Introduction
The etiology of prostate cancer is largely unknown,
with the exception of a few known risk factors such as advancing
age, intact-tight androgen metabolism, ethnicity and genetic
background (1,2). For the maintenance of prostate
growth, the complex equilibrium between cell growth, proliferation
and apoptosis-inducing factors is essential and fluctuations in
this balance may lead to tumorigenesis and cancer. In prostate
cancer progression, loss or suppression of apoptosis has been
heavily implicated. Apoptosis induction is considered an effective
therapeutic approach for the treatment of prostate tumors (3).
Investigations into the role of cell cycle
regulatory molecules in the induction of apoptosis in cancer have
provided a better understanding of the biology of cancer cells.
Cell cycle control is crucial for normal cell growth and
differentiation and is regulated by cyclin-dependent kinases
(CDKs). Activity of CDKs is suppressed by CDK inhibitors. A
universal inhibitor of cyclin-dependent kinases (CDK2, CDK3, CDK4,
and CDK6) is p21, also known as CDKN1A, WAF1, CAP20, Cip1 and Sdi1
(4). Cell cycle inhibitory effects
of p21 may be attributed, not only to its ability to bind CDKs, but
also to its binding to proliferating cell nuclear antigen (PCNA),
resulting in inhibition of cell cycle progression in either G1 and
G2, or in the S-phase (5). This
protein also has other important functions including the regulation
of apoptosis. When cells are exposed to genotoxic agents, p53
protein is activated via phosphorylation and binds to
p53-responsive elements of the p21 gene (6). p21 was initially discovered as a
p53-target gene, but has since been suggested to play a role as a
downstream effector of other tumor suppressors, including BRCA1,
TGF-β and Wnt-1 (5,7). Although activation of p21 is crucial
in mediating p53-dependent cell growth arrest, it is not essential
for p53-mediated apoptosis, as suggested by the fact that p53 is
able to induce apoptosis in cells lacking p21 (8). p21 has a dual role, and in addition,
can assume both pro- or anti-apoptotic functions in response to
anti-tumor agents, depending on the cell type and context (5,9).
The gene for p21 is localized on chromosome 6p21.2.
It consists of three exons and two introns and encodes a 21-kDa
protein. The translation region lies mainly in exon 2 (10). Mutations or single nucleotide
polymorphisms (SNPs) in the p21 gene may result in
alteration of p21 expression and/or activity, thereby modulating
susceptibility to cancer (11–13).
A total of 40 SNPs have been identified in the p21 gene and
their frequency depends on ethnicity and geography. From these
SNPs, 35 are intronic, 7 have an allele frequency >10% (~1.3 kb
upstream of exon 2) and 4 are detected within the 3′ untranslated
region (3′UTR). The most commonly studied polymorphism of
p21 is Ser31Arg. A substitution of C to A in the third base
of codon 31 of p21 (p21C98A, rs1801270) results in a serine
to arginine amino acid substitution in the DNA-binding zinc finger
motif of the protein (14). Only
one of the SNPs within the 3′UTR, p21 C70T (rs1059234), has
an allele frequency >10% and causes a single C to T substitution
20 nucleotides downstream of the stop codon at exon 3. Therefore,
p21 C98A and p21 C70T are thought to alter p21
function (15).
These p21 SNPs have been associated with a
range of human cancers in various populations (14,16–20).
We have found four other studies (21–24)
in connection with prostate cancer and p21 genetic
variation, but only one of these studies has focused on the p21
C70T polymorphism and prostate cancer (24).
The present pilot study is therefore the first to
investigate the correlation between DNA variants within the 3′UTR
of the p21 gene (p21 C70T) and susceptibility to
prostate cancer within the Slovak population. The study also
attempted to establish a potential association of these gene
variations with smoking status and tumor-specific
clinicopathological characteristics or serum prostate-specific
antigen (PSA) levels and Gleason score.
Materials and methods
Study subjects and samples
This study included 118 newly diagnosed prostate
cancer patients and 130 healthy controls under the age of 50.
Patients were recruited between May 2009 and December 2011 at the
Department of Urology when undergoing regular prostate cancer
screening. Final diagnoses of cases were confirmed by routine
histopathological examination. Control subjects were cancer-free
individuals randomly selected from a cancer screening program for
early detection of prostate cancer conducted in the same region
during the period when the case patients were recruited. The
selection criteria for the controls included absence of individual
history of cancer, and controls were frequency matched to case
patients based on age. Cases and controls were interviewed with
regard to age, smoking status (i.e., habitual smokers and
never-smokers), previous and/or current prostate disease, and
history of incidence of cancer and other chronic diseases. The
studied population is described in Table I. The present study was approved by
the Ethics Board of Jessenius Faculty of Medicine, Comenius
University, and written informed consent was obtained from all
individuals prior to inclusion in the study.
 | Table IPrincipal characteristics of the
control and prostate cancer patient groups. |
Table I
Principal characteristics of the
control and prostate cancer patient groups.
|
Characteristics | Controls (n=130) n
(%) | Cases (n=118) n
(%) |
|---|
| Age in years
(median, range) | 59 (50–78) | 66 (53–84) |
| Smoking status | | |
| Never smokers | 102 (78.5) | 83 (70) |
| Smokers | 28 (21.5) | 35 (30) |
| Serum PSA at
diagnosis | | |
| <10 ng/ml | 130 (100) | 53 (45) |
| ≥10 ng/ml | 0 | 45 (38) |
| Unknown | 0 | 20 (17) |
| Gleason score
(grade 1 + 2) | | |
| <7 | N/A | 33 (28) |
| ≥7 | N/A | 85 (72) |
DNA isolation
Peripheral venous blood was collected in 2 ml
heparinized tubes and the specimens were immediately stored at
−20°C for genotyping. In cases and controls, genomic DNA was
isolated from peripheral leukocytes by proteinase K digestion,
phenol/chloroform extraction and ethanol precipitation before it
was dissolved in TE buffer (pH 7.5) and stored at −20°C until
genotype analysis.
PCR assay for p21 C70T genotyping
The p21 C70T polymorphism was determined by
PCR-RFLP as described by Taghavi et al(16). Briefly, the amplification reactions
were carried out in a 25 μl volume consisting of 100 ng genomic
DNA, 25 pmol of each primer (forward: 5′-CCCAGGGAAGGGTGTCCTG-3′ and
reverse: 5′-GGGCGGCCAGGGTATGTAC-3′), 200 μmol/l deoxynucleoside
triphosphates and 1 unit of Taq polymerase in 10X PCR buffer
composed of 16.6 mmol/l (NH4)2SO4
and 20.0 mmol/l MgCl2, pH 8.8. The cycling conditions
were 94°C for 5 min, followed by 33 cycles at 95°C for 30 sec,
61.5°C for 30 sec, and 72°C for 30 sec with a final cycle at 72°C
for 5 min. A 10 μl aliquot of the appropriate PCR product was
digested with 2 units of the restriction enzyme PstI
(Fermentas Co., Denmark) at 37°C for 30 min and separated on
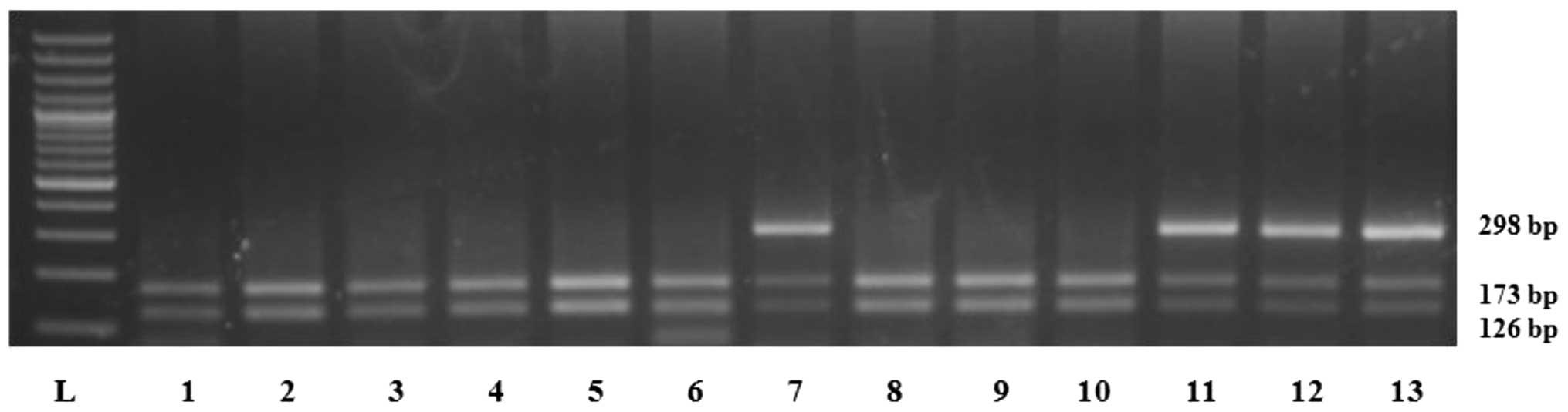
ethidium bromide-stained 3% agarose gel. The intact PstI
site (in the wild-type allele) generated two 126 and 173 bp
fragments. The loss of the PstI site (C to T polymorphism)
yielded a 298 bp fragment (Fig.
1). Genotypes were verified by repeating PCR-RFLP on 40 random
samples.
Statistical analysis
Genotype frequencies for the p21 C70T
polymorphism were calculated for the prostate cancer patient and
healthy control groups. Comparisons of genotype distribution and
its association with prostate cancer susceptibility, smoking status
and clinical data were performed using the Chi-square test with all
odds ratio (OR) values being adjusted for age, which is considered
to be a confounder. Statistical analysis was performed using
StatsDirect statistical package version 2.7.8 (http://www.statsdirect.com). Quantitative variables
including age and smoking status were compared by the Student's
t-test. All P-values cited were two-sided and P<0.05 was
considered statistically significant.
Results
General characteristics of cases and
controls
The frequency distribution of the general
characteristics of the 118 prostate cancer patients and 130
controls is shown in Table I. A
significant difference was observed between cases and controls in
age and serum PSA levels at the time of diagnosis (p<0.001). In
the group of 118 patients, 33 patients (28%) had a Gleason score
<7 and the remaining 85 patients (72%) had a Gleason score ≥7.
More smokers were observed among cases than among controls (30 vs.
21.5%).
Genotype frequencies of p21 C70T
polymorphism
The genotype frequencies of the p21 C70T
polymorphism are shown in Table
II. The distribution of p21 C70T genotypes in either
group was in agreement with the Hardy-Weinberg equilibrium
(p>0.05; data not shown). The CC and CT genotype
frequencies were 88 and 12% among cases and 83 and 17% among
controls, respectively. No TT genotype was observed in this
polymorphism. The p21 CT genotype was associated with
decreased risk for the development of prostate cancer (OR=0.66; 95%
CI, 0.32–1.36; p>0.05) compared with the CC genotype.
 | Table IIDistribution of the p21 C70T
polymorphism. |
Table II
Distribution of the p21 C70T
polymorphism.
| Genotypes | Controls n (%) | Cases n (%) | OR (95% CI) | P-value |
|---|
| CC | 108 (83) | 104 (88) | 1.00 (ref.) | - |
| CT | 22 (17) | 14 (12) | 0.66
(0.32–1.36) | NS |
Smoking status in cases and controls
The smoking status (never and ever smokers) in cases
and controls was also analyzed with respect to its association with
genotypes (Table III). Smokers
with the p21 C70T common genotype (CC) or
heterozygous mutant (CT) genotype were positively associated
with prostate cancer risk (OR=1.48; 95% CI, 0.80–2.76 and OR=1.15;
95% CI, 0.27–4.77, respectively; p>0.05). The presence of the
CT genotype in never-smokers was associated with decreased
prostate cancer risk (OR=0.64; 95% CI, 0.27–1.47).
 | Table IIIRisk of prostate cancer associated
with p21 C70T genotypes and cigarette smoking in cases and
controls. |
Table III
Risk of prostate cancer associated
with p21 C70T genotypes and cigarette smoking in cases and
controls.
| Genotype |
Never-smokersa | Smokersa |
|---|
| CC | 73/84 | 31/24 |
| OR (95% CI) | 1.00 (ref.) | 1.48
(0.80–2.76) |
| CT | 10/18 | 4/4 |
| OR (95% CI) | 0.64
(0.27–1.47) | 1.15
(0.27–4.77) |
Serum PSA levels and Gleason score in
prostate cancer patients
The association of the p21 C70T genotypes
with serum PSA levels and Gleason score in 118 prostate cancer
patients was analyzed (Table IV).
Cases with serum PSA levels ≥10 ng/ml and the CT genotype
tended to have significantly decreased prostate cancer risk
(OR=0.16; 95% CI, 0.03–0.75; p>0.05) in comparison to cases with
serum PSA levels <10 ng/ml and the CC genotype. The OR
for high-grade (Gleason score ≥7) versus low-grade (Gleason score
<7) disease was 1.48 (95% CI 0.39–5.71) for the CT
genotype relative to the CC genotype.
 | Table IVAssociation of p21 C70T
polymorphism with serum PSA and Gleason score in prostate cancer
patients (n=118). |
Table IV
Association of p21 C70T
polymorphism with serum PSA and Gleason score in prostate cancer
patients (n=118).
| p21
C70T |
|---|
|
|
|---|
| CC n
(%) | CT n
(%) |
|---|
| Total prostate
cancer, n (%) | 104 (88) | 14 (12) |
| Serum PSA at
diagnosis |
| <10 ng/ml | 41 (77.4) | 12 (22.6) |
| ≥10 ng/ml | 43 (95.5) | 2 (4.5) |
| OR (95% CI) | 1.00 | 0.16
(0.03–0.75) |
| P-value | - | 0.02 |
| Gleason score |
| <7 | 30 (91) | 3 (9) |
| ≥7 | 74 (87) | 11 (13) |
| OR (95% CI) | 1.00 | 1.48
(0.39–5.71) |
| P-value | - | NS |
Discussion
Cell cycle deregulation is common in human cancer,
and alteration of p21, the critical cell cycle regulator, is
involved in the development of a number of human malignancies
(9,17). The p21 C70T polymorphism is
thought to cause a functional change in p21, and as this
polymorphism lies in a crucial region for cell differentiation,
proliferation may increase cancer risk by altering messenger RNA
stability, which, in turn, may affect protein expression and
activity (25,26). Certain studies suggest that this
polymorphism has a functional significance and most likely
contributes to genetic susceptibility to several types of cancer
(15,25). However, other studies have also
demonstrated no association between polymorphisms and cancer risk
(16,27). A possible explanation for the
discrepancies between these studies may be due to non-random
sampling, limited sample size, different molecular mechanisms
involved in carcinogenesis, ethnic disparity and/or linkage
disequilibrium of this polymorphism with other functional variants
at the susceptibility loci.
Our results suggest that the p21 C70T
polymorphism may not be relevant as a risk factor for prostate
cancer development due to a non-significant protective effect of
the p21 CT polymorphic variant on prostate cancer risk. The
prevalence rate of CC homozygosity and CT
heterozygosity was 83 and 17%, respectively, in our Caucasian
control subjects. These frequencies are similar to the frequencies
observed in other studies on p21 C70T polymorphism in
identical populations (15,24).
Only one case-control study by Kibel et al
evaluated the association of the p21 C70T polymorphism with
the risk of advanced prostate carcinoma in a European-American
population (24). The authors of
that study demonstrated that polymorphic genotypes CT and
TT were associated with an increased risk of advanced
prostate carcinoma compared with the CC genotype (OR=2.24;
95% CI, 1.02–4.95) and these genotypes were more strongly
associated with more aggressive metastatic disease
(androgen-independent disease or mortality from metastatic prostate
carcinoma) (OR=2.88; 95% CI, 1.19–6.97).
To the best of our knowledge, this is the first
study to examine the association of the p21 C70T
polymorphism, prostate cancer risk and smoking status. It is well
known that differences in genetic background may not only affect
the susceptibility of individuals to cancer, but may also modify
the effects of environmental carcinogens (16,28).
In the present study, we have shown that smokers with the
p21 homozygote variant genotype (CC) and heterozygous
mutant (CT) genotype exhibited higher prostate cancer risk,
while non-smokers with the CT genotype had a reduced risk.
The finding of a greater risk in ever-smokers also suggests that
the p21 variant genotypes has an impact on cell cycle
control induced by DNA damage caused by carcinogens in tobacco
smoke (29). We hypothesized that
the balance between cell cycle arrest and apoptosis may be shifted
to cell cycle arrest and lead to more mutations and genomic
instability in individuals heavily exposed to carcinogens, as cells
with incompletely repaired genomes may not be removed by
apoptosis.
Results of previous studies have demonstrated that
p21 protein expression in prostate cancer is associated with poor
clinical outcome following treatment for localized disease, such as
radical prostatectomy or radiation therapy (30,31).
The immunohistochemical study of Omar et al showed that
expression of the p21 protein did not correlate with patient age at
diagnosis, pretreatment serum PSA, histologic grade and clinical
stage (32). However, to the best
of our knowledge, no study has previously evaluated the effect of
the p21 C70T polymorphism on the serum PSA levels and
Gleason score in prostate cancer patients. Compared to patients
with the CC genotype who had serum PSA levels <10 ng/ml,
patients who were CT genotype carriers and had serum PSA
levels ≥10 ng/ml had a significant 0.16-fold decreased prostate
cancer risk. Patients with a Gleason score >7 and the variant
genotype (CT) did not show a significantly higher risk
compared to patients who carried wild-type genotype (CC) and
had a Gleason score <7. These findings suggest that the p21
CT genotype may be a protective factor for prostate cancer
development, particularly for patients with a serum PSA level
>10 ng/ml. The precise manner in which this polymorphism affects
serum PSA levels and prostate cancer development, however, may
require more detailed in vitro and in vivo
studies.
It has been shown that the p21 C70T
polymorphism always occurs together with a more common p21
polymorphism on codon 31 (p21 C98A) (15), however, no studies are currently
available in connection with prostate cancer. There are three
studies that have shown an association between the p21 C98A
polymorphism and prostate cancer development in various populations
(21–23). Liu et al performed a
meta-analysis of 33,120 cancer cases and 44,954 controls from 49
publications with 66 individual case-control studies for this
polymorphism (14). The authors of
that study demonstrated that the p21 31Arg allele is a
low-penetrant risk factor for cancer development, particularly
among Caucasians. When stratifying by cancer types, significantly
increased risks were observed for breast cancer and ‘other cancers’
among Caucasians and significantly decreased risks were observed
for oesophageal and gastric cancer among Asians.
Therefore, we conclude the following: a) there was a
decreased risk of prostate cancer with the p21 CT genotype
and a protective effect of the T allele; b) smokers carrying the
CC and CT genotypes were at an increased risk of
prostate cancer compared to non-smokers with the CC
genotype; c) a significantly decreased prostate cancer risk was
observed in men with serum PSA levels ≥10 ng/ml and p21 CT
genotype; and d) in a case analysis according to Gleason score, no
significant trend in OR from the CT towards the CC
genotype between low-grade versus high-grade disease was detected.
A complete interpretation of our results is limited at present, as
we concentrated on only one functionally active p21
polymorphism. Due to the complexity with multiple genetic and
environmental factors in the development of prostate cancer, large
population studies are required that are able to take into account
all variables including association with other polymorphisms in
genes for DNA repair, cell cycle control and apoptosis systems,
exposure to environmental factors, ethnic and demographic features.
With this in mind, future studies are likely to concentrate on
larger population sizes in order to validate any possible
interactions and to be able to understand likely interactions and
their relevance for prostate cancer development. Such studies
should help to elucidate any impact and interaction of this
polymorphism with other polymorphisms in the p21 gene and
cell cycle regulatory genes (p53, p27) with overall respect
to prostate cancer risk assessment.
Acknowledgements
This study was supported by the Ministry of Health
of the Slovak Republic under the projects 2007/45-UK-10 ‘Genetic
polymorphism of xenobiotic metabolising enzymes and susceptibility
to prostate cancer in the Slovak population’, MH SR 2007/57-UK-17
and by VEGA grant 1/426207. The study was also supported by the
project ‘Center of Excellence for Research on Personalized Therapy
(CEVYPET)’, code 2622012053, co-financed from EU sources and
European Regional Development Fund. The authors are grateful to Dr
N.A. Yeboah for critical reading of the manuscript and wish to
thank Mrs. M. Martinčeková for her technical assistance.
References
|
1
|
Grönberg H: Prostate cancer epidemiology.
Lancet. 361:859–864. 2003.PubMed/NCBI
|
|
2
|
Sivonova MK, Dobrota D, Matakova T, et al:
Microsomal epoxide hydrolase polymorphisms, cigarette smoking and
prostate cancer risk in the Slovak population. Neoplasma. 59:79–84.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan N, Adhami VM and Mukhtar H: Apoptosis
by dietary agents for prevention and treatment of prostate cancer.
Endocr Relat Cancer. 17:39–52. 2010. View Article : Google Scholar
|
|
4
|
Gartel AL and Tyner AL: The role of the
cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer
Ther. 1:639–649. 2002.PubMed/NCBI
|
|
5
|
Liu S, Bishop WR and Liu M: Differential
effects of cell cycle regulatory protein p21(WAF1/Cip1) on
apoptosis and sensitivity to cancer chemotherapy. Drug Resist
Updat. 6:183–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suzuki H, Ito R, Ikeda K and Tamura TA:
TATA-binding protein (TBP)-like protein is required for
p53-dependent transcriptional activation of an upstream promoter of
the p21Waf1/Cip1 gene. J Biol Chem. 282:19792–19803. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Englert C, Maheswaran S, Garvin AJ,
Kreidberg J and Haber DA: Induction of p21 by the Wilms' tumor
suppressor gene WT1. Cancer Res. 57:1429–1434. 1997.
|
|
8
|
Deng C, Zhang P, Harper JW, Elledge SJ and
Leder P: Mice lacking p21CIP1/WAF1 undergo normal development, but
are defective in G1 checkpoint control. Cell. 82:675–684. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gartel AL: The conflicting roles of the
cdk inhibitor p21(CIP1/WAF1) in apoptosis. Leuk Res. 29:1237–1238.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Deiry WS, Tokino T, Velculescu VE, et
al: WAF1, a potential mediator of p53 tumor suppression. Cell.
75:817–825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma H, Zhou Z, Wei S and Wei Q: Association
between p21 Ser31Arg polymorphism and cancer risk: a meta-analysis.
Chin J Cancer. 30:254–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gravina S, Lescai F, Hurteau G, et al:
Identification of single nucleotide polymorphisms in the p21
(CDKN1A) gene and correlations with longevity in the Italian
population. Aging (Albany NY). 1:470–480. 2009.PubMed/NCBI
|
|
13
|
Keshava C, Frye BL, Wolff MS, McCanlies EC
and Weston A: Waf-1 (p21) and p53 polymorphisms in breast cancer.
Cancer Epidemiol Biomarkers Prev. 11:127–130. 2002.PubMed/NCBI
|
|
14
|
Liu F, Li B, Wei Y, et al: P21 codon 31
polymorphism associated with cancer among white people: evidence
from a meta-analysis involving 78,074 subjects. Mutagenesis.
26:513–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li G, Liu Z, Sturgis EM, et al: Genetic
polymorphisms of p21 are associated with risk of squamous cell
carcinoma of the head and neck. Carcinogenesis. 26:1596–1602. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taghavi N, Biramijamal F, Abbaszadegan MR,
Khademi H, Sotoudeh M and Khoshbakht S: P21(waf1/cip1) gene
polymorphisms and possible interaction with cigarette smoking in
esophageal squamous cell carcinoma in northeastern Iran: a
preliminary study. Arch Iran Med. 13:235–242. 2010.PubMed/NCBI
|
|
17
|
Lin G, Fang F, Yu XJ and Yu L:
Meta-analysis of the relationship between p21 Ser31Arg polymorphism
and lung cancer susceptibility. Genet Mol Res. 10:2449–2456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shirai O, Ohmiya N, Taguchi A, et al: P53,
p21, and p73 gene polymorphisms in gastric carcinoma.
Hepatogastroenterology. 57:1595–1601. 2010.PubMed/NCBI
|
|
19
|
Ebner F, Schremmer-Danninger E and Rehbock
J: The role of TP53 and p21 gene polymorphisms in breast cancer
biology in a well specified and characterized German cohort. J
Cancer Res Clin Oncol. 136:1369–1375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang W, Qi Q, Zhang H, et al: p21
Waf1/Cip1 polymorphisms and risk of esophageal cancer. Ann Surg
Oncol. 17:1453–1458. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang SP, Wu WJ, Chang WS, et al: p53
Codon 72 and p21 codon 31 polymorphisms in prostate cancer. Cancer
Epidemiol Biomarkers Prev. 13:2217–2224. 2004.PubMed/NCBI
|
|
22
|
Facher EA, Becich MJ, Deka A and Law JC:
Association between human cancer and two polymorphisms occurring
together in the p21Waf1/Cip1 cyclin-dependent kinase inhibitor
gene. Cancer. 79:2424–2429. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao X, Chen YQ, Wu N, et al: Somatic
mutations of the WAF1/CIP1 gene in primary prostate cancer.
Oncogene. 11:1395–1398. 1995.PubMed/NCBI
|
|
24
|
Kibel AS, Suarez BK, Belani J, et al:
CDKN1A and CDKN1B polymorphisms and risk of advanced prostate
carcinoma. Cancer Res. 63:2033–2036. 2003.PubMed/NCBI
|
|
25
|
Wang Z, Sturgis EM, Zhang F, et al:
Genetic variants of p27 and p21 as predictors for risk of second
primary malignancy in patients with index squamous cell carcinoma
of head and neck. Mol Cancer. 11:172012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rastinejad F and Blau HM: Genetic
complementation reveals a novel regulatory role for 3′ untranslated
regions in growth and differentiation. Cell. 72:903–917. 1993.
|
|
27
|
Valentin MD, Canalle R, Queiroz Rde P and
Tone LG: Frequency of polymorphisms and protein expression of
cyclin-dependent kinase inhibitor 1A (CDKN1A) in central nervous
system tumors. Sao Paulo Med J. 127:288–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sivonová M, Waczulíková I, Dobrota D, et
al: Polymorphisms of glutathione-S-transferase M1, T1, P1 and the
risk of prostate cancer: a case-control study. J Exp Clin Cancer
Res. 28:322009.PubMed/NCBI
|
|
29
|
Lei D, Sturgis EM, Liu Z, Zafereo ME, Wei
Q and Li G: Genetic polymorphisms of p21 and risk of second primary
malignancy in patients with index squamous cell carcinoma of the
head and neck. Carcinogenesis. 31:222–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aaltomaa S, Lipponen P, Eskelinen M,
Ala-Opas M and Kosma VM: Prognostic value and expression of
p21(waf1/cip1) protein in prostate cancer. Prostate. 39:8–15. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baretton GB, Klenk U, Diebold J, Schmeller
N and Lohrs U: Proliferation and apoptosis-associated factors in
advanced prostatic carcinomas before and after androgen deprivation
therapy: prognostic significance of p21/WAF1/CIP1 expression. Br J
Cancer. 80:546–555. 1999. View Article : Google Scholar
|
|
32
|
Omar EA, Behlouli H, Chevalier S and
Aprikian AG: Relationship of p21(WAF-I) protein expression with
prognosis in advanced prostate cancer treated by androgen ablation.
Prostate. 49:191–199. 2001. View Article : Google Scholar : PubMed/NCBI
|