Introduction
Nasal polyps are small abnormal lesions or bags that
protrude from the nasal cavity, and are characterized by edematous
stroma following progression of dense eosinophilia to a thickened
basement membrane in epithelium (1). Although usually harmless, nasal
polyposis may cause significant airway obstruction including
infection of nose or sinuses, and is known to be associated with
bronchial asthma by occurring more frequently in asthma and
aspirin-hypersensitive patients (2,3).
Although the exact mechanism for the origin of nasal polyps remains
unknown, allergies, infections, inflammation and genetic factors
are considered to contribute to the onset of this condition
(4–6).
The human class II, major histocompatibility
complex, transactivator (CIITA) encodes a major
histocompatibility complex (MHC) class II transactivator and is
essential for MHC class II molecules (7). The full length of the human
CIITA gene is ~48 kb and is located on chromosome 16p13. The
gene contains 20 exons and 4 alternative promoters with
tissue-specific transcription (8).
CIITA is often referred to as the master control factor of
MHC II molecules due to its regulating activity at the
transcriptional level (9). Since
abnormal immune responses and immunodeficiency are associated with
lack of expression of MHC II genes, the function of
CIITA, which interacts other transcription factors in the
MHC II promoter region, is considered to be a master factor in
immune response (8,10,11).
Wang et al(12) reported
that MHC class II antigens were more frequently detected in polyps
in atopic and cystic fibrosis patients, suggesting that the MHC
class II antigens in airway epithelial cells of nasal polyps are
able to be regulated by interferon γ at the transcriptional
level.
Polymorphisms in CIITA are reportedly
associated with several immune diseases such as multiple sclerosis,
Addison’s disease, and systemic lupus erythematosus (7,13,14).
CIITA and MHC II genes are involved in immune
responses. Subsequently, we hypothesized that the CIITA gene
is a marker for the development of nasal polyps in asthmatic
patients. The aim of this case-control study was to investigate the
association between CIITA polymorphisms and the presence of
nasal polyps in asthmatic patients.
Materials and methods
Subjects
A total of 467 asthmatics were recruited from the
Soonchunhyang University hospitals in Seoul and Bucheon (Korea),
both of which are under the Asthma Genome Research. The
participants provided written informed consent, and the study
protocols were approved by the Institutional Review Board of the
Hospital. Following the guidelines of Global Initiative for Asthma
(GINA), asthma was diagnosed as previously described (15). Twenty-four common inhalant
allergens were used in a skin-prick test (Bencard Co. Ltd.,
Brentford, UK), and atopy was defined as at least a 3-mm wheal
reaction to any of the allergens. Furthermore, total immunoglobulin
E (IgE) was measured using the CAP system (Pharmacia Diagnostics,
Uppsala, Sweden). Asthmatics with endoscopically visible polyps
present in the middle nasal meatus were classified as
polyp-positive cases, while the remaining individuals were
identified as polyp-negative controls.
To distinguish aspirin-exacerbated respiratory
disease (AERD) from aspirin-tolerant asthma (ATA) patients, all the
asthma patients underwent oral aspirin challenge (OAC) that was
performed according to previously described methods (15). Asthmatics exhibiting ≥20% decrease
in forced expiratory volume in 1 sec (FEV1) or a 15–19%
decrease in FEV1 with naso-ocular or cutaneous reactions
comprised the AERD group, whereas those demonstrating <15%
decrease in FEV1 without naso-ocular or cutaneous
reactions comprised the ATA group. The clinical profile of the
patients included in this study is summarized in Table I. Results from the
aspirin-provocation test demonstrated significant differences in
the mean score of aspirin-induced the reduced rate of
FEV1 between polyp-positive asthma patients and
polyp-negative controls (12.22 vs. 6.78%, P<0.0001).
Additionally, the positive rate of aspirin intolerance and the
history of aspirin hypersensitivity were also found to be
significantly prevalent in the two groups (Table I).
 | Table IClinical profile of asthmatic
patients (n=467). |
Table I
Clinical profile of asthmatic
patients (n=467).
| Clinical
profile | Polyp-positive | Polyp-negative | P-value |
|---|
| No. of
subjects | 158 | 309 | |
| Age (years), mean
(range) | 46.24
(17.93–76.86) | 47.00
(15.40–77.88) | 0.56 |
| Gender
(male/female), n | 55/103 | 100/209 | 0.60 |
| Total smoker
(current smoker; ex-smoker) (%) | 27.21 (11.39;
15.82) | 27.83 (11.33;
16.50) | 0.93 |
| Body mass index
(kg/m2) | 23.95±3.00 | 24.53±3.51 | 0.06 |
| Percentage decrease
of FEV1 by aspirin provocation | 12.22±14.39 | 6.78±11.46 |
<0.0001 |
| Positive rate of
aspirin intolerance (%) | 41.77 | 15.53 |
<0.0001 |
| Positive rate of
the history of aspirin hypersensitivity (%) | 30.52 | 8.55 |
<0.0001 |
| Blood eosinophil
(%) | 6.92±6.23 | 5.98±6.00 | 0.12 |
| PC20 methacholine
(mg/ml) | 5.83±8.87 | 6.88±8.62 | 0.23 |
| Total IgE
(IU/ml) | 298.35±469.67 | 368.39±654.01 | 0.19 |
| FEV1 (%
predicted) | 89.67±15.76 | 91.79±17.33 | 0.18 |
| FVC (%
predicted) | 89.02±12.65 | 87.68±14.56 | 0.30 |
| Positive rate of
skin test (%) | 51.90 | 57.61 | 0.24 |
Selection and genotyping of single
nucleotide polymorphisms (SNPs)
Eighteen common SNPs with minor allele frequencies
(MAF) >0.05 were selected for genotyping in the Asian population
(Chinese and Japanese) from the International HapMap database
(http://hapmap.ncbi.nlm.nih.gov/index.html.en). Genomic
DNA was isolated from the blood of patients using the
Wizard® Genomic DNA Purification kit (Promega, Madison,
WI, USA). Genotyping was performed using TaqMan assay on the ABI
PRISM® 7900HT sequence detection system (Applied
Biosystems, Foster City, CA, USA). Genotyped data quality was
assessed by duplicate DNA checking (n=10; rate of concordance in
duplicates >99%).
Statistical analysis
Differences in the genotype distributions of
CIITA variations in polyp-positive asthma cases and
polyp-negative asthma controls were analyzed using logistic models
adjusted for the age of initial diagnosis (continuous value),
gender (male, 0; female, 1), smoking status (non-smoker, 0;
ex-smoker, 1; smoker, 2) and atopy (absence, 0; presence, 1) to
eliminate confounding variables that might influence findings. AERD
status was also controlled for the logistic analysis of the overall
asthmatic patients. Data were managed on the Statistical Analysis
System (SAS) version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Statistical power of single associations was
determined using the Power for Genetic Association Analyses (PGA)
software (16), and multiple
testing corrections were calculated using the effective number of
independent marker loci (Meff) that accounts for the eigenvalue
spectral decomposition (SpD) of all the genotypes represented in
the correlation matrix (17) that
was extracted from the SNPSpD program (Meff value =16.5417).
Results
Characteristics of study subjects
The clinical profile of the included patients showed
that the positive rate of aspirin intolerance and of the history of
aspirin hypersensitivity were significantly higher in the nasal
polyp-positive asthmatic patients compared to the polyp-negative
controls. In addition, the reduced rate of FEV1
decreased by aspirin provocation was 2-fold higher in the nasal
polyp-positive patients compared to the polyp-negative controls
(P<0.0001, Table I). These
findings indicate that nasal polyposis is associated with aspirin
hypersensitivity in asthma.
Distribution of CIITA polymorphisms
A total of 18 SNPs in the CIITA gene were
successfully genotyped. Most of the polymorphisms were localized in
the non-coding regions of the gene (introns). Notably, 3 SNPs were
located in coding regions (exons), and 2 SNPs (rs4774 and
rs7201430) were non-synonymous variations that induced amino
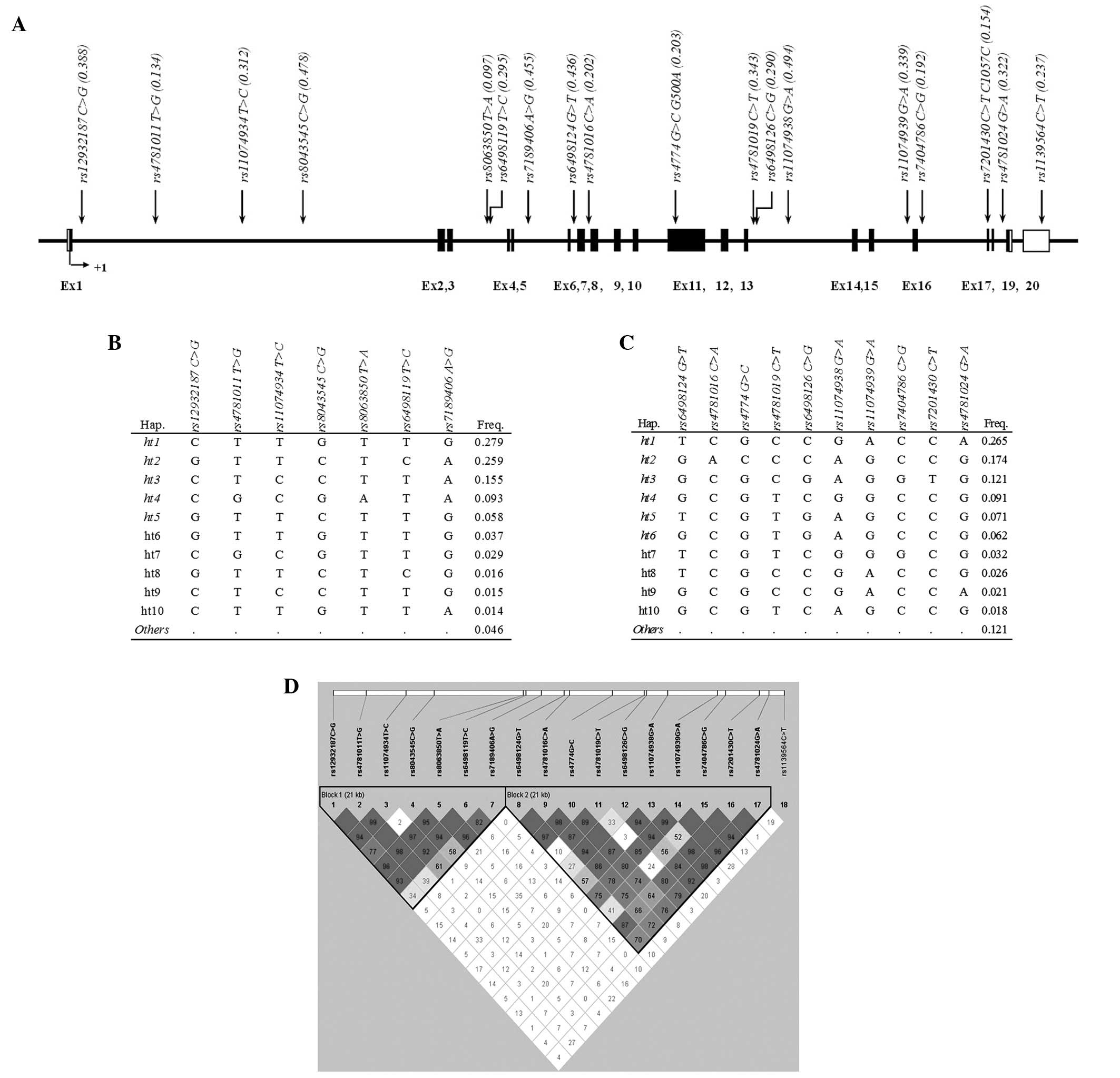
acid change by base substitution. Fig.
1A shows the position and MAF of each SNP. Two linkage
disequilibrium (LD) blocks were inferred from the pairwise
comparison of the genotype polymorphisms, and 11 major haplotypes
(frequency >0.05) were examined for association with the
presence of nasal polyps among asthmatic patients (Fig. 1B-D).
Associations of CIITA variants with nasal
polyposis
Table II shows the
results of the logistic analyses of variants in CIITA gene
with nasal polyps in all the asthmatic patients. Two SNPs
(rs12932187 and rs11074938) and 2 haplotypes
(CIITA_BL1_ht2 and CIITA_BL1_ht5) were demonstrated
to be associated with nasal polyps (P=0.001–0.01, OR=0.53–2.35
depending on the genetic model). When multiple testing correction
was conducted with a Meff of 16.5417, a SNP (rs12932187)
retained the associations with nasal polyps
(Pcorr=0.02). Since nasal polyposis is usually
accompanied with aspirin hypersensitivity in asthma, further
comparison of this association in AERD and ATA subgroups was
performed. As a result, one SNP (rs1139564) was demonstrated
to have a nominal association with nasal polyps in the AERD group
(P=0.01 in co-dominant model; P=0.02 in dominant model). However,
the association was not retained after multiple testing corrections
(Table III).
 | Table IIAssociation of significant
CIITA variants with nasal polyps in all the asthmatic
patients (n=467). |
Table II
Association of significant
CIITA variants with nasal polyps in all the asthmatic
patients (n=467).
| MAF | Co-dominant | Dominant | Recessive | |
|---|
|
|
|
|
| |
|---|
| SNP/haplotype | Polyp-positive
(n=158) | Polyp-negative
(n=309) | OR (95% CI) | Pa |
Pcorrb | OR (95% CI) | Pa |
Pcorrb | OR (95% CI) | Pa |
Pcorrb | Statistical
power |
|---|
|
rs12932187 | 0.456 | 0.359 | 1.42
(1.08–1.88) | 0.01 | NS | 1.25
(0.83–1.90) | 0.29 | - | 2.35
(1.41–3.90) | 0.001 | 0.02 | 82.0 |
|
rs4781011 | 0.127 | 0.129 | 0.93
(0.61–1.42) | 0.73 | - | 0.95
(0.59–1.52) | 0.83 | - | 0.63
(0.12–3.43) | 0.60 | - | 57.5 |
|
rs11074934 | 0.285 | 0.309 | 0.88
(0.65–1.19) | 0.40 | - | 0.93
(0.62–1.39) | 0.71 | - | 0.64
(0.31–1.30) | 0.22 | - | 80.2 |
|
rs8043545 | 0.449 | 0.494 | 0.85
(0.64–1.12) | 0.24 | - | 0.71
(0.46–1.11) | 0.13 | - | 0.91
(0.56–1.47) | 0.69 | - | 82.3 |
|
rs8063850 | 0.085 | 0.097 | 0.84
(0.50–1.40) | 0.50 | - | 0.86
(0.51–1.46) | 0.58 | - | - | - | - | 48.3 |
|
rs6498119 | 0.320 | 0.280 | 1.14
(0.85–1.54) | 0.38 | - | 1.00
(0.67–1.50) | 0.99 | - | 1.83
(0.98–3.41) | 0.06 | - | 78.6 |
|
rs7189406 | 0.443 | 0.468 | 0.95
(0.71–1.27) | 0.72 | - | 0.95
(0.61–1.48) | 0.82 | - | 0.91
(0.55–1.51) | 0.71 | - | 82.7 |
|
CIITA_BL1_ht1 | 0.237 | 0.299 | 0.77
(0.56–1.07) | 0.12 | - | 0.75
(0.50–1.12) | 0.16 | - | 0.64
(0.28–1.43) | 0.27 | - | 79.7 |
|
CIITA_BL1_ht2 | 0.288 | 0.244 | 1.19
(0.88–1.62) | 0.26 | - | 1.07
(0.71–1.60) | 0.75 | - | 2.03
(1.03–3.99) | 0.04 | NS | 75.9 |
|
CIITA_BL1_ht3 | 0.136 | 0.155 | 0.85
(0.58–1.26) | 0.43 | - | 0.89
(0.57–1.41) | 0.63 | - | 0.46
(0.12–1.75) | 0.25 | - | 63.4 |
|
CIITA_BL1_ht4 | 0.082 | 0.092 | 0.86
(0.51–1.44) | 0.56 | - | 0.88
(0.51–1.51) | 0.64 | - | - | - | - | 46.7 |
|
CIITA_BL1_ht5 | 0.082 | 0.055 | 1.71
(0.99–2.95) | 0.05 | NS | 1.67
(0.92–3.02) | 0.09 | - | 6.32
(0.56–71.23) | 0.14 | - | 32.2 |
|
rs6498124 | 0.440 | 0.450 | 1.09
(0.81–1.45) | 0.58 | - | 1.05
(0.68–1.62) | 0.83 | - | 1.21
(0.73–2.01) | 0.46 | - | 82.9 |
|
rs4781016 | 0.180 | 0.212 | 0.80
(0.56–1.15) | 0.24 | - | 0.86
(0.57–1.31) | 0.49 | - | 0.34
(0.09–1.23) | 0.10 | - | 72.5 |
| rs4774 | 0.184 | 0.214 | 0.81
(0.57–1.17) | 0.26 | - | 0.83
(0.54–1.26) | 0.38 | - | 0.53
(0.17–1.69) | 0.28 | - | 72.7 |
|
rs4781019 | 0.342 | 0.327 | 1.11
(0.81–1.52) | 0.52 | - | 1.04
(0.69–1.56) | 0.86 | - | 1.46
(0.75–2.85) | 0.26 | - | 81.0 |
|
rs6498126 | 0.269 | 0.285 | 0.93
(0.66–1.30) | 0.66 | - | 0.78
(0.52–1.18) | 0.24 | - | 1.77
(0.78–3.99) | 0.17 | - | 78.9 |
|
rs11074938 | 0.446 | 0.503 | 0.76
(0.56–1.02) | 0.07 | - | 0.53
(0.33–0.83) | 0.006 | NS | 0.97
(0.59–1.59) | 0.90 | - | 82.2 |
|
rs11074939 | 0.377 | 0.341 | 1.23
(0.90–1.67) | 0.19 | - | 1.19
(0.79–1.80) | 0.40 | - | 1.57
(0.85–2.90) | 0.15 | - | 81.5 |
|
rs7404786 | 0.199 | 0.186 | 1.02
(0.71–1.48) | 0.90 | - | 1.03
(0.67–1.57) | 0.90 | - | 1.03
(0.35–3.10) | 0.95 | - | 68.9 |
|
rs7201430 | 0.161 | 0.147 | 1.00
(0.67–1.50) | 1.00 | - | 0.98
(0.63–1.54) | 0.94 | - | 1.18
(0.30–4.59) | 0.81 | - | 61.8 |
|
rs4781024 | 0.358 | 0.322 | 1.22
(0.89–1.66) | 0.21 | - | 1.22
(0.81–1.84) | 0.34 | - | 1.45
(0.76–2.77) | 0.26 | - | 80.8 |
|
rs1139564 | 0.241 | 0.227 | 1.07
(0.77–1.50) | 0.69 | - | 1.12
(0.74–1.68) | 0.59 | - | 0.94
(0.38–2.32) | 0.90 | - | 74.2 |
|
CIITA_BL2_ht1 | 0.285 | 0.269 | 1.22
(0.88–1.69) | 0.24 | - | 1.36
(0.91–2.04) | 0.14 | - | 0.96
(0.41–2.22) | 0.92 | - | 77.9 |
|
CIITA_BL2_ht2 | 0.152 | 0.183 | 0.81
(0.55–1.19) | 0.28 | - | 0.82
(0.53–1.28) | 0.39 | - | 0.51
(0.13–1.90) | 0.31 | - | 68.4 |
|
CIITA_BL2_ht3 | 0.127 | 0.121 | 0.97
(0.63–1.50) | 0.89 | - | 0.97
(0.60–1.57) | 0.90 | - | 0.90
(0.16–4.96) | 0.90 | - | 55.5 |
|
CIITA_BL2_ht4 | 0.111 | 0.086 | 1.22
(0.75–1.97) | 0.42 | - | 1.25
(0.74–2.12) | 0.40 | - | 1.10
(0.16–7.55) | 0.93 | - | 44.6 |
|
CIITA_BL2_ht5 | 0.057 | 0.079 | 0.77
(0.43–1.38) | 0.38 | - | 0.80
(0.44–1.47) | 0.48 | - | - | - | - | 42.0 |
|
CIITA_BL2_ht6 | 0.054 | 0.058 | 1.01
(0.54–1.87) | 0.98 | - | 0.88
(0.45–1.72) | 0.71 | - | - | - | - | 33.5 |
 | Table IIIAssociation of significant
CIITA variants with nasal polyps in AERD patients
(n=114). |
Table III
Association of significant
CIITA variants with nasal polyps in AERD patients
(n=114).
| MAF | Co-dominant | Dominant | Recessive | |
|---|
|
|
|
|
| |
|---|
| SNP/haplotype | Polyp-positive
(n=66) | Polyp-negative
(n=48) | OR (95% CI) | Pa |
Pcorrb | OR (95% CI) | Pa |
Pcorrb | OR (95% CI) | Pa |
Pcorrb | Statistical
power |
|---|
|
rs12932187 | 0.455 | 0.396 | 1.29
(0.75–2.23) | 0.36 | - | 1.21
(0.53–2.73) | 0.65 | - | 1.76
(0.64–4.82) | 0.27 | - | 31.7 |
|
rs4781011 | 0.144 | 0.135 | 1.03
(0.47–2.29) | 0.93 | - | 1.08
(0.44–2.60) | 0.87 | - | 0.72
(0.04–12.94) | 0.82 | - | 19.3 |
|
rs11074934 | 0.303 | 0.313 | 0.95
(0.56–1.63) | 0.86 | - | 0.87
(0.40–1.85) | 0.71 | - | 1.12
(0.36–3.47) | 0.85 | - | 29.7 |
|
rs8043545 | 0.462 | 0.458 | 1.00
(0.55–1.82) | 1.00 | - | 1.00
(0.41–2.44) | 0.99 | - | 1.00
(0.36–2.77) | 1.00 | - | 32.1 |
|
rs8063850 | 0.098 | 0.094 | 0.94
(0.35–2.55) | 0.91 | - | 0.94
(0.35–2.55) | 0.91 | - | - | - | - | 15.5 |
|
rs6498119 | 0.311 | 0.365 | 0.80
(0.46–1.40) | 0.43 | - | 0.66
(0.30–1.42) | 0.28 | - | 0.99
(0.31–3.17) | 0.99 | - | 31.1 |
|
rs7189406 | 0.439 | 0.385 | 1.34
(0.74–2.43) | 0.34 | - | 1.23
(0.53–2.84) | 0.64 | - | 2.01
(0.62–6.48) | 0.24 | - | 31.5 |
|
CIITA_BL1_ht1 | 0.220 | 0.250 | 0.88
(0.47–1.65) | 0.68 | - | 0.81
(0.37–1.78) | 0.59 | - | 1.05
(0.22–5.07) | 0.96 | - | 27.1 |
|
CIITA_BL1_ht2 | 0.280 | 0.302 | 0.91
(0.50–1.66) | 0.76 | - | 0.77
(0.36–1.65) | 0.50 | - | 1.50
(0.35–6.48) | 0.59 | - | 29.3 |
|
CIITA_BL1_ht3 | 0.136 | 0.167 | 0.81
(0.39–1.69) | 0.58 | - | 0.77
(0.32–1.89) | 0.57 | - | 0.77
(0.10–6.03) | 0.80 | - | 21.8 |
|
CIITA_BL1_ht4 | 0.091 | 0.094 | 0.88
(0.32–2.39) | 0.80 | - | 0.88
(0.32–2.39) | 0.80 | - | - | - | - | 15.5 |
|
CIITA_BL1_ht5 | 0.061 | 0.031 | 2.23
(0.54–9.21) | 0.27 | - | 2.23
(0.54–9.21) | 0.27 | - | - | - | - | 8.7 |
|
rs6498124 | 0.379 | 0.354 | 1.15
(0.63–2.11) | 0.64 | - | 0.92
(0.42–2.03) | 0.83 | - | 2.54
(0.62–10.49) | 0.20 | - | 30.9 |
|
rs4781016 | 0.205 | 0.198 | 1.06
(0.53–2.10) | 0.87 | - | 1.12
(0.50–2.50) | 0.78 | - | 0.77
(0.10–6.09) | 0.81 | - | 24.0 |
| rs4774 | 0.205 | 0.198 | 1.04
(0.53–2.06) | 0.91 | - | 0.93
(0.42–2.06) | 0.86 | - | 2.56
(0.24–26.95) | 0.44 | - | 24.0 |
|
rs4781019 | 0.326 | 0.292 | 1.12
(0.64–1.99) | 0.69 | - | 0.92
(0.43–1.98) | 0.83 | - | 2.31
(0.58–9.15) | 0.23 | - | 29.0 |
|
rs6498126 | 0.265 | 0.260 | 1.09
(0.55–2.13) | 0.81 | - | 0.93
(0.43–2.03) | 0.85 | - | 3.49
(0.36–34.28) | 0.28 | - | 27.6 |
|
rs11074938 | 0.485 | 0.469 | 1.10
(0.61–1.97) | 0.75 | - | 0.88
(0.36–2.17) | 0.78 | - | 1.52
(0.56–4.16) | 0.41 | - | 32.1 |
|
rs11074939 | 0.326 | 0.365 | 0.81
(0.43–1.52) | 0.51 | - | 0.63
(0.28–1.40) | 0.26 | - | 1.53
(0.35–6.71) | 0.58 | - | 31.1 |
|
rs7404786 | 0.220 | 0.198 | 1.10
(0.55–2.23) | 0.78 | - | 1.42
(0.61–3.28) | 0.42 | - | 0.35
(0.05–2.35) | 0.28 | - | 24.0 |
|
rs7201430 | 0.189 | 0.177 | 1.08
(0.51–2.29) | 0.84 | - | 1.27
(0.54–2.99) | 0.58 | - | 0.34
(0.03–3.98) | 0.39 | - | 22.6 |
|
rs4781024 | 0.318 | 0.344 | 0.83
(0.44–1.58) | 0.57 | - | 0.69
(0.31–1.54) | 0.37 | - | 1.34
(0.29–6.18) | 0.71 | - | 30.7 |
|
rs1139564 | 0.303 | 0.156 | 2.45
(1.21–4.93) | 0.01 | NS | 2.66
(1.19–5.94) | 0.02 | NS | 4.97
(0.53–46.20) | 0.16 | - | 21.0 |
|
CIITA_BL2_ht1 | 0.242 | 0.219 | 1.20
(0.61–2.36) | 0.60 | - | 1.07
(0.49–2.36) | 0.87 | - | 3.26
(0.33–32.01) | 0.31 | - | 25.4 |
|
CIITA_BL2_ht2 | 0.167 | 0.156 | 1.09
(0.52–2.27) | 0.82 | - | 1.04
(0.45–2.40) | 0.93 | - | 1.90
(0.16–23.15) | 0.61 | - | 21.0 |
|
CIITA_BL2_ht3 | 0.136 | 0.156 | 0.86
(0.38–1.95) | 0.71 | - | 0.96
(0.40–2.29) | 0.92 | - | - | - | - | 21.0 |
|
CIITA_BL2_ht4 | 0.136 | 0.115 | 1.23
(0.54–2.81) | 0.63 | - | 1.11
(0.45–2.73) | 0.83 | - | - | - | - | 17.5 |
|
CIITA_BL2_ht5 | 0.045 | 0.052 | 0.92
(0.25–3.39) | 0.90 | - | 0.92
(0.25–3.39) | 0.90 | - | - | - | - | 11.1 |
|
CIITA_BL2_ht6 | 0.045 | 0.042 | 1.16
(0.34–3.93) | 0.81 | - | 0.95
(0.23–3.83) | 0.94 | - | - | - | - | 10.0 |
In further association analysis, 4 CIITA
variations (rs12932187, rs6498119, rs11074938
and CIITA_BL1_ht2) were significantly associated with nasal
polyps (P=0.001–0.05, OR=0.45–2.61 depending on the genetic model;
Table IV) in ATA patients. Of
these variations, the association of 2 variations
(rs12932187 and CIITA_BL1_ht2) were retained
following multiple testing corrections (Pcorr=0.02 in
rs12932187 and Pcorr=0.04 in
CIITA_BL1_ht2; Table IV).
These findings showed that CIITA polymorphisms were more
significantly associated with nasal polyposis in ATA compared to
AERD patients.
 | Table IVAssociation of significant
CIITA variants with nasal polyps in ATA patients
(n=353). |
Table IV
Association of significant
CIITA variants with nasal polyps in ATA patients
(n=353).
| MAF | Co-dominant | Dominant | Recessive | |
|---|
|
|
|
|
| |
|---|
| SNP/haplotype | Polyp-positive
(n=92) | Polyp-negative
(n=261) | OR (95% CI) | Pa |
Pcorrb | OR (95% CI) | Pa |
Pcorrb | OR (95% CI) | Pa |
Pcorrb | Statistical
power |
|---|
|
rs12932187 | 0.457 | 0.352 | 1.49
(1.07–2.07) | 0.02 | NS | 1.29
(0.79–2.11) | 0.31 | - | 2.61
(1.46–4.67) | 0.001 | 0.02 | 64.2 |
|
rs4781011 | 0.114 | 0.128 | 0.88
(0.52–1.48) | 0.62 | - | 0.89
(0.50–1.58) | 0.70 | - | 0.57
(0.07–4.96) | 0.61 | - | 41.7 |
|
rs11074934 | 0.272 | 0.308 | 0.83
(0.57–1.22) | 0.34 | - | 0.93
(0.58–1.50) | 0.78 | - | 0.41
(0.14–1.20) | 0.10 | - | 62.4 |
|
rs8043545 | 0.440 | 0.500 | 0.80
(0.58–1.11) | 0.18 | - | 0.65
(0.39–1.08) | 0.09 | - | 0.86
(0.49–1.50) | 0.59 | - | 64.3 |
|
rs8063850 | 0.076 | 0.098 | 0.76
(0.40–1.42) | 0.38 | - | 0.78
(0.41–1.50) | 0.45 | - | - | - | - | 35.0 |
|
rs6498119 | 0.326 | 0.264 | 1.31
(0.93–1.86) | 0.13 | - | 1.18
(0.73–1.90) | 0.50 | - | 2.28
(1.12–4.67) | 0.02 | NS | 59.7 |
|
rs7189406 | 0.446 | 0.483 | 0.86
(0.62–1.21) | 0.39 | - | 0.87
(0.51–1.46) | 0.59 | - | 0.77
(0.43–1.39) | 0.39 | - | 64.6 |
|
CIITA_BL1_ht1 | 0.250 | 0.308 | 0.74
(0.50–1.09) | 0.12 | - | 0.73
(0.45–1.18) | 0.20 | - | 0.54
(0.20–1.45) | 0.22 | - | 62.4 |
|
CIITA_BL1_ht2 | 0.293 | 0.234 | 1.31
(0.92–1.87) | 0.13 | - | 1.23
(0.76–1.99) | 0.40 | - | 2.16
(1.02–4.60) | 0.05 | NS | 57.1 |
|
CIITA_BL1_ht3 | 0.136 | 0.153 | 0.87
(0.54–1.40) | 0.57 | - | 0.94
(0.55–1.62) | 0.82 | - | 0.31
(0.04–2.46) | 0.27 | - | 46.4 |
|
CIITA_BL1_ht4 | 0.076 | 0.092 | 0.81
(0.43–1.52) | 0.51 | - | 0.84
(0.44–1.62) | 0.61 | - | - | - | - | 33.5 |
|
CIITA_BL1_ht5 | 0.098 | 0.059 | 1.68
(0.93–3.05) | 0.09 | - | 1.63
(0.84–3.16) | 0.15 | - | 6.10
(0.54–68.76) | 0.14 | - | 24.4 |
|
rs6498124 | 0.484 | 0.467 | 1.07
(0.76–1.49) | 0.71 | - | 1.12
(0.66–1.91) | 0.67 | - | 1.05
(0.60–1.85) | 0.86 | - | 64.9 |
|
rs4781016 | 0.163 | 0.215 | 0.72
(0.46–1.12) | 0.14 | - | 0.78
(0.47–1.29) | 0.33 | - | 0.18
(0.02–1.37) | 0.10 | - | 55.1 |
| rs4774 | 0.168 | 0.216 | 0.73
(0.47–1.14) | 0.16 | - | 0.79
(0.48–1.31) | 0.36 | - | 0.19
(0.02–1.46) | 0.11 | - | 55.2 |
|
rs4781019 | 0.353 | 0.333 | 1.11
(0.76–1.62) | 0.60 | - | 1.11
(0.68–1.81) | 0.67 | - | 1.20
(0.53–2.73) | 0.66 | - | 63.5 |
|
rs6498126 | 0.272 | 0.289 | 0.90
(0.60–1.34) | 0.59 | - | 0.76
(0.46–1.23) | 0.26 | - | 1.51
(0.61–3.74) | 0.37 | - | 61.4 |
|
rs11074938 | 0.418 | 0.510 | 0.66
(0.46–0.94) | 0.02 | NS | 0.45
(0.27–0.76) | 0.003 | 0.04 | 0.83
(0.46–1.49) | 0.52 | - | 64.0 |
|
rs11074939 | 0.413 | 0.337 | 1.40
(0.99–1.99) | 0.06 | - | 1.53
(0.93–2.52) | 0.09 | - | 1.58
(0.80–3.11) | 0.18 | - | 63.7 |
|
rs7404786 | 0.185 | 0.184 | 1.01
(0.65–1.56) | 0.97 | - | 0.94
(0.56–1.56) | 0.80 | - | 1.64
(0.47–5.77) | 0.44 | - | 51.2 |
|
rs7201430 | 0.141 | 0.142 | 0.99
(0.61–1.61) | 0.96 | - | 0.90
(0.52–1.56) | 0.71 | - | 2.14
(0.46–9.89) | 0.33 | - | 44.4 |
|
rs4781024 | 0.386 | 0.318 | 1.36
(0.96–1.95) | 0.09 | - | 1.48
(0.91–2.42) | 0.12 | - | 1.52
(0.74–3.10) | 0.26 | - | 62.9 |
|
rs1139564 | 0.196 | 0.239 | 0.78
(0.51–1.18) | 0.24 | - | 0.79
(0.48–1.29) | 0.34 | - | 0.52
(0.15–1.84) | 0.31 | - | 57.6 |
|
CIITA_BL2_ht1 | 0.315 | 0.278 | 1.22
(0.84–1.79) | 0.30 | - | 1.49
(0.92–2.41) | 0.11 | - | 0.71
(0.26–1.97) | 0.51 | - | 60.7 |
|
CIITA_BL2_ht2 | 0.141 | 0.188 | 0.71
(0.45–1.14) | 0.16 | - | 0.75
(0.44–1.27) | 0.28 | - | 0.24
(0.03–1.90) | 0.18 | - | 51.8 |
|
CIITA_BL2_ht3 | 0.120 | 0.115 | 1.04
(0.62–1.75) | 0.88 | - | 1.01
(0.57–1.82) | 0.96 | - | 1.41
(0.25–7.87) | 0.70 | - | 38.9 |
|
CIITA_BL2_ht4 | 0.092 | 0.080 | 1.21
(0.67–2.20) | 0.53 | - | 1.36
(0.71–2.58) | 0.35 | - | - | - | - | 30.4 |
|
CIITA_BL2_ht5 | 0.065 | 0.084 | 0.75
(0.39–1.45) | 0.39 | - | 0.79
(0.39–1.58) | 0.50 | - | - | - | - | 31.4 |
|
CIITA_BL2_ht6 | 0.060 | 0.061 | 0.97
(0.47–2.01) | 0.93 | - | 0.87
(0.40–1.87) | 0.71 | - | - | - | - | 25.0 |
Discussion
Although small polyps in the nasal may be harmless,
the development of nasal polyps could cause breathing difficulties
and infections. As a result, it is important to clarify the exact
mechanisms underlying nasal polyps pathogenesis. Nasal polyps often
occur with asthma and aspirin intolerance. Additionally, since the
formation of nasal polyps has been related to various immune
responses, cytokines and the MHC system have been considered to be
important molecules for the development of nasal polyps (18,19).
Although the development of nasal polyps has been associated with
AERD and ATA, the exact genetic factors in the pathogenesis of
nasal polyps have yet to be adequately elucidated.
To investigate causal genetic factor showing
associations between genetic effect and the development of nasal
polyps, numerous studies have been performed thus far on candidate
genes of nasal polyps including interleukins (ILs), TNF-α,
NOS2A, IFN, CD14, CCL5, CCL2, GSTT1, GSTM1, MMPs and MHC class
II genes (5,20–26).
Most studies were conducted in Caucasian population, with the
exception of one study investigating Taiwanese and Chinese
population (n=75–933 in all subjects; n=75–245 in nasal polyp
subjects) (5,20–26).
Among the variations in the tested candidate genes, -308 G/A
in TNF-α (P=0.02), rs17561 in IL1A (P=0.02),
(CCTTT)n in NOS2A (P=0.001), rs3939286 in
IL33 (P=0.004), HLA-DR7-DQA1*0201 and
HLA-DQB1*0202 haplotype, -174 G/C in IL6
(P=0.03) and rs3918242 in MMP-9 (P=0.02) were
significantly associated with the formation of nasal polyps.
However, no associations also were identified in CD14, CCL2,
CCL5, TNF-β1, deletion in GSTT1 and GSTM1, and
MMP-2. Although numerous studies have been performed to
identify genetic factors for nasal polyps, these studies were
mainly performed on Caucasian and not Asian populations. Moreover,
additional association studies on genes in the MHC region, which is
considered an important immunologic factor for the formation of
nasal polyps, are needed. CIITA, which is located on
chromosome 16p13 and distinguished from the MHC cluster (27), plays an important role in the
physiological regulation of the expression of MHC class II genes.
The mutational alteration of CIITA is able to cause a
complete lack of MHC class II expression in all tissues (28). Furthermore, CIITA SNPs have
been reported to be associated with immune diseases such as
multiple sclerosis (7) and
persistent hepatitis B virus (HBV) infection (29).
We investigated 18 polymorphisms in the CIITA
gene and carried out a case-control association analysis based on
three genetic models of inheritance in asthmatic patients,
classified into AERD and ATA groups. The results demonstrated that
a total of four SNPs were significantly associated with the
presence of nasal polyps in the overall asthmatic, AERD and ATA
groups. The results indicate that the genetic effect of
CIITA variations is likely to contribute to the formation of
nasal polyps in asthmatic patients. Furthermore, two variations
(rs12932187 and CIITA_BL1_ht2) were shown to be
associated with nasal polyps in ATA (Pcorr=0.02–0.04;
Table IV) compared to AERD
patients (P>0.05 for the variations). By contrast, the
association signals of rs1139564 were higher in the AERD
(P=0.01) compared to the ATA group. These findings indicate that
the four CIITA SNPs are potential markers of genetic
susceptibility to nasal polyposis between AERD and ATA groups.
Results of the present study have shown nominal
correlation between nasal polyposis and aspirin hypersensitivity in
asthma (Tables II and IV). Although this suggests that
CIITA potentially contributes to the development of nasal
polyps in AERD patients, the modest signal may also suggest a
putative relationship. Factors such as the decrease of the sample
size in the AERD group might influence the findings. Therefore,
additional large-scale studies as well as functional evaluations
that provide useful information for the pathogenesis of nasal polyp
development, are needed.
The association between CIITA variations and
nasal polyposis in asthmatic patients was investigated. To the best
of our knowledge, this study is the first to investigate the
association between CIITA variations and nasal polyposis in
allergic and non-allergic asthmatic patients. It was found that
rs12932187 and rs11074938 may be susceptibility
markers of inflammation of the nasal passages. However, the average
statistical power to detect the effect sizes of the significantly
associated SNPs was 67.89%, suggesting an insufficient sample size.
Therefore, further studies comparing patients with healthy subjects
are needed to confirm the conclusions of the present study.
Acknowledgements
This study was supported by the Korea Science and
Engineering Foundation (KOSEF), funded by the Korean Government
(MEST) (nos. 2009-0080157 and 2011-0004453), and a grant was
provided by the Korea Healthcare Technology R&D Project,
Ministry for Health, Welfare and Family Affairs, Republic of Korea
(A010249). This study was also supported by the Sogang University
Research Grant of 2011 (SRF-201114006.01). The DNA samples were
generously provided by the Soonchunhyang University, Bucheon
Hospital Biobank, a member of the National Biobank of Korea,
supported by the Ministry of Health, Welfare and Family Affairs,
Republic of Korea.
References
|
1
|
Ediger D, Sin BA, Heper A, Anadolu Y and
Misirligil Z: Airway inflammation in nasal polyposis:
immunopathological aspects of relation to asthma. Clin Exp Allergy.
35:319–326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hedman J, Kaprio J, Poussa T and Nieminen
MM: Prevalence of asthma, aspirin intolerance, nasal polyposis and
chronic obstructive pulmonary disease in a population-based study.
Int J Epidemiol. 28:717–722. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Munoz del Castillo F, Jurado-Ramos A,
Fernandez-Conde BL, et al: Allergenic profile of nasal polyposis. J
Investig Allergol Clin Immunol. 19:110–116. 2009.
|
|
4
|
Asero R and Bottazzi G: Hypersensitivity
to molds in patients with nasal polyposis: a clinical study. J
Allergy Clin Immunol. 105:186–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Molnar-Gabor E, Endreffy E and Rozsasi A:
HLA-DRB1, -DQA1, and -DQB1 genotypes in patients with nasal
polyposis. Laryngoscope. 110:422–425. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pang YT, Eskici O and Wilson JA: Nasal
polyposis: role of subclinical delayed food hypersensitivity.
Otolaryngol Head Neck Surg. 122:298–301. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bronson PG, Caillier S, Ramsay PP, et al:
CIITA variation in the presence of HLA-DRB1*1501 increases risk for
multiple sclerosis. Hum Mol Genet. 19:2331–2340. 2010.
|
|
8
|
LeibundGut-Landmann S, Waldburger JM,
Krawczyk M, et al: Mini-review: specificity and expression of
CIITA, the master regulator of MHC class II genes. Eur J Immunol.
34:1513–1525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harton JA and Ting JP: Class II
transactivator: mastering the art of major histocompatibility
complex expression. Mol Cell Biol. 20:6185–6194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu XS, Linhoff MW, Li G, Chin KC, Maity
SN and Ting JP: Transcriptional scaffold: CIITA interacts with
NF-Y, RFX, and CREB to cause stereospecific regulation of the class
II major histocompatibility complex promoter. Mol Cell Biol.
20:6051–6061. 2000. View Article : Google Scholar
|
|
11
|
Mumtaz M, Lofgren S, Hugander A and
Dimberg J: Polymorphism in MHC class II transactivator gene is not
associated with susceptibility to colorectal cancer in Swedish
patients. Anticancer Res. 28:1789–1791. 2008.PubMed/NCBI
|
|
12
|
Wang D, Levasseur-Acker GM, Jankowski R,
et al: HLA class II antigens and T lymphocytes in human nasal
epithelial cells. Modulation of the HLA class II gene transcripts
by gamma interferon. Clin Exp Allergy. 27:306–314. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koizumi K, Okamoto H, Iikuni N, et al:
Single nucleotide polymorphisms in the gene encoding the major
histocompatibility complex class II transactivator (CIITA) in
systemic lupus erythematosus. Ann Rheum Dis. 64:947–950. 2005.
View Article : Google Scholar
|
|
14
|
Skinningsrud B, Husebye ES, Pearce SH, et
al: Polymorphisms in CLEC16A and CIITA at 16p13 are associated with
primary adrenal insufficiency. J Clin Endocrinol Metab.
93:3310–3317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pasaje CF, Bae JS, Park BL, et al:
Association analysis of DTD1 gene variations with
aspirin-intolerance in asthmatics. Int J Mol Med. 28:129–137.
2011.PubMed/NCBI
|
|
16
|
Menashe I, Rosenberg PS and Chen BE: PGA:
power calculator for case-control genetic association analyses. BMC
Genet. 9:362008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nyholt DR: A simple correction for
multiple testing for single-nucleotide polymorphisms in linkage
disequilibrium with each other. Am J Hum Genet. 74:765–769. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kramer MF and Rasp G: Nasal polyposis:
eosinophils and interleukin-5. Allergy. 54:669–680. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karjalainen J, Joki-Erkkila VP, Hulkkonen
J, et al: The IL1A genotype is associated with nasal polyposis in
asthmatic adults. Allergy. 58:393–396. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Erbek SS, Yurtcu E, Erbek S, Atac FB,
Sahin FI and Cakmak O: Proinflammatory cytokine single nucleotide
polymorphisms in nasal polyposis. Arch Otolaryngol Head Neck Surg.
133:705–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang LF, Chien CY, Tai CF, Kuo WR, Hsi E
and Juo SH: Matrix metalloproteinase-9 gene polymorphisms in nasal
polyposis. BMC Med Genet. 11:852010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erbek SS, Yurtcu E, Erbek S and Sahin FI:
Matrix metalloproteinase-9 promoter gene polymorphism (−1562C>T)
in nasal polyposis. Am J Rhinol Allergy. 23:568–570. 2009.
|
|
23
|
Arbag H, Cora T, Acar H, Ozturk K, Sari F
and Ulusoy B: Lack of association between the
glutathione-s-transferase genes (GSTT1 and GSTM1) and nasal
polyposis. Rhinology. 44:14–18. 2006.PubMed/NCBI
|
|
24
|
Bernstein JM, Anon JB, Rontal M, Conroy J,
Wang C and Sucheston L: Genetic polymorphisms in chronic
hyperplastic sinusitis with nasal polyposis. Laryngoscope.
119:1258–1264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mfuna Endam L, Cormier C, Bosse Y,
Filali-Mouhim A and Desrosiers M: Association of IL1A, IL1B, and
TNF gene polymorphisms with chronic rhinosinusitis with and without
nasal polyposis: A replication study. Arch Otolaryngol Head Neck
Surg. 136:187–192. 2010.PubMed/NCBI
|
|
26
|
Pascual M, Sanz C, Isidoro-Garcia M, et
al: (CCTTT)n polymorphism of NOS2A in nasal polyposis and asthma: a
case-control study. J Investig Allergol Clin Immunol. 18:239–244.
2008.PubMed/NCBI
|
|
27
|
Mach B, Steimle V, Martinez-Soria E and
Reith W: Regulation of MHC class II genes: lessons from a disease.
Annu Rev Immunol. 14:301–331. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Otten LA, Steimle V, Bontron S and Mach B:
Quantitative control of MHC class II expression by the
transactivator CIITA. Eur J Immunol. 28:473–478. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He Y, Zhao Y, Zhang S, et al: Not
polymorphism but methylation of class II transactivator gene
promoter IV associated with persistent HBV infection. J Clin Virol.
37:282–286. 2006. View Article : Google Scholar : PubMed/NCBI
|