20 min
when no drugs were injected, *P<0.05; (B) GE% for 20
min following the intraperitoneal administration of ghrelin.
P-values indicate differences of gastric emptying rates between
control and model mice when the same dose or different doses of
drugs were administered. aP<0.01,
a,bP<0.01, b,cP<0.01;
a′P>0.05, a′,b′P>0.05,
b′,c′P>0.05, a′,c′P>0.05;
a,a′P<0.01, b,b′P<0.01,
c,c′P<0.01, n=4 per condition. Mean ± SEM. |
Organ bath
Smooth muscle strips
When EFS (4 Hz) was applied in vitro, the
contractile response of the smooth muscle strips was lower in the
strips from the Ghsr−/− mice than in those from the
Ghsr+/+ mice (Fig.
2A).
In the strips from the Ghsr+/+ mice,
ghrelin (0.01, 0.1, 0.5 and 1.0 μM) increased the amplitude of
contraction or relaxation of the strips in a dose-dependent manner
in the presence of EFS, while in the strips from the
Ghsr−/− mice, this effect was not observed (Fig. 2A). There were statistically
significant differences in the contractile amplitudes of the strips
from the two types of mice when EFS and EFS plus ghrelin were
applied (Fig. 2B and C).
Isolated stomach
The changes of the intragastric pressure levels were
lower in the Ghsr−/− mice than in the Ghsr+/+
mice when carbachol (50, 100, 200, 400 and 600 nM) was applied to
the isolated stomach (Fig. 3A).
There were statistically significant differences in the
intragastric pressure levels when different concentrations of
carbachol were administered (Fig.
3B).
Immunofluorescent staining of gastric muscle
layer nerve cells
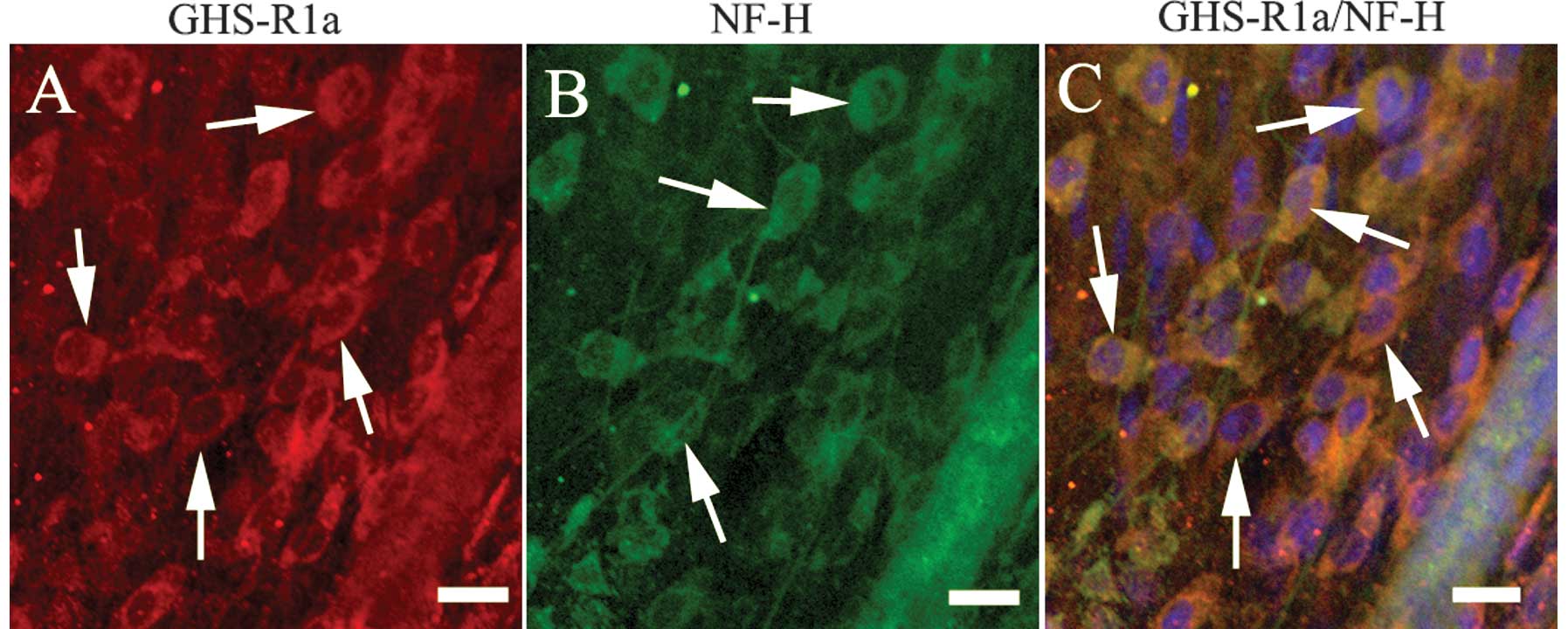
Immunofluorescent staining indicated that GHS-R1as
(red fluorescence) were present in the Ghsr+/+ mice
(Fig. 4A) but not in the
Ghsr−/− mice (data not shown). In the Ghsr+/+
mice, GHS-R1as were mainly located on the membrane and the
cytoplasms of the nerve cells in the gastric antrum muscle plexus
(Fig. 4C).
Immunofluorescent staining of the nerve cells in the
gastric antrum muscle layer in the Ghsr+/+ and
Ghsr−/− mice is shown in Fig. 5A. The number of nerve cells in the
gastric antrum muscle layer was decreased in the Ghsr−/−
mice (Fig. 5B).
Discussion
In previous studies, the administration of ghrelin
via the central nervous system has been demonstrated to have a
pronounced effect on appetite and the motility of the
gastrointestinal tract (27–29).
Centrally, ghrelin acts through activation of GHS-Rs in the
hypothalamus. These effects are mediated by the vagal nerve
(30). When the vagal nerve is
severed, central effects are eliminated (31). The peripheral administration of
ghrelin also enhances the motility of the gastrointestinal tract
(32–34). The peripheral effects of ghrelin
may be caused by the activation of GHS-Rs on the vagal nerve
(14) and gastrointestinal enteric
plexus (35). Ghrelin exerts its
effects by activating GHS-Rs in central or peripheral tissues
(2–5).
In Ghsr−/− mice, GHS-Rs are defective due
to the knockout of GHS-R genomic DNA. This is likely to influence a
number of the effects mediated by ghrelin, including the promotion
of gastrointestinal tract motility. Sun et al reported that
the body weights of Ghsr−/− mice, regardless of high fat
or regular diet, were slightly lower than those of their wild-type
littermates (P<0.05) (18), and
food intake following fasting was identical in Ghsr+/+
and Ghsr−/− mice, indicating that the absence of the
Ghsr in obese mice does not prevent weight gain following weight
loss. Some of these results were obtained from obese rather than
non-obese mice. Thus, there may be differences in food intake and
gastric motility in non-obese mice, and further studies should be
conducted.
Our experimental results in vivo demonstrated
that gastric emptying rates were reduced in Ghsr−/−
mice. Ghrelin promoted gastric emptying rates in a dose-dependent
manner in Ghsr+/+ mice. In Ghsr−/− mice, no
effect of ghrelin on gastric emptying rates was observed. In
Ghsr+/+ mice, GHS-Rs are expressed normally, allowing
ghrelin to exert its biological functions by activating GHS-Rs in a
dose-dependent manner, while in Ghsr−/− mice, the
gastric emptying induced by ghrelin was eliminated, which may
involve changes to the central and peripheral channels. The absence
of the GHS-Rs in the central system may attenuate the effect of the
hypothalamus on gastric motility, while the absence of GHS-Rs in
the stomach may eliminate the peripheral effect of ghrelin on
gastric motility. Ghrelin was administered at physiological
stimulus doses of 20, 40 and 80 μg/kg. Under physiological
conditions, the plasma levels of ghrelin in rats are 500–2000
pmol/l (36). Fujino et al
reported that the injection of 1.5 nmol ghrelin in rats resulted in
an ~600 pmol/l increase in plasma ghrelin concentrations (14). This result is supported by our
finding that the plasma concentrations of ghrelin were ~2400 pmol/l
following the administration of 80 μg/kg ghrelin, which approached
physiological concentrations.
In vitro, EFS induced a contractile response
in smooth muscle strips, and the amplitudes of contraction or
relaxation of the strips differed between the Ghsr+/+
and Ghsr−/− mice. The different contractile responses of
the smooth muscle strips, we hypothesize, may relate to the
functions and states of the smooth muscle cells or nerve cells in
the muscle layers. The effects of ghrelin on the isolated strips
were the same as those observed in vivo. These results
indicate that ghrelin is able to induce a contractile response in
smooth muscle strips from Ghsr+/+ mice only when the
strips are stimulated through excitatory nerves. In vivo,
ghrelin is not able to induce a contractile response directly, but
enhances the amplitudes of contraction or relaxation of the strips
induced by EFS. In Ghsr−/− mice, the absence of the Ghsr
in the strips may negate the effect of ghrelin on the contractile
response of the smooth muscle strips.
In vitro, ghrelin played a
gastroprokinetic-like role when smooth muscle strips were
stimulated by an electrical field (24). This finding suggests that ghrelin
exerts its effect only in response to excitatory nerve impulses or
changes in membrane potential, i.e., it is able to enhance but not
directly induce smooth muscle cell contraction. In the current
study, we found that a single administration of ghrelin did not
affect intragastric pressure levels. No effects were observed even
though the increased dose of ghrelin exceeded physiological
concentrations 50-fold (data not shown), which is in accordance
with results previously obtained from gastric smooth muscle strips
(24). This may be as a result of
the effect of ghrelin on gastric motility being mediated by the
activation of GHS-Rs located on nerve cells in the gastric plexus,
i.e., when the stomach was isolated, the motor nerves were cut off
and the functions of the gastric plexus downstream from the motor
nerves were suppressed. Intragastric pressure levels were lower in
the Ghsr−/− mice than in the Ghsr+/+ mice
when the same dose of carbachol was administered, suggesting that
the reactive ability of gastric muscle layers to carbachol was
altered in the Ghsr−/− mice. This may be related to the
deficiency of GHS-Rs and the functions and states of the smooth
muscle cells or nerve cells in the gastric plexus. In
Ghsr+/+ mice, the effect of carbachol was likely to be
enhanced by ghrelin (37,38), which is secreted by mucous X/A-like
cells (9).
Fluorescent staining results supported the results
obtained from the in vivo and in vitro studies. The
fluorescent staining of gastric muscle tissues indicated that
GHS-Rs were mainly located on nerve cells in the gastric plexus.
This result morphologically supports the previous theory that
ghrelin acts through the nerve cells in the gastrointestinal plexus
(24,39). GHS-Rs were not detected in muscle
layers in the Ghsr−/− mice, which demonstrated the
reliability of the knockout of GHS-R genomic DNA. Thus, loss of
GHS-Rs eliminated the gastroprokinetic-like role mediated by
ghrelin in the Ghsr−/− mice, and the central and
peripheral effects of ghrelin were also eliminated. The number of
nerve cells in the gastric antrum muscle layers was decreased in
the Ghsr−/− mice, which may affect the release of
excitatory neurotransmitters, i.e., the quantity of
neurotransmitters released from nerve cells in the gastric plexus
may be decreased in Ghsr−/− mice when the same intensity
of EFS and concentration of carbachol are applied. The decrease in
the number of nerve cells in the gastric plexus and the loss of
GHS-Rs in muscle layers may together lead to the decrease in the
contractility of gastric smooth muscle to EFS and carbachol in
vitro. Since the functions and states of the smooth muscle
cells themselves are unknown, further studies should be
performed.
In summary, GHS-R-deficient mice were a platform to
study the effects of the ghrelin receptors. In the present study,
the ghrelin receptor deficiency weakened gastric motility. The
knockout of ghrelin receptor genomic DNA may affect the development
of nerve cells in the gastric plexus and lead to a reduction in the
number of nerve cells. The loss of ghrelin receptors in muscle
layers and of nerve cells in the gastric plexus may together
attenuate gastric motility. However, more studies should be carried
out to clarify the mechanisms.
Acknowledgements
The authors are grateful to the Shanghai Research
Center for Model Organisms for participation in the study. This
study was supported in part by the Physiology Laboratory, Shanghai
Jiaotong University and was supported by the National Natural
Science Foundation of China (No. 30400429).
References
|
1
|
van der Lely AJ, Tschöp M, Heiman ML and
Ghigo E: Biological, physiological, pathophysiological, and
pharmacological aspects of ghrelin. Endocr Rev. 25:426–457.
2004.PubMed/NCBI
|
|
2
|
Guan XM, Yu H, Palyha OC, et al:
Distribution of mRNA encoding the growth hormone secretagogue
receptor in brain and peripheral tissues. Brain Res Mol Brain Res.
48:23–29. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shuto Y, Shibasaki T, Wada K, et al:
Generation of polyclonal antiserum against the growth hormone
secretagogue receptor (GHS-R): evidence that the GHS-R exists in
the hypothalamus, pituitary and stomach of rats. Life Sci.
68:991–996. 2001. View Article : Google Scholar
|
|
4
|
Kojima M, Hosoda H and Kangawa K:
Purification and distribution of ghrelin: the natural endogenous
ligand for the growth hormone secretagogue receptor. Horm Res.
56(Suppl 1): S93–S97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yokote R, Sato M, Matsubara S, et al:
Molecular cloning and gene expression of growth hormone-releasing
peptide receptor in rat tissues. Peptides. 19:15–20. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Date Y, Kojima M, Hosoda H, et al:
Ghrelin, a novel growth hormone-releasing acylated peptide, is
synthesized in a distinct endocrine cell type in the
gastrointestinal tracts of rats and humans. Endocrinology.
141:4255–4261. 2000.PubMed/NCBI
|
|
7
|
Kamegai J, Tamura H, Shimizu T, et al:
Central effect of ghrelin, an endogenous growth hormone
secretagogue, on hypothalamic peptide gene expression.
Endocrinology. 141:4797–4800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mori K, Yoshimoto A, Takaya K, et al:
Kidney produces a novel acylated peptide, ghrelin. FEBS Lett.
486:213–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kojima M, Hosoda H, Date Y, et al: Ghrelin
is a growth-hormone-releasing acylated peptide from stomach.
Nature. 402:656–660. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dimaraki EV and Jaffe CA: Role of
endogenous ghrelin in growth hormone secretion, appetite regulation
and metabolism. Rev Endocr Metab Disord. 7:237–249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arakawa M, Suzuki H, Minegishi Y, et al:
Enhanced ghrelin expression and subsequent acid secretion in mice
with genetic H(2)-receptor knockout. J Gastroenterol. 42:711–718.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asakawa A, Inui A, Fujimiya M, et al:
Stomach regulates energy balance via acylated ghrelin and desacyl
ghrelin. Gut. 54:18–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagaya N and Kangawa K: Ghrelin, a novel
growth hormone-releasing peptide, in the treatment of chronic heart
failure. Regul Pept. 114:71–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujino K, Inui A, Asakawa A, et al:
Ghrelin induces fasted motor activity of the gastrointestinal tract
in conscious fed rats. J Physiol. 550:227–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tack J, Depoortere I, Bisschops R, et al:
Influence of ghrelin on interdigestive gastrointestinal motility in
humans. Gut. 55:327–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taniguchi H, Ariga H, Zheng J, et al:
Effects of ghrelin on interdigestive contractions of the rat
gastrointestinal tract. World J Gastroenterol. 14:6299–6302. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trudel L, Tomasetto C, Rio MC, et al:
Ghrelin/motilin-related peptide is a potent prokinetic to reverse
gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver
Physiol. 282:G948–G952. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Butte NF, Garcia JM and Smith RG:
Characterization of adult ghrelin and ghrelin receptor knockout
mice under positive and negative energy balance. Endocrinology.
149:843–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Wang P, Zheng H and Smith RG:
Ghrelin stimulation of growth hormone release and appetite is
mediated through the growth hormone secretagogue receptor. Proc
Natl Acad Sci USA. 101:4679–4684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen D, Zhao CM, Håkanson R, et al:
Altered control of gastric acid secretion in
gastrin-cholecystokinin double mutant mice. Gastroenterology.
126:476–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicklas W, Baneux P, Boot R, et al:
Recommendations for the health monitoring of rodent and rabbit
colonies in breeding and experimental units. Lab Anim. 36:20–42.
2002. View Article : Google Scholar
|
|
22
|
Scarpignato C, Capovilla T and Bertaccini
G: Action of caerulein on gastric emptying of the conscious rat.
Arch Int Pharmacodyn Ther. 246:286–294. 1980.PubMed/NCBI
|
|
23
|
Amira S, Soufane S and Gharzouli K: Effect
of sodium fluoride on gastric emptying and intestinal transit in
mice. Exp Toxicol Pathol. 57:59–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kitazawa T, De Smet B, Verbeke K, et al:
Gastric motor effects of peptide and non-peptide ghrelin agonists
in mice in vivo and in vitro. Gut. 54:1078–1084. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura T, Onaga T and Kitazawa T:
Ghrelin stimulates gastric motility of the guinea pig through
activation of a capsaicin-sensitive neural pathway: in vivo and in
vitro functional studies. Neurogastroenterol Motil. 22:446–452.
2010. View Article : Google Scholar
|
|
26
|
Mulè F, Amato A, Baldassano S and Serio R:
Involvement of CB1 and CB2 receptors in the modulation of
cholinergic neurotransmission in mouse gastric preparations.
Pharmacol Res. 56:185–192. 2007.PubMed/NCBI
|
|
27
|
Kamiji MM, Troncon LE, Suen VM and de
Oliveira RB: Gastrointestinal transit, appetite, and energy balance
in gastrectomized patients. Am J Clin Nutri. 89:231–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kobashi M, Yanagihara M, Fujita M, et al:
Fourth ventricular administration of ghrelin induces relaxation of
the proximal stomach in the rat. Am J Physiol Regul Integr Comp
Physiol. 296:R217–R223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tups A, Helwig M, Khorooshi RM, et al:
Circulating ghrelin levels and central ghrelin receptor expression
are elevated in response to food deprivation in a seasonal mammal
(Phodopus sungorus). J Neuroendocrinol. 16:922–928. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ammori JB, Zhang WZ, Li JY, et al: Effects
of ghrelin on neuronal survival in cells derived from dorsal motor
nucleus of the vagus. Surgery. 144:159–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kamiji MM, Troncon LE, Antunes-Rodrigues
J, et al: Ghrelin and PYY(3–36) in gastrectomized and vagotomized
patients: relations with appetite, energy intake and resting energy
expenditure. Eur J Clin Nutr. 64:845–852. 2010.
|
|
32
|
Edholm T, Levin F, Hellström PM and
Schmidt PT: Ghrelin stimulates motility in the small intestine of
rats through intrinsic cholinergic neurons. Regul Pept. 121:25–30.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobelt P, Tebbe JJ, Tjandra I, et al: CCK
inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol
Regul Integr Comp Physiol. 288:R751–R758. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kleinz MJ, Maguire JJ, Skepper JN and
Davenport AP: Functional and immunocytochemical evidence for a role
of ghrelin and des-octanoyl ghrelin in the regulation of vascular
tone in man. Cardiovasc Res. 69:227–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murakami N, Hayashida T, Kuroiwa T, et al:
Role for central ghrelin in food intake and secretion profile of
stomach ghrelin in rats. J Endocrinol. 174:283–288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bassil AK, Dass NB and Sanger GJ: The
prokinetic-like activity of ghrelin in rat isolated stomach is
mediated via cholinergic and tachykininergic motor neurones. Eur J
Pharmacol. 544:146–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu WC, Wang ZG, Wang WG, et al:
Therapeutic effects of ghrelin and growth hormone releasing peptide
6 on gastroparesis in streptozotocin-induced diabetic guinea pigs
in vivo and in vitro. Chinese Med J (Engl). 121:1183–1188.
2008.PubMed/NCBI
|
|
38
|
Dass NB, Munonyara M, Bassil AK, et al:
Growth hormone secretagogue receptors in rat and human
gastrointestinal tract and the effects of ghrelin. Neuroscience.
120:443–453. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Depoortere I, De Winter B, Thijs T, et al:
Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and
motilin in rats in vivo and in vitro. Eur J Pharmacol. 515:160–168.
2005. View Article : Google Scholar : PubMed/NCBI
|