Introduction
Every year, 0.3–0.7% of live births worldwide are
affected by congenital limb deformities caused by abnormalities in
soft tissue which lead to varus (inclined inward), adductus
(axially rotated) and equinus (pointed downward) fixation of the
foot. This limb deformity is known as idiopathic congenital talipes
equinovarus (ICTEV) (1–2). Neurological, muscular, bony, vascular
and connective tissue mechanisms have been hypothesized to be
involved in the pathology of ICTEV; however, the specific roles of
these mechanisms in ICTEV remain unclear. Previous studies in twins
and various ethnicities, as well as pedigree analyses, have
indicated that a genetic basis in combination with additional
environmental factors is responsible for ICTEV (2–6). It
is becoming increasingly clear that genetic factors are important
for the pathogenesis of ICTEV. Furthermore, genes associated with
limb development, including homeobox D13 (HOXD13),
collagen, type IX, alpha 1 (COL9A1) and GLI family
zinc finger 3 (GLI3), are significantly associated with
ICTEV (7–9).
We previously performed transmission disequilibrium
test analysis, identifying that the COL9A1 gene on
chromosome 6q12–13 is associated with ICTEV. Increased expression
of COL9A1 was identified in abductor hallucis muscle samples
of ICTEV patients compared with control samples, indicating that
the COL9A1 gene may be an important susceptibility gene for
ICTEV (7). Mutation screening
experiments found no mutations in the exons and the upstream
sequence of the COL9A1 gene in ICTEV patients. ICTEV
patients may exhibit increased expression of COL9A1 but not
with mutations in the exons and the upstream regions of
COL9A1(7). At present, the
cause of the high expression levels of COL9A1 in muscle
samples of ICTEV patients remains unclear.
Specific in vitro and in vivo studies
have revealed that the SRY (sex-determining region
Y)-box 9 (SOX9) transcription factor binds to
consensus sequence pairs in the upstream region of the
COL2A1 and COL9A1 genes and regulates expression
(10–16). SOX9 is a protein of the HMG-box
DNA-binding protein class, which recognize the CCTTGAG sequence.
SOX9 is important for chondrocyte differentiation and the
transcriptional regulation of the Anti-Müllerian Hormone, as well
as steroidogenic factor-1 (17).
Deficiencies in SOX9 result in a skeletal malformation
syndrome (camptomelic dysplasia) and are also frequently associated
with sex reversal (18). In a
previous study, expression of COL9A1 was found to be
regulated by SOX9 in chondrocytes (14). Thus, we hypothesized that there is
a correlation between SOX9 and high expression levels of
COL9A1 in muscle samples of ICTEV patients.
Materials and methods
Patients and normal individuals
Abductor hallucis muscle samples were obtained from
15 ICTEV patients (12 males and 3 females) aged 4–12 years
(average, 6.6 years old). Medial plantar release was performed on
the patients following failure of conservative treatment. Abductor
hallucis muscle control samples were obtained from 2 fresh cadavers
(2 males) and 7 trauma patients (3 males and 4 females) aged 5–11
years (average, 7.5 years old). All fresh samples were stored at
-80°C as soon as possible. Peripheral blood samples were obtained
from 84 ICTEV patients (50 males and 34 females) aged 3–12 years
(average, 6.2 years old). The blood samples were stored at -20°C.
All patients were recruited at the Department of Pediatric
Orthopedic Surgery (Second Affiliated Hospital of China Medical
University, Shenyang, China). Informed consent was obtained from
the parents of the patients and the study was approved by the China
Medical University Ethics Committee. The probands revealed the
typical ICTEV phenotype [varus (inclined inward), adductus (axially
rotated) and equinus (pointed downward) fixation of the foot].
Analysis of SOX9 and COL9A1 expression in
muscle samples by immunofluorescence using confocal laser scanning
microscopy
Muscle samples were embedded in paraffin and fixed
in 4% paraformaldehyde overnight at 4°C. Tissues were sectioned
into 4-μm slices. Specific slides were stained with hematoxylin and
eosin (H&E). Immunofluorescence was performed according to the
manufacturer’s instructions (Wuhan Boster Biological Technology,
Ltd., Wuhan, China). To quench nonspecific binding, sections were
incubated in normal goat serum for 1 h at room temperature.
Sections were then incubated in rabbit anti-human SOX9 polyclonal
and mouse anti-human COL9A1 monoclonal antibodies (both 1:50; Santa
Cruz Biotechnoloy, Inc., Santa Cruz, CA, USA) overnight at room
temperature and washed 3–4 times in 0.01 mol/l PBS. Sections were
hybridized with 4′,6-diamidino-2-phenylindole, FITC-labeled goat
anti-rabbit immunoglobulins and trimethyl rhodamine
isothiocyanate-labeled goat anti-mouse immunoglobulins (all
1:1,000; Wuhan Boster Biological Technology, Ltd., Wuhan, China)
for 1 h at 37°C in the dark and then washed 3–4 times in 0.01 mol/l
PBS. Confocal laser scanning microscopy (CLSM) was used to analyze
protein expression of SOX9 and COL9A1.
Mutational analysis of the SOX9 gene in
blood samples by denaturing gradient gel electrophoresis
(DGGE)
Genomic DNA was extracted from 84 fresh blood
samples obtained from ICTEV patients using a blood DNA extraction
kit (Tiangen Biotech, Beijing, China) according to the
manufacturer’s instructions. Six primer pairs (presented in
Table I) were designed to amplify
SOX9 exons 1–3 and the 5′ flanking sequence. Polymerase
chain reaction (PCR) products of SOX9 were detected by DGGE,
a sensitive method to separate alleles based on differences in
melting behavior (19). DNA was
visualized following ethidium bromide staining by UV
transillumination and images were captured using a Polaroid
camera.
 | Table IPCR primer sequences of
SOX9. |
Table I
PCR primer sequences of
SOX9.
| Region | Direction | Primer sequence
(5′->3′) | Annealing temperature
(°C) | Product size
(bp) |
|---|
| Promoter 1 | F* |
TTACAAACCAAGTGACCGGC | 55 | 495 |
| R |
TGCCTGCAAAAGTGCTTAGA | | |
| Promoter 2 | F* |
TTATTACGGAGGAACAGCGG | 62 | 588 |
| R |
CTTTCGGCTCTCCAACTCC | | |
| Promoter 3 | F* |
GCTCTAAGGTGAGGCGGAGT | 56 | 445 |
| R |
ATGAAGGGGTCCAGGAGATT | | |
| Exon 1 | F* |
GCTGGTTTGAGAGGCAGAAA | 58 | 497 |
| R |
CAACACAGAGAATATGACCCCA | | |
| Exon 2 | F* |
CTTCAGCCATGGACAGTTCC | 58 | 340 |
| R |
CAACTCCCTTCTCTGGCTGT | | |
| Exon 3 | F* |
ACTCCGCCAGAGTGGAGCGT | 58 | 573 |
| R |
TTTACGCGCCTGGAGCGAGC | | |
Analysis of SOX9 mRNA expression in
muscle samples by real-time PCR (RT-PCR)
RT-PCR was performed to evaluate differences in RNA
expression levels of SOX9. RNA was extracted from the muscle
samples using a tissue RNA kit (Tiangen, China) and cDNA was
synthesized using a reverse transcription kit (Promega Corporation,
Madison, WI, USA) according to the manufacturer’s instructions.
RT-PCR amplification was performed in 25-μl reaction volumes with
12.5 μl SYBR Premix Ex Taq, 9.5 μl deionized water, 0.5 μl (initial
concentration, 10 μM) of each primer and 2 μl cDNA according to the
manufacturer’s instructions (Advanced Biotechnologies Inc.,
Columbia, MD, USA). Amplification was performed by pre-denaturation
at 95°C for 10 sec, followed by 40 rounds of denaturation at 95°C
for 5 sec and annealing and extension at 58°C for 20 sec. Primer
sequences were as follows: SOX9, 5′-ACT CGC CAC ACT CCT CCT
C -3′ (forward) and 5′-CCC TCT CGC TTC AGG TCA-3′ (reverse);
β-actin, 5′-CCC AGA GCA AGA GAG GCA-3′ (forward) and 5′-GGG
AGC CAC ACG CAG-3′ (reverse). Data were analyzed using the
2-ΔΔCT method.
Analysis of SOX9 protein expression in
muscle samples by western blot analysis
Western blot analysis was used to evaluate levels of
SOX9 protein expression. Cytoplasmic protein was extracted
from the abductor hallucis muscle samples from ICTEV patients and
controls using a cytoplasmic and nuclear protein extraction kit
(Active Motif, Carlsbad, CA, USA) according to the manufacturer’s
instructions. The protein concentration was determined
spectrophotometrically (Unico, Dayton, NJ, USA) at 280 nm (20). Sample buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) was added to the cytoplasmic protein
samples and the solution was loaded onto a 6% polyacrylamide gel.
Following protein separation, the polyacrylamide gel was
electroblotted onto a PVDF membrane (Millipore, Billerica, MA,
USA). Nonspecific binding sites were blocked with 3% bovine serum
albumin (Sigma, Poole, UK) in TBST buffer [20 mM Tris-buffered
saline, 0.047% Tween (pH 7.4)] overnight at 4°C. The membrane was
incubated for 3 h with rabbit anti-human SOX9 polyclonal antibody
(1:50; Santa Cruz Biotechnoloy, Inc.) at room temperature and
washed for 40 min in TBST. The membrane was then incubated for 2 h
at room temperature with a goat anti-rabbit IgG horseradish
peroxidase-conjugated antibody (1:4,000; Davis, CA, USA). Protein
bands were visualized using modified enhanced chemiluminescence
(Tiangen Biotech, China). Quantification of relative band densities
was performed using standard densitometry scanning techniques.
Statistical analysis
Data are expressed as mean ± SD and analyzed with
SPSS (v13.0, SPSS, Inc., Chicago, IL, USA). Differences between two
groups were analyzed using the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Correlation was estimated using the Spearman’s rank method.
Results
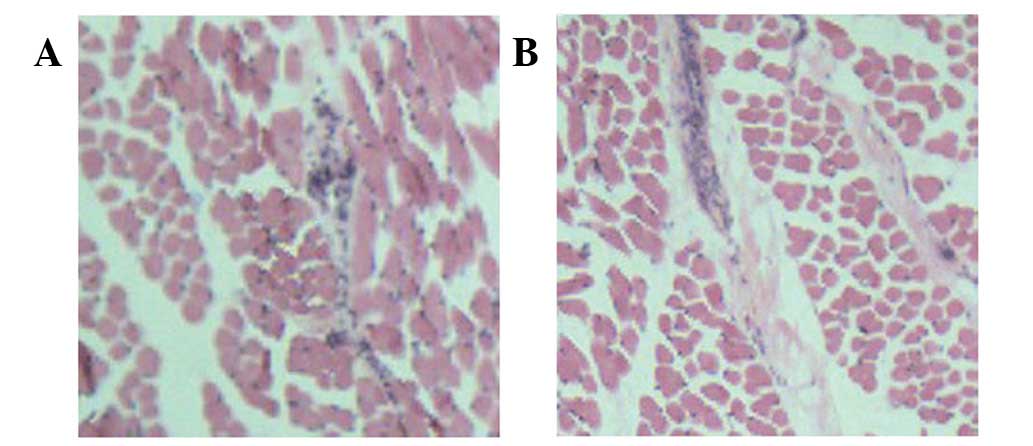
Light microscopy revealed disorder in the tissue
structure of the H&E-stained muscle samples. The presence of
increased fibrous tissue in the ICTEV muscle samples was indicative
of fibrosis (Fig. 1).
Immunofluorescence by CLSM revealed that high expression of
SOX9 and COL9A1 was colocalized to the connective
tissue of the sarcolemma, endomysium and muscle membrane of ICTEV
patients (Fig. 2). The results
indicated that increased expression of COL9A1 may be
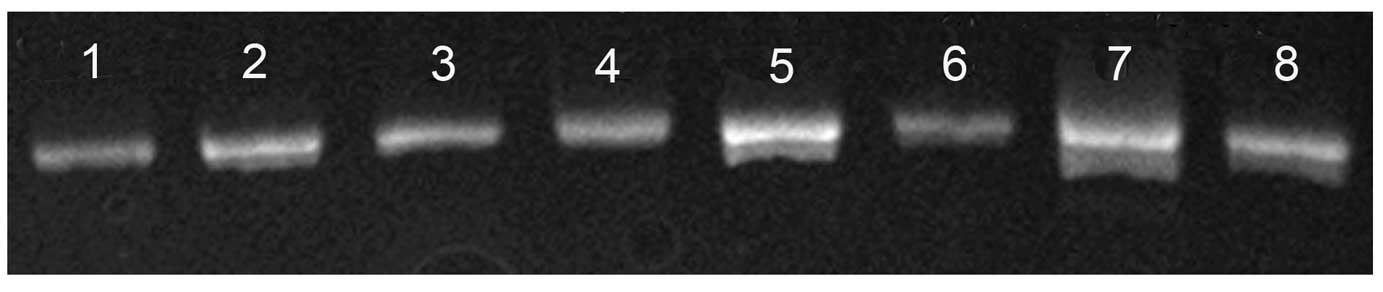
associated with SOX9. No mutations were observed in the 5′
flanking sequence or exons 1–3 of SOX9 in the blood samples of 84
ICTEV patients (Fig. 3). As
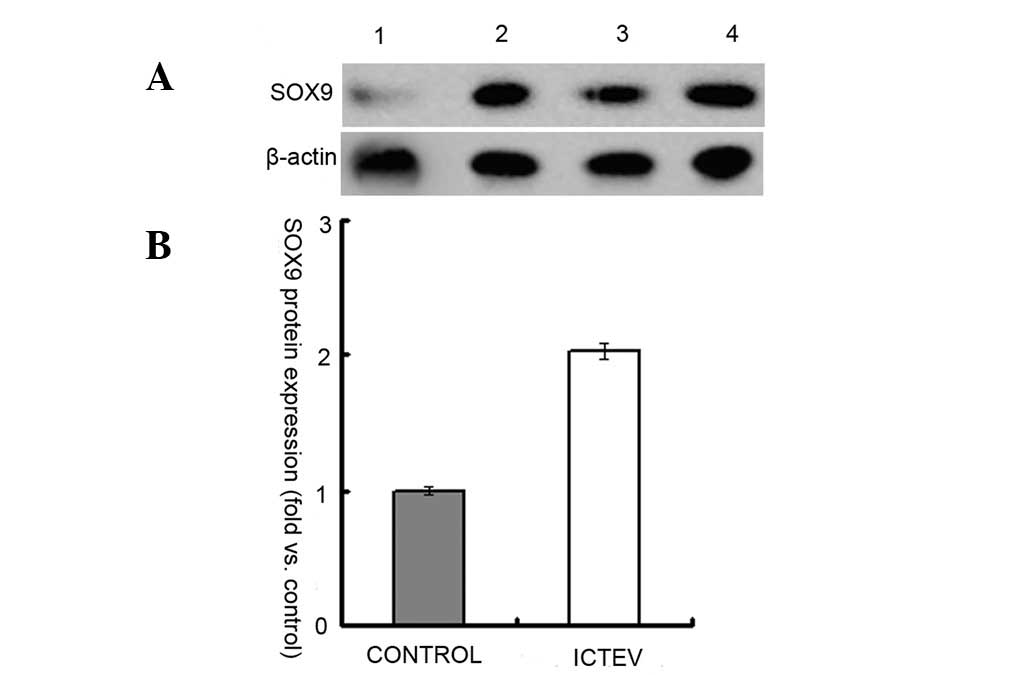
demonstrated in Figs. 4 and
5, RNA and protein expression
levels of SOX9 in the case samples were ~2 fold those of the
controls. The correlation between mRNA and protein expression was
positive, with a Spearman’s rank correlation coefficient of
0.58.
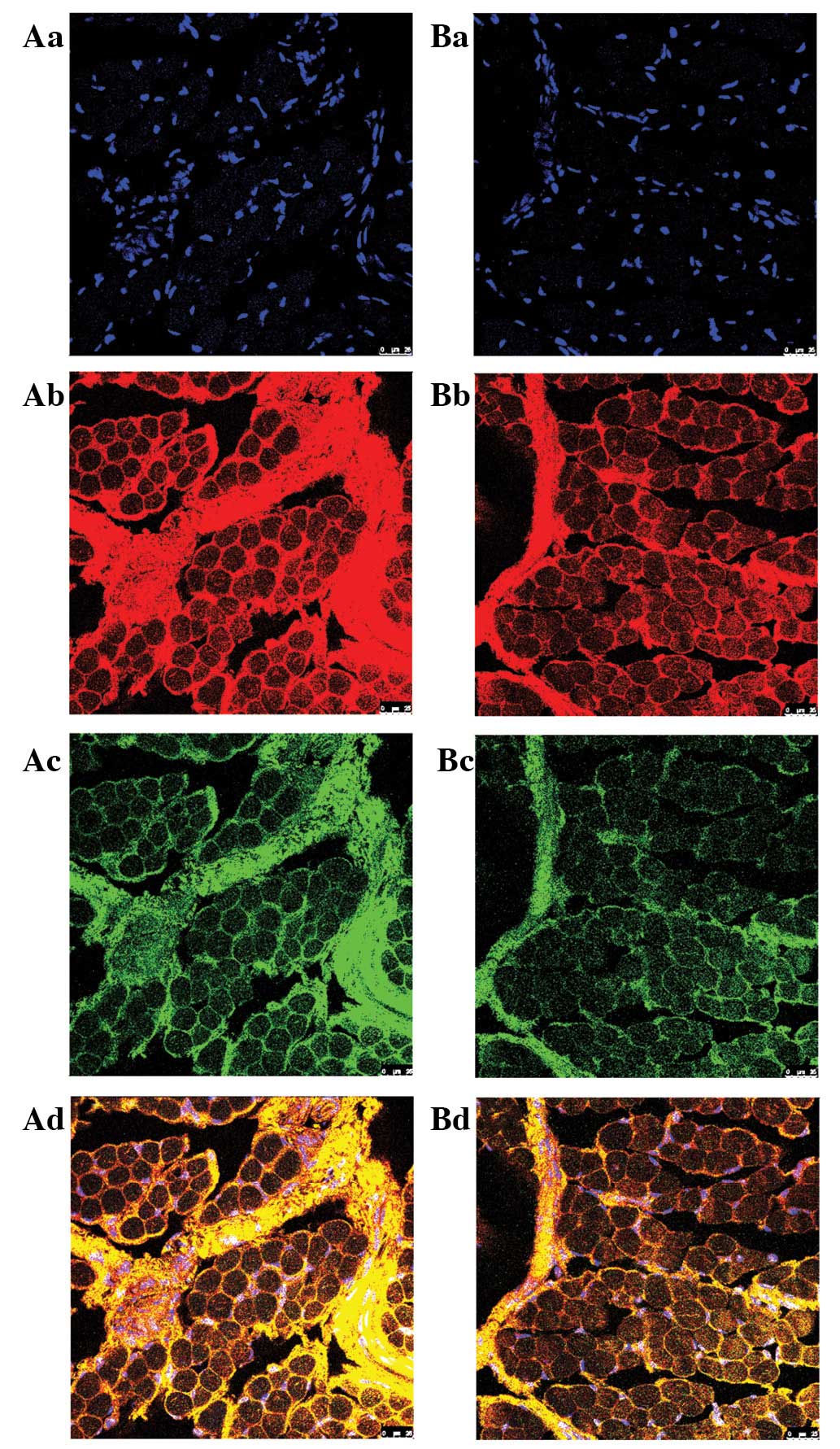 | Figure 2Analysis of SOX9 and COL9A1 in
abductor hallucis muscle samples of ICTEV patients by
immunofluorescence using CLSM. (A) ICTEV patients and (B) control.
(a) Blue fluorescence, nuclei; (b) red fluorescence, COL9A1
protein; (c) green fluorescence, SOX9 protein; and (d) yellow
fluorescence, merged images. High expression of SOX9 and COL9A1 was
colocalized to the connective tissue of the sarcolemma, endomysium
and muscle membrane of the ICTEV patient (magnification, ×400).
ICTEV, idiopathic congenital talipes equinovarus; COL9A1, collagen,
type IX, alpha 1; SOX9, SRY (sex-determining region Y)-box 9. |
Discussion
Varus foot deformity in ICTEV patients is believed
to be caused by contracture of medial soft tissue in the foot,
including the deltoid and talonavicular ligaments and posterior
tibial muscle. An additional cause of varus foot may be the
shortening of the soft tissue at the bottom of the foot, including
the plantar fascia, abductor hallucis and flexor digitorum brevis.
Medial plantar release is a recognized surgical technique for the
treatment of clubfoot. However, the technique is reserved for
children who have already reached walking age and have no reducible
deformity or those for whom conservative treatment has failed. The
abductor hallucis muscle samples are the contracture tissues
removed from the ICTEV patients by medial plantar release. Fibrosis
was observed under light microscope in the connective tissues of
the muscle samples from ICTEV patients. In a previous study,
Ippolito and Ponseti hypothesized that retraction fibrosis of the
distal muscles occurs in ICTEV patients (21). In addition, Ippolito identified
increased fibrosis of muscle tissue in 4 aborted fetuses with
clubfoot (22). It is generally
accepted that fibrosis of muscle tissue is often caused by
increased collagen components. Ionasescu et al also
identified increased collagen synthesis in clubfeet (23).
Our previous study revealed that genes on chromosome
6q12–13 may be associated with ICTEV. Further analysis also
indicated that COL9A1, in this chromosomal region, is a
candidate gene for ICTEV based on extended transmission
disequilibrium testing and was expressed in significantly higher
quantities in the abductor hallucis muscle of ICTEV compared with
control samples. These observations indicated that the
COL9A1 gene may be an important susceptibility gene in the
development of ICTEV. The COL9A1 gene encodes one of the
three α chains of type IX collagen, which is a minor collagen
component that makes up 5–20% of cartilage as well as type II
collagen. Type IX collagen gene mutations result in multiple
epiphyseal dysplasia with osteochondritis dissecans and a mild
myopathy (24). A previous study
in which intramuscular injection of bone morphogenetic proteins was
performed to induce ectopic bone formation demonstrated that
non-myogenic cells residing in the fascia of skeletal muscle have a
marked chondrogenic potential and may represent a novel donor cell
source for cartilage regeneration and repair (25).
In the present study, increased levels of
COL9A1 protein were identified in the connective tissue of
the epimysium, sarcolemma, endomysium and muscle membrane of ICTEV
patients. This observation revealed that the potentially
chondrogenic cells residing in the fascia of the abductor hallucis
muscle may have been induced to differentiate into chondrocytes by
a specific stimulation factor and therefore express high levels of
the COL9A1 gene. Excessive production of COL9A1 may lead to
retraction fibrosis of the abductor hallucis muscle and the
supporting connective tissues. This may represent a possible
causative factor for varus foot in ICTEV patients. Fibrosis may be
generated by chronic damage, including muscular dystrophies. We
hypothesized that the high expression of the COL9A1 gene in
the connective tissue of the abductor hallucis muscle results from
regulation of cartilage development by important transcription
factors. Specific studies have reported that the SOX9 transcription
factor binds to consensus sequence pairs in the COL2A1,
COL9A1 and COL27A1 genes and regulates their
expression. Recently, Gu et al reported that the earliest
steps of chondrogenesis may be determined by the balance between
Twist1 and SOX9 expression (26).
In the current study, SOX9 and COL9A1 protein was
identified to be colocalized to the connective tissue of the
sarcolemma, endomysium and muscle membrane of ICTEV patients. These
results indicate a correlation between SOX9 and high
expression of COL9A1 in the muscle samples of ICTEV
patients. In addition, the promoter and coding regions of
SOX9 were analyzed for mutations in blood samples of 84
ICTEV patients using DGGE; however, no mutation was found. Such
mutations may be revealed in larger sample sizes. Present results
also revealed that the mRNA and protein expression levels of
SOX9 in the abductor hallucis muscle samples of ICTEV
patients were overexpressed 2-fold compared with those of the
controls. mRNA levels in the ICTEV patients were correlated with
those of the protein. We hypothesized that increased binding of the
SOX9 transcription factor to the upstream sequence in COL9A1
upregulates COL9A1 levels in the abductor hallucis of ICTEV
patients. The significance of this potentially important mechanism
remains to be determined. Although the present study used a small
sample of ICTEV patients, it is reasonable to conclude that high
levels of SOX9 may play a role in the development of
ICTEV.
Previously, soft tissue release surgery was
considered to be the best treatment method for ICTEV. However,
although extensive soft tissue release provides definitive
correction, the surgical procedure is associated with a number of
poor, long-term complications, including muscle weakness, stiffness
of ankle and subtalar joints, arthritis, pain and residual
deformity (27). Thus, surgical
soft tissue release has been gradually replaced by the Ponseti
method of clubfoot manipulation and casting, Achilles tendon
tenotomy and foot abduction bracing, which are associated with
excellent short-term as well as long-term results (28). With alterations in surgical
approach, soft tissue samples of ICTEV patients have become
increasingly rare. In view of the relatively small number of
samples in the present study, the results require confirmation in
studies with larger sample sizes.
Acknowledgements
The present study was supported by a grant from the
National Key Research Project of China (no. 30973140).
References
|
1
|
Dietz F: The genetics of idiopathic
clubfoot. Clin Orthop Relat Res. 401:39–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cardy AH, Barker S, Chesney D, Sharp L,
Maffulli N and Miedzybrodzka Z: Pedigree analysis and
epidemiological features of idiopathic congenital talipes
equinovarus in the United Kingdom: a case-control study. BMC
Musculoskelet Disord. 8:622007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rebbeck TR, Dietz FR, Murray JC and Buetow
KH: A single-gene explanation for the probability of having
idiopathic talipes equinovarus. Am J Hum Genet. 53:1051–1063.
1993.PubMed/NCBI
|
|
4
|
Wynne-Davies R: Genetic and environmental
factors in the etiology of talipes equinovarus. Clin Orthop Relat
Res. 84:9–13. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapman C, Stott NS, Port RV and Nicol RO:
Genetics of clubfoot in Maori and Pacific people. J Med Genet.
37:680–683. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miedzybrodzka Z: Congenital talipes
equinovarus (clubfoot): a disorder of the foot but not the hand. J
Anat. 202:37–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu LY, Jin CL, Cao DH, Zhao N, Lin CK and
Sun KL: Analysis of association between COL9A1 gene and idiopathic
congenital talipes equinovarus. Yi Chuan. 29:427–432. 2007.(In
Chinese).
|
|
8
|
Wang LL, Fu WN, Li-Ling J, Li ZG, Li LY
and Sun KL: HOXD13 may play a role in idiopathic congenital
clubfoot by regulating the expression of FHL1. Cytogenet Genome
Res. 121:189–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao D, Jin C, Ren M, Lin C, Zhang X and
Zhao N: The expression of Gli3, regulated by HOXD13, may play a
role in idiopathic congenital talipes equinovarus. BMC
Musculoskelet Disord. 10:1422009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Q, Eberspaecher H, Lefebvre V and De
Crombrugghe B: Parallel expression of Sox9 and Col2a1 in cells
undergoing chondrogenesis. Dev Dyn. 209:377–386. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sock E, Pagon RA, Keymolen K, Lissens W,
Wegner M and Scherer G: Loss of DNA-dependent dimerization of the
transcription factor SOX9 as a cause for campomelic dysplasia. Hum
Mol Genet. 12:1439–1447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernard P, Tang P, Liu S, Dewing P, Harley
VR and Vilain E: Dimerization of SOX9 is required for
chondrogenesis, but not for sex determination. Hum Mol Genet.
12:1755–1765. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ng LJ, Wheatley S, Muscat GE,
Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS
and Koopman P: SOX9 binds DNA, activates transcription and
coexpresses with type II collagen during chondrogenesis in the
mouse. Dev Biol. 183:108–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang P, Jimenez SA and Stokes DG:
Regulation of human COL9A1 gene expression. Activation of the
proximal region by SOX9. J Biol Chem. 278:117–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rentsendorj O, Nagy A, Sinkó I, Daraba A,
Barta E and Kiss I: Highly conserved proximal promoter element
harbouring paired Sox9-binding sites contributes to the tissue- and
developmental stage-specific activity of the matrilin-1 gene.
Biochem J. 389:705–716. 2005. View Article : Google Scholar
|
|
16
|
Jenkins E, Moss JB, Pace JM and
Bridgewater LC: The new collagen gene COL27A1 contains
SOX9-responsive enhancer elements. Matrix Biol. 24:177–184. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ross AJ, Tilman C, Yao H, MacLaughlin D
and Capel B: AMH induces mesonephric cell migration in XX gonads.
Mol Cell Endocrinol. 211:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jakubiczka S, Schröder C, Ullmann R, et
al: Translocation and deletion around SOX9 in a patient with
acampomelic campomelic dysplasia and sex reversal. Sex Dev.
4:143–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Hout AH, van den Ouweland AM, van
der Luijt RB, et al: A DGGE system for comprehensive mutation
screening of BRCA1 and BRCA2: application in a Dutch cancer clinic
setting. Hum Mutat. 27:654–666. 2006.PubMed/NCBI
|
|
20
|
Kalb VF Jr and Bernlohr RW: A new
spectrophotometric assay for protein in cell extracts. Anal
Biochem. 82:362–371. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ippolito E and Ponseti IV: Congenital club
foot in the human fetus. A histological study. J Bone Joint Surg
Am. 62:8–22. 1980.PubMed/NCBI
|
|
22
|
Ippolito E: Update on pathologic anatomy
of clubfoot. J Pediatr Orthop B. 4:17–24. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ionasescu V, Maynard JA, Ponseti IV and
Zellweger H: The role of collagen in the pathogenesis of idiopathic
clubfoot. Biochemical and electron microscopic correlations. Helv
Paediatr Acta. 29:305–314. 1974.PubMed/NCBI
|
|
24
|
Jackson GC, Marcus-Soekarman D,
Stolte-Dijkstra I, Verrips A, Taylor JA and Briggs MD: Type IX
collagen gene mutations can result in multiple epiphyseal dysplasia
that is associated with osteochondritis dissecans and a mild
myopathy. Am J Med GenetA. 152:863–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li G, Zheng B, Meszaros LB, Vella JB, Usas
A, Matsumoto T and Huard J: Identification and characterization of
chondrogenic progenitor cells in the fascia of postnatal skeletal
muscle. J Mol Cell Biol. 3:369–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu S, Boyer TG and Naski MC: Basic
helix-loop-helix transcription factor twist1 inhibits the
transactivation function of the master chondrogenic regulator Sox9.
J Biol Chem. 287:21082–21092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ippolito E, Farsetti P, Caterini R and
Tudisco C: Long-term comparative results in patients with
congenital clubfoot treated with two different protocols. J Bone
Joint Surg Am. 85:1286–1294. 2003.PubMed/NCBI
|
|
28
|
Ponseti IV: Treatment of congenital club
foot. J Bone Joint Surg Am. 74:448–454. 1992.PubMed/NCBI
|