Introduction
Breast cancer is the most commonly diagnosed cancer
in women worldwide, with an estimated 1.4 million new cases and
458,000 deaths in 2008 (1,2). Breast cancer is the second leading
cause of cancer-related mortality in woman, while its incidence in
the developing world is on the rise (3). Notably, incidence has increased 6.8%
annually during the last 6 years in Korea, leading to an increase
in mortality (4).
Epithelial-to-mesenchymal transition (EMT) is the
process whereby epithelial cells transform to a mesenchymal state.
EMT is an essential process during embryonic development, wound
healing, inflammation and fibrosis as well as tumorigenesis
(5–7). During the EMT process, cancer cells
lose epithelial markers, such as E-cadherin and occuludins, and
acquire mesenchymal markers, such as vimentin, fibronectin,
N-cadherin and matrix metalloproteinases (MMPs). MMPs facilitate
migration, invasion and metastasis in cancer (5,7–9).
Cell migration is involved in tumor invasion and metastasis.
Degradation of the extracellular matrix (ECM) by MMPs is understood
to be a pre-requisite for cell migration to native or provisional
tissue matrix based on studies of several systems (10,11).
Focal adhesion kinase (FAK) is a 125-kDa
non-receptor tyrosine kinase localized to sites of integrin
clustering, termed focal adhesions (2,12).
FAK activation leads to a number of cell processes including cell
attachment, migration, invasion, proliferation and survival
(12,13). Furthermore, in breast cancer, the
FAK gene is amplified and its protein is overexpressed (14).
Recent studies have reported that natural bioactive
compounds derived from plants exhibit anticancer effects by
suppressing the EMT process (3,15,16).
Indole-3-carbinol (I3C) is naturally found in vegetables of the
Cruciferae family such as broccoli, brussel sprouts and
cauliflower (17,18). I3C autolytically breaks down into
indole glucosinolates and indole[3,2-b] carbazole (ICZ), an
acid-derived condensation metabolite (19,20).
Epidemiological studies have reported that high dietary intake of
cruciferous vegetables is associated with lower cancer risk. In
particular, I3C strongly suppresses development and growth of
breast cancer (17,18).
In the present study, the effects of I3C and ICZ on
migration in two human breast cancer cell lines, MCF-7 and
MDA-MB231 were examined. The mRNA expression of E-cadherin,
vimentin, FAK and MMP activity was also investigated.
Materials and methods
Cell culture and I3C and ICZ
treatment
MCF-7 and MDA-MB231 breast cancer cells were
purchased from ATCC (American Type Culture Collection, Manassas,
VA, USA) and maintained in RPMI-1640 supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin.
Cells were then incubated in a standard humidified incubator with
5% CO2 at 37°C. I3C and ICZ were purchased from
Sigma-Aldrich (St. Louis, MO, USA) (Fig. 1). Stock solutions of I3C and ICZ
were prepared in ethanol and diluted into the growth medium such
that the final concentration of ethanol did not exceed 0.1%
(v/v).
Determination of cell viability by MTT
assay
Cells were cultured in a 96-well plate at a density
of 1×105/ml. After 24 h, cells were treated with various
concentrations of I3C and ICZ and incubated for 48 h. An MTT
solution [0.5 mg/ml phosphate-buffered saline (PBS)] was then added
to each well, and cells were incubated for 4 h. The supernatants
were carefully removed, and formazan crystals were dissolved in
dimethyl sulfoxide (DMSO). Absorbance was measured at 570 nm on a
microplate reader. The cell viability was determined relative to
untreated control cells.
Wound healing assay
Cells were grown to confluence on 35-mm culture
dishes. The monolayer was then scratched with a sterile pipette tip
and was washed with PBS to remove cellular debris. Cells were then
grown in RPMI-1640 containing 5% FBS in either I3C or ICZ (0, 1,
2.5, 5 or 10 μM). After 48 h, cell migration was observed under a
microscope and photographed.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from breast cancer cells
using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (1
μg) was reverse-transcribed using the AccessQuick™ RT-PCR system
(Promega, Madison, WI, USA). The primer sequences used for
amplification of the sense and antisense strands were: FAK,
5′-GCGCTGGCTGGAAA AAGAGGAA-3′ and 5′-TCGGTGGGTGCTGGCTGGT AGG-3′;
E-cadherin, 5′-ACATTGTCACCTCGCAGAC-3′ and
5′-GCGGATTGTAGAAGTCTTGG-3′; vimentin, 5′-TGG CACGTCTTGACCTTGAA-3′
and 5′-GGTCATCGTGATGC TGAGAA-3′; GAPDH, 5′-CGGAGTCAACGGATTTGGT
CGTAT-3′ and 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. PCR was carried out
under the following conditions: 30 cycles for FAK at 94°C for 30
sec, 65°C for 90 sec and 72°C for 30 sec; 30 cycles for E-cadherin
and vimentin at 94°C for 45 sec, 58°C for 45 sec, and 72°C for 45
sec; and 25 cycles for GAPDH at 94°C for 30 sec, 60°C for 30 sec
and 72°C for 30 sec. The PCR products were separated on 1.5% (w/v)
agarose gel. Data analyses were carried out using a 7500 system
with the SDS software version 1.3.1 (Applied Biosystems Inc.,
Foster City, CA, USA).
Gelatin zymography
The MMP activity was measured using gelatin
zymography. Cells (5×105) were seeded in a 6-well plate
and incubated for 24 h. Then, cells were treated with I3C and ICZ
(0, 1, 2.5, 5 or 10 μM) and conditioned medium was collected. Equal
volumes of conditioned medium were separated by electrophoresis on
10% SDS-polyacrylamide gel containing 0.1% gelatin. Following
electrophoresis, the gels were washed with 2.5% Triton X-100 to
remove SDS and were then incubated in developing buffer (50 mM
Tris-HCl, 10 mM CaCl2, 150 mM NaCl, 0.02%
NaN3, pH 7.5) at 37°C for 18 h. Gels were stained with
0.25% Coomassie Brilliant Blue R-250 (Pierce Biotechnology,
Rockford, IL, USA) and de-stained. Gelatinase activity was
visualized as clear bands.
Statistical analyses
The experimental results are presented as the means
± standard deviation (SD). The significance of treatment effects
was assessed using Tukey's multiple range tests following one-way
ANOVA using the SAS software (SAS Institute Inc., Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell viability
The cytotoxicity of I3C and ICZ on breast cancer
cells was measured using an MTT assay. As shown in Fig. 2, I3C and ICZ did not affect
viability of MCF-7 and MDA-MB231 breast cancer cells at
concentrations ranging from 1 to 10 μM.
Effect of I3C and ICZ on migration
To examine the effect of I3C and ICZ on human breast
cancer cell migration, a wound-healing assay was performed. As
shown in Fig. 3A, I3C and ICZ at 5
and 10 μM effectively inhibited the MCF-7 cell migration after 48 h
in a dose-dependent manner compared to untreated control cells.
However, I3C and ICZ only slightly inhibited migration in MDA-MB231
(Fig. 3B). Thus, the subsequent
experiments were performed using MCF-7 cells.
Effect of I3C and ICZ on mRNA expression
of E-cadherin, vimentin and FAK
Aggressive breast cancers exhibit phenotypic
characteristics of EMT (8).
Therefore, to determine whether or not I3C and ICZ inhibit the EMT
process, mRNA expression of E-cadherin and vimentin was examined.
As shown in Fig. 4, E-cadherin
mRNA expression significantly increased (1.42- and 1.78-fold) with
10 μM I3C and ICZ, respectively, compared to untreated control
cells. In addition, vimentin mRNA expression dramatically decreased
(by 52 and 45%) at 10 μM I3C and ICZ, respectively, compared to
untreated control cells. FAK has been known to be involved in cell
interactions with ECM proteins for migration and enhancement of
cell spreading through tyrosine phosphorylation (17). In this study, to determine whether
FAK is a target of I3C and ICZ in the inhibition of breast cancer
cell migration, mRNA expression of FAK was measured using RT-PCR.
Using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the
internal control, the mRNA expression of FAK was dose-dependently
reduced by 44 and 34%, in 10 μM I3C- and ICZ-treated cells,
respectively.
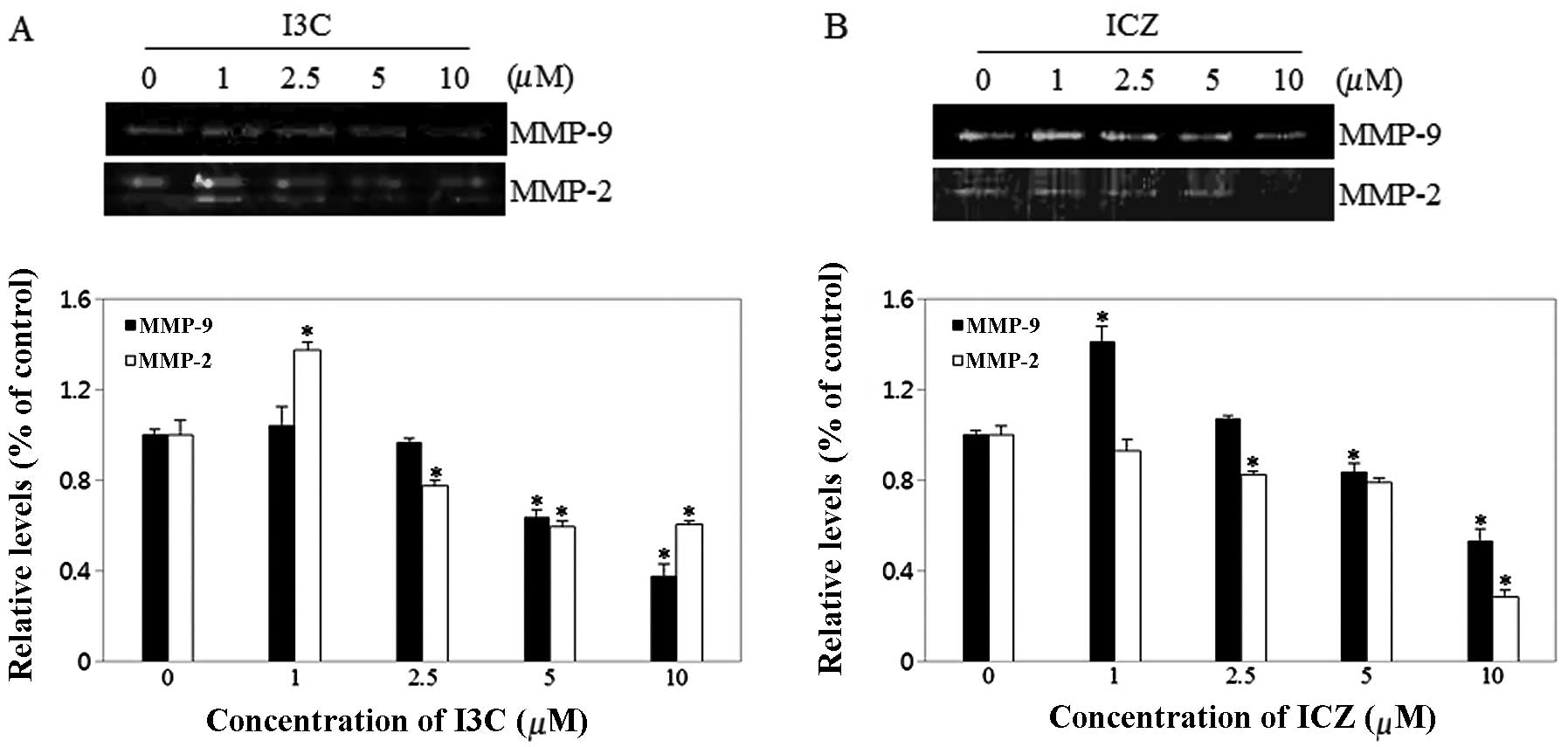
Effect of I3C and ICZ on MMP
activity
Cell migration may be promoted by overexpression of
MMPs (11). Therefore, MMP-2 and
-9 activity was measured using zymography following treatment with
various concentrations of I3C and ICZ. As shown in Fig. 5, MMP-2 and -9 activity of MCF-7
cells was noticeably downregulated by I3C and ICZ in a
dose-dependent manner. In particular, I3C levels of 10 μM inhibited
MMP-9 and -2 activity by 63 and 40%, respectively, compared to
untreated control cells. ICZ levels of 10 μM significantly
suppressed MMP-9 and -2 activity by 47 and 72%, respectively,
compared to untreated control cells.
Discussion
Cell migration is a highly integrated multistep
process including detachment, translocation and protrusion. It is
involved in various biological processes, such as embryogenesis,
cancer and chronic inflammatory diseases (21,22).
Recently, studies of breast and other types of cancers have
suggested that EMT may be a potential mechanism by which epithelial
tumor cells acquire a more motile and invasive phenotype and the
ability to escape from the primary tumor (23,24).
In other words, EMT has been implicated in cancer migration,
invasion and metastasis and therefore may also represent a major
mechanism of tumor progression (5,24).
EMT is characterized by a downregulation of E-cadherin with the
concomitant acquisition of vimentin and secretion of MMPs (25). In particular, E-cadherin is
regarded as a ‘master’ controller of the epithelial/mesenchymal
phenotype switch due to its repression being sufficient to induce
and to complete EMT, and its re-activation potentially resulting in
the reverse process, mesenchymal-epithelial transition (MET)
(23). In this study, I3C and ICZ
significantly increased E-cadherin mRNA expression in MCF-7 cells.
In their study, Meng et al(17) demonstrated that similar effects of
I3C were observed in other breast cancer cell lines, such as T-47D
and MDA-MB468. In addition, I3C and ICZ significantly attenuated
mesenchymal markers, such as vimentin. Thus, our findings
demonstrate that I3C and ICZ inhibit cell migration through
suppression of EMT.
MMPs (a family of zinc-dependent endopeptidases) are
highly involved in the degradation and reconstruction of ECM
(26,27). The misregulation of MMPs is
widespread in several pathological settings and especially in
cancer, whereby MMP overexpression contributes to tumorigenesis and
tumor progression through multiple mechanisms (28). In particular, MMP-1, -2 and -9 are
highly expressed in breast carcinoma cell lines, and studies
suggest that MMPs play a critical role in breast cancer invasion,
metastasis and tumor angiogenesis (14). Moreover, cancer cells that undergo
EMT may produce more MMPs, and elevated levels of MMPs may directly
induce EMT with enhanced invasion and metastasis (28,29).
Therefore, we analyzed MMP-2 and -9 activity and revealed decreases
in MMP-2 and -9 activity associated with I3C and ICZ treatment.
This finding is consistent with other reports that showed that I3C
inhibited MMP-2 expression (27).
The present study showed that downregulation of MMP-2 and -9
activity, a reduction of EMT and consequential inhibition of
migration in breast cancer cells are associated with I3C and ICZ
treatment.
However, increased levels of FAK play a crucial role
in various biological processes such as embryonic development, cell
adhesion, survival, differentiation, migration, invasion and
angiogenesis (2,30,31).
FAK-/- fibroblasts derived from FAK knockdown mouse embryos yield a
significant decrease in cell migration compared to the cells from
wild-type mice (12,32). Moreover, recent studies suggest
that FAK may be a critical mediator in TGF-β-induced EMT in
hepatocytes and renal tubular epithelial cells (33,34).
According to Deng et al(34), FAK may induce the loss of
E-cadherin and the decrease in MMP-9 secretion (34). In the present study, subsequent to
treatment with ICZ and ICZ, FAK mRNA expression was significantly
decreased in a dose-dependent manner. These data demonstrate that
I3C and ICZ significantly inhibit the EMT process and cell
migration through reduction of mRNA expression of FAK in MCF-7
cells. Previous studies report that I3C and ICZ exhibit anticancer
activity in various cancer cell lines (17,27,35,36).
However, to the best of our knowledge, this study is the first to
indicate that I3C and ICZ inhibit migration through suppression of
the EMT process and FAK expression in MCF-7 breast cancer
cells.
In conclusion, treatment with I3C and ICZ
significantly inhibits the migration of breast cancer cells by
suppression of the EMT process and by reduction of MMP-2 and -9
activity through repression of FAK mRNA expression. Our findings
suggest that I3C and ICZ are potential target compounds for the
inhibition of cancer cell migration.
Acknowledgements
The present study was supported by a grant from the
Kyung Hee University in 2009 (no. KHU-20100157).
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo M and Guan JL: Focal adhesion kinase:
a prominent determinant in breast cancer initiation, progression
and metastasis. Cancer Lett. 289:127–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu YL, Wu LY, Hou MF, Tsai EM, Lee JN,
Liang HL, Jong YJ, Hung CH and Kuo PL: Glabridin, an isoflavan from
licorice root, inhibits migration, invasion and angiogenesis of
MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal
adhesion kinase/Rho signaling pathway. Mol Nutr Food Res.
55:318–327. 2011. View Article : Google Scholar
|
|
4
|
Park SK, Kim Y, Kang D, Jung EJ and Yoo
KY: Risk factors and control strategies for the rapidly rising rate
of breast cancer in Korea. J Breast Cancer. 14:79–87. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hay ED: The mesenchymal cell, its role in
the embryo, and the remarkable signaling mechanisms that create it.
Dev Dyn. 233:706–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarkar FH, Li Y, Wang Z and Kong D:
Pancreatic cancer stem cells and EMT in drug resistance and
metastasis. Minerva Chir. 64:489–500. 2009.PubMed/NCBI
|
|
8
|
Hardy KM, Booth BW, Hendrix MJ, Salomon DS
and Strizzi L: ErbB/EGF signaling and EMT in mammary development
and breast cancer. J Mammary Gland Biol Neoplasia. 15:191–199.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Foroni C, Massimo B, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stetler-Stevenson WG, Aznavoorian S and
Liotta LA: Tumor cell interactions with the extracellular matrix
during invasion and metastasis. Annu Rev Cell Biol. 9:541–573.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nabeshima K, Inoue T, Shimao Y and
Sameshima T: Matrix metalloproteinases in tumor invasion: role for
cell migration. Pathol Int. 52:255–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Nimwegen MJ and van de Water B: Focal
adhesion kinase: a potential target in cancer therapy. Biochem
Pharmacol. 73:597–609. 2007.PubMed/NCBI
|
|
14
|
Cortes-Reynosa P, Robledo T, Macias-Silva
M, Wu SV and Salazar EP: Src kinase regulates metalloproteinase-9
secretion induced by type IV collagen in MCF-7 human breast cancer
cells. Matrix Biol. 27:220–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen PN, Chu SC, Kuo WH, Chou MY, Lin JK
and Hsieh YS: Epigallocatechin-3 gallate inhibits invasion,
epithelial-mesenchymal transition, and tumor growth in oral cancer
cells. Agric Food Chem. 59:3836–3844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vergara D, Valente CM, Tinelli A,
Siciliano C, Lorusso V, Acierno R, Giovinazzo G, Santino A,
Storelli C and Maffia M: Resveratrol inhibits the epidermal growth
factor-induced epithelial mesenchymal transition in MCF-7 cells.
Cancer Lett. 310:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng Q, Goldberg ID, Rosen EM and Fan S:
Inhibitory effects of Indole-3-carbinol on invasion and migration
in human breast cancer cells. Breast Cancer Res Treat. 63:147–152.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anderton MJ, Manson MM, Verschoyle RD,
Gescher A, Lamb JH, Farmer PB, Steward WP and Williams ML:
Pharmacokinetics and tissue disposition of indole-3-carbinol and
its acid condensation products after oral administration to mice.
Clin Cancer Res. 10:5233–5241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Wormke M, Safe SH and Bjeldanes LF:
Indolo[3,2-b]carbazole: a dietary-derived factor that exhibits both
antiestrogenic and estrogenic activity. J Natl Cancer Inst.
86:1758–1765. 1994.
|
|
20
|
Chen YH, Dai HJ and Chang HP: Suppression
of inducible nitric oxide production by indole and isothiocyanate
derivatives from Brassica plants in stimulated macrophages.
Planta Med. 69:696–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lauffenburger DA and Horwitz AF: Cell
migration: a physically integrated molecular process. Cell.
84:359–369. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li T, Li YG and Pu DM: Matrix
metalloproteinase-2 and -9 expression correlated with angiogenesis
in human adenomyosis. Gynecol Obstet Invest. 62:229–235. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hung WC and Chang HC: Indole-3-carbinol
inhibits Sp1-induced matrix metalloproteinase-2 expression to
attenuate migration and invasion of breast cancer cells. J Agric
Food Chem. 57:76–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesecnchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CY, Tsai PH, Kandaswami CC, Lee PP,
Huang CJ, Hwang JJ and Lee MT: Matrix metalloproteinase-9
cooperates with transcription factor Snail to induce
epithelial-mesenchymal transition. Cancer Sci. 102:815–827. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cary LA, Chang JF and Guan JL: Stimulation
of cell migration by overexpression of focal adhesion kinase and
its association with Src and Fyn. J Cell Sci. 109:1787–1794.
1996.PubMed/NCBI
|
|
31
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Deliv Rev. 63:610–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ilic D, Furuta Y, Kanazawa S, Takeda N,
Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M and Yamamoto T:
Reduced cell motility and enhanced focal adhesion contact formation
in cells from FAK-deficient mice. Nature. 377:539–544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cicchini C, Laudadio I, Citarella F,
Carazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L and
Tripodi M: TGFbeta-induced EMT requires focal adhesion kinase (FAK)
signaling. Exp Cell Res. 314:143–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng B, Yang X, Liu J, He F, Zhu Z and
Zhang C: Focal adhesion kinase mediates TGF-beta1-induced renal
tubular epithelial-to-mesenchymal transition in vitro. Mol Cell
Biochem. 340:21–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonnesen C, Eggleston IM and Hayes JD:
Dietary indoles and isothiocyanates that are generated from
cruciferous vegetables can both stimulate apoptosis and confer
protection against DNA damage in human colon cell lines. Cancer
Res. 61:6120–6130. 2001.PubMed/NCBI
|
|
36
|
Bradlow HL: Indole-3-carbinol as a
chemoprotective agent in breast and prostate cancer. In Vivo.
22:441–445. 2008.PubMed/NCBI
|