Introduction
Neoplasms of the salivary glands constitute 3–10% of
all tumors of the head and neck (1), and are located in the parotid gland
in 34–86% of cases (2,3). Among parotid tumors, benign tumors
are more common than malignant ones (4,5).
Parotidectomy is commonly used in the treatment of gland tumors; it
is the first choice to treat gland tumors (5–7).
However, complications, such as Frey syndrome (8), transient or permanent facial nerve
paresis (9), cosmetic
disfigurement (10), pain and
discomfort, and subsequent xerostomia, can reduce the quality of
life of patients after parotidectomy (11). Among these complications, Frey
syndrome has the highest incidence, from 11 to 95% (12–14).
Frey syndrome was first described by Łucja (Lucie)
Frey in 1923 (15,16). Andre Thomas in 1927 and later Ford
and Woodhall in 1938 postulated the theory of aberrant regeneration
of the sectioned parasympathetic fibers that regrow to innervate
the vessels and sweat glands of the skin overlying the parotid to
explain the symptoms (15,17). Although Frey syndrome is not
life-threatening, the surveys show that Frey syndrome and concave
facial deformity are identified as the most serious self-perceived
sequelae with significant potential negative social and
psychological implications, which result in discomfort worsening
with time (7,11,12,18,19).
Thus, the goals of parotidectomy are to remove the primary tumor,
prevent severe functional loss, avoid cosmetic defects, and
particularly to prevent Frey syndrome.
The therapeutic and preventive methods for Frey
syndrome can be subdivided into surgical and non-surgical
modalities. Non-surgical techniques used to prevent Frey syndrome
include drugs; the most common is a local injection of botulinum
toxin. Yet, this has many side effects, including mild and
temporary muscle weakness and pain at the injection site, easy
recrudescence, and the procedure also frequently affects the
quantity and quality of salivary flow (20,21).
The major surgical techniques include implantation
of a ‘barrier’, including autogenous vascularized tissue [such as
the sternocleidomastoid muscle flap (22), temporoparietal fascia rotational
flap (23) and the superficial
muscular aponeurotic system (24)], non-vascularized tissue [such as
fascia lata (25) and dermis fat
grafts (26)], and synthetic
biomaterials [such as expanded polytef (27)]. However, these surgical management
options are limited by at least one of the following factors: i)
the need for a second operation (with its associated potential
morbidity), ii) the need for an additional donor site, iii)
prolonged period of time under general anesthesia, iv) insufficient
amount of tissue to cover the wound surface completely, v)
inability to reduce the incidence of Frey syndrome (28), and vi) the higher incidence of
wound infection, rejection reaction or other postoperative
complications (29).
A ‘barrier’ that is able to demonstrate efficacy
with regard to preventing Frey syndrome, while at the same time
eliminating the disadvantages mentioned above would be prefered and
is now being considered. AlloDerm®, in addition to
serving as a nerve barrier, may serve as an effective soft tissue
augmentation device in the head and neck region (30,31).
Since it undergoes fibrous tissue ingrowth, has extensive sources,
convenient manufacturing, efficient industrialization and virtually
no side effects, it is considered as an ideal barrier in the field
of oral health to date (32).
The first published study on the utilization of
AlloDerm in humans was conducted by MacKinnon in 1997 (33), and it has been widely used in this
field and reported in many research articles (34–41).
It is a new type of biological material, yet its long-term side
effects are unknown. Therefore, we performed this systematic review
and meta-analysis in order to evaluate the effectiveness and safety
of AlloDerm to prevent Frey syndrome after parotidectomy.
Materials and methods
Selection of studies
A comprehensive systematic search was conducted by
two independent reviewers (X.T.Z. and M.Z.L.) in PubMed (1966 to
May 2011), Embase (1974 to May 2011), the Cochrane Library (issue
4, 2011) and the ISI Web of Knowledge databases (1994 to May 2011)
for relevant citations. In addition, we searched the reference
lists of all known primary and review articles. All identified
articles were screened for cross-references; no language
restrictions were imposed.
The search terms were combined with (‘Frey syndrome’
OR ‘Frey’s syndrome’ OR ‘gustatory sweating’ OR ‘auriculotemporal
syndrome’) AND (‘AlloDerm’ OR ‘acellular tissue patch’ OR
‘acellular dermal matrix’ OR ‘acellular dermal’ OR ‘acellular
dermis’ OR ‘Permacol’ OR ‘porcine dermal matrix’ OR ‘allograft
dermis’ OR ‘allograft dermal matrix’), then duplicated results were
removed. The remaining citations were displayed and examined.
Inclusion and exclusion criteria
Eligibility was determined by two independent
reviewers (X.J.T. and W.H.), with consensus from the third reviewer
(W.D.L.), on the basis of information found in the article’s title,
abstract or full text. Studies were included in the review if they
met the following criteria: randomized controlled trials (RCTs) and
quasi-RCTs of all the articles, the study patients included adults
(≥18 years) who were diagnosed with parotid tumors and had received
a partial or total parotidectomy with facial nerve preservation,
AlloDerm was applied as the experimental group and a placebo
(blank) as the control group. Studies that included patients with
previous surgical procedures in the parotid area or with previous
radiotherapy were excluded. Review ariticles, commentaries,
guidelines and letters were also excluded.
Data extraction
The data were extracted by two reviewers (X.J.W and
Y.M.N.) independently. In case of discrepancies, a third reviewer
(W.D.L.) was consulted and, after agreement, a consensus was
reached. Data were extracted on publication data (the first
author’s last name, year of publication and country of population
studied), sample size, patient characteristics (mean age and sex
ratio), study design, follow-up period, outcome measures and method
of measurement. Authors were contacted by e-mail for additional
information if data was unavailable.
The primary outcome measure was the incidence of
Frey syndrome (objective or subjective). Secondary outcomes
included facial contour, adverse events (wound infection and
rejection), other postoperative complications (seroma or sialocele,
salivary fistula and facial nerve paralysis) that were noted when
they were reported in the studies.
Quality assessment
We assessed the study quality using the Cochrane
Handbook’s evalution tool for assessing the risk of bias (42) and the Jadad scoring system
(43). It was also conducted by
two independent reviewers (X.T.Z. and M.Z.L.) and an agreement was
reached after consulting a third reviewer (Y.G.).
The Cochrane Handbook’s evalution tool included:
Sequence generation – Was the allocation sequence adequately
generated? Allocation concealment – Was allocation adequately
concealed? Blinding – Was knowledge of the allocated intervention
adequately blinded during the study? Blinding of participants and
personnel? Blinding of outcome assessors? Incomplete outcome data –
Were incomplete outcome data adequately addressed? Selective
outcome reporting – Were reports of the study free of suggestion of
selective outcome reporting? Other sources of bias – Was the study
apparently free of other problems that could put it at a high risk
of bias?
The Jadad scoring system included: Was the study
described as randomized? Was the method used to generate the
sequence of randomization described and appropriate? Was the study
described as double-blind? Was the method of double-blinding
described and appropriate? Was there a description of withdrawals
and dropouts?
This is a five-point scale, with low-quality studies
having a score of ≤2 and high-quality studies a score of at least
3.
Statistical analysis
Data were processed in accordance with the Cochrane
Handbook (42). Intervention
effects were expressed with relative risks (RRs) and associated 95%
confidence intervals (CIs) for dichotomous data and mean
differences (MDs) and 95% CIs for continuous data, respectively.
Heterogeneity among studies was informally assessed by visual
inspection of forest plots, and formally estimated using the
Chi-square test and I2 test (both P>0.05 and
I2<50% indicated there was no evidence of
heterogeneity between the pooled studies) (44). The fixed-effects model was first
used for meta-analyses; if there was heterogeneity, the
random-effects model was used. Publication bias was tested from
separate funnel plots (when the number of included studies was ≥9).
Symmetry of and outlying results on, the funnel plots implied lack
of bias, whereas asymmetry would imply that the results were
subject to reporting or publication bias (45). All statistical analyses were
performed using Review Manager (version 5.0.2; The Cochrane
Collaboration). Description analysis was performed when the
quantitative data could not be pooled. The data were entered into
Review Manager by X.T.Z., and M.N.Y. checked data entry.
Results
Characteristics and quality of the
included studies
A database search yielded 25 publications, of which
both of the reviewers considered 14 to be potentially eligible. We
excluded 9 of the articles during the second phase of the inclusion
process, 2 were not controlled (39,41),
3 were case reports (33,40,46)
and 1 was a meta-analysis (47).
One was not done up to Frey syndrome (48) and 2 were commentaries (49,50).
The remaining 5 articles were included in the meta-analysis
(34–38). A summary of the study selection
process is presented in Fig. 1.
There were 3 studies published in English (34,36,37)
and 2 in Chinese (35,38). A total of 409 participants were
included. The sample size ranged from 20 to 168, and the follow-up
period ranged from 5 to 39 months. All of the studies had similar
eligibility criteria (Table
I).
 | Table ICharacteristics and Jadad scores of
the included studies. |
Table I
Characteristics and Jadad scores of
the included studies.
| Author/(Ref.) | Year and
country | Group | No. | Mean age
(years) | Gender (M:F) | Interventions | Study period
(years) | Follow-up
(months) | Measurement | Outcomes | Jadad scores |
|---|
| Govindaraj et
al (34) | 2001 USA | E | 32 | 51.7 | 12:20 | AlloDerm | 1997–1999 | ≥6 | Subjective | Frey syndrome | 1 |
| C | 32 | 49.8 | 14:18 | Blank | Objective | Facial contour
Complications |
| Sinha et al
(36) | 2003 USA | E | 10 | ≥18 | Not reported | AlloDerm | Not reported | ≥12 | Subjective | Frey syndrome | 1 |
| C | 10 | | | Blank | Objective | Complications |
| Yu et al
(38) | 2007 China | E | 30 | 45 | Not reported | AlloDerm | 2005–2006 | 5–14 | Objective | Frey syndrome | 1 |
| C | 27 | 43 | | Blank | | |
| Ye et al
(37) | 2008 China | E | 64 | 42 | Not reported | AlloDerm | 2004–2005 | 11–27 | Subjective | Frey syndrome | 1 |
| C | 104 | | | Blank | Objective | Facial contour
Complications |
| Li et al
(35) | 2006 China | E | 50 | 40 | 20:30 | AlloDerm | 2001–2004 | 10–39 | Subjective | Frey syndrome | 3 |
| C | 50 | | 21:29 | Blank | | Complications |
There was good agreement between the reviewers in
regards to the validity assessments. All studies were clinical
controlled trials, and the methodological quality of the included
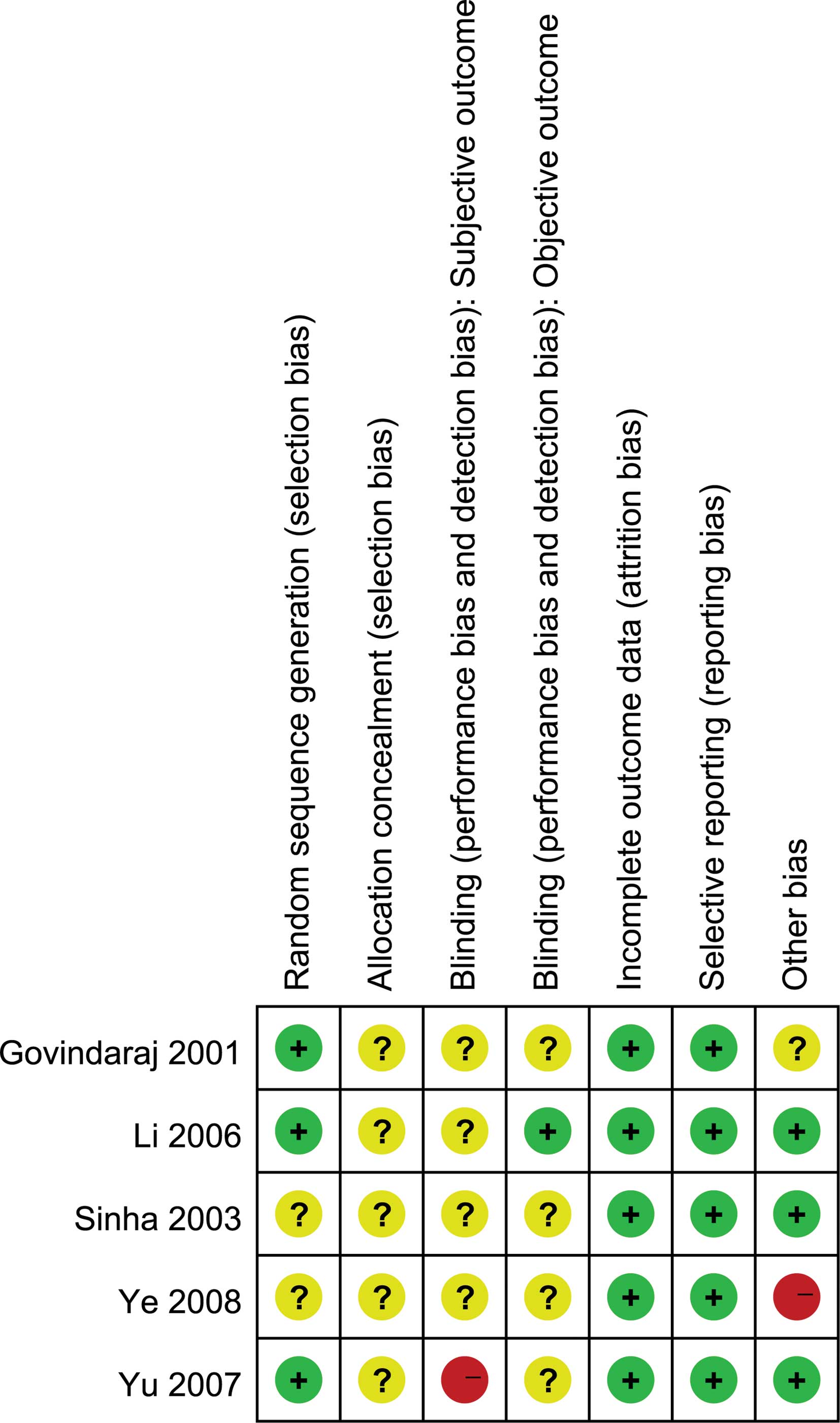
trials ranged from poor to excellent (Table I). Fig. 2 shows the risk of bias summary; the
majority had adequate patient follow-up, and the main study biases
may be caused by sample size, randomization, the procedure for
concealing the treatment allocation and blinding (as it is not
feasible to blind staff in these studies, blinding of investigators
is feasible). For example, only 1 study (35) mentioned randomization and blinding,
but did not describe how the random allocation sequence was
generated.
Frey syndrome
We extracted data for the incidence of Frey syndrome
from the included 5 studies. Four studies reported data of the
objective incidence (34,36–38),
for which outcomes were consistent across studies (heterogeneity
P=0.49, I2=0%), so the fixed effects model was used. The
result showed that there was a significant trend toward lower
incidence in the AlloDerm group (Fig.
3; RR=0.15, 95% CI 0.08–0.30; P<0.00001).
Four studies reported data involving subjective
incidence (34–37); there was significant heterogeneity
(P=0.01, I2=71%), therefore, the random effects model
was used. The result also showed that there was a significant trend
toward lower incidence in the AlloDerm group (RR=0.16, 95% CI
0.09–0.28; P<0.00001). We explored possible causes of
heterogeneity, taking into account the imbalance of patients
between the two groups in one trial (37). When we excluded this trial from the
analysis, the heterogeneity disappeared (Fig. 4; P=0.83, I2=0%), and the
trend toward the AlloDerm group weakened (Fig. 4; RR=0.32, 95% CI 0.19–0.57;
P<0.00001).
Facial contour
Two trials reported the perception of cosmetic
appearance and facial symmetry (36,37).
They found that using AlloDerm to fill in the parotid bed resulted
in better cosmesis and restoration of good soft tissue contour at
the surgical site (symmetrical, with fine scars), while the scars
of the control patients were noticeable, locally depressed and with
wrinkled skin.
Adverse events
Adverse events in the original studies consisted of
wound infection and rejection. Four studies reported data on wound
infection (34–37); there was no significant trend
toward increased incidence in the AlloDerm group (Fig. 5; RR=3.00, 95% CI 0.14–65.90;
P=0.49). The reason for the adverse event was streptococcus
species, not the AlloDerm (36).
Four studies reported data for rejection (34–37),
but all of them reported that there were no cases of implant
extrusion that occurred in the AlloDerm group.
Other postoperative complications
Other postoperative complications in the original
studies consisted of seroma/sialocele, saliva fistula and facial
nerve paralysis. Fig. 6 summarizes
the results.
Three studies reported data regarding
seroma/sialocele (34–36), for which outcomes were consistent
across studies (P=0.46, I2=0%). There was no significant
trend toward an increase in incidence in the AlloDerm group
(RR=1.36, 95% CI 0.66–2.80; P=0.40).
Two studies reported data regarding salivary fistula
(36,37), the result revealed that the
incidence was lower in the AlloDerm group and the difference had
statistical significance (RR=0.09, 95% CI 0.01–0.66; P=0.02).
Three studies reported data concerning facial nerve
paralysis (34–36); there was no significant trend
toward a lower incidence in the AlloDerm group (RR=0.96, 95% CI
0.84–1.09; P=0.51).
Discussion
Principal findings
Our systematic review identified 5 studies that
addressed the relative impact of AlloDerm and a blank control. We
found that the use of AlloDerm was associated with significant
reductions in Frey syndrome (both subjective and objective
symptoms) after parotidectomy.
The period of onset of Frey syndrome is more than
3–6 months after surgery: 3 years (51), 8 and a half years (52) and, at present, the longest is 14
years (53) as reported in
research articles. The follow-up period ranged from 5 to 39 months
in the included studies, so we believe that the preliminary results
are reliable.
The present meta-analysis confirms that, despite
various confounding factors, AlloDerm does decrease the occurrence
of Frey syndrome after parotidectomy by 85% (objective) and 68%
(subjective). We also found a possible trend that AlloDerm may
decrease the incidences of salivary fistula and facial nerve
paralysis, and improve facial contour. Although AlloDerm has
several potential adverse effects, they are minimal compared to
Frey syndrome and other complications, and these effects are able
to be prevented or solved easily.
Strengths and limitations
Our study is more precise than previous ones
(47). Previously published
meta-analyses have focused on the preventive effect of surgical
techniques, and carried out a search only in PubMed for
English-language studies. They included many surgical techniques
(AlloDerm was one of them), yet no subgroup analysis was performed,
although heterogeneity was high. Therefore, the authors suggested
that further studies were necessary to stratify differences among
the various available techniques. Also, they did not take into
account adverse events or other complications.
Our study also has limitations. Firstly, the number
of studies contributing substantial data to the meta-analysis was
small; therefore, we could not fully assess the effects of
important clinical factors that may have influenced outcomes.
Possible problems with concealment, lack of blinding and loss of
follow-up could have introduced bias. The sample sizes were quite
few, thus we could not adequately assess effects. Finally,
potential limitations of any meta-analysis is the ‘file-drawer’
effect, in which studies with negative results may remain
unpublished, thus biasing the literature toward positive
findings.
Clinical and policy implications
Our results may have potential implications for
clinical practice and health policy. Frey syndrome is a widely
recognized sequela of parotidectomy; there exist many strategies
for both its prevention and treatment. Before the development of
AlloDerm, previous strategies were able to effectively prevent Frey
syndrome (47), but had various
disadvantages (20,21,28,29).
Our results, although based on only 5 randomized controlled trials,
indicate that it is plausible that AlloDerm delivers a clinically
significant reduction in preventing Frey syndrome, without adverse
effects. It may also reduce salivary fistula and facial nerve
paralysis, and improve facial contour.
Shuman and Bradford (54) supported the fact that surgeons are
obligated to inform patients regarding the significance of Frey
syndrome prior to surgery. Yet, to date the process of informed
consent and pre-operative decision-making has posed a potential
ethical quandary. Many studies have also come to the conclusion
that Frey syndrome interferes with the quality of life of patients
(7,11,12,18,19).
Therefore, surgeons and policy makers need to address the clinical
importance and value of the heighted focus in relation to the goals
of treatment, so that AlloDerm may meet their and their patients’
expectations.
Implications for research
The methods in trials were limited by the inability
to blind clinical staff to the method of operation, yet the
blinding of investigators to evaluate and collect outcome data is
feasible. Unfortunately, only 1 study performed blinding of
investigators (35). Therefore it
is possible that the decisions and actions of the clinicians could
have been influenced, resulting in biased estimates of treatment
effect.
We also found that AlloDerm may reduce salivary
fistula and facial nerve paralysis, and improve facial contour. As
a result, future studies should follow the CONSORT 2010 statement
(55) to design and report, in
order to provide high-quality evidence.
In conclusion, evidence from the included studies
suggests that use of AlloDerm results in decreased total incidence
of Frey syndrome. Evidence also suggests that AlloDerm improves
facial contour, may reduce salivary fistula and facial nerve
paralysis, without adverse events. Yet limited data from the
included studies is currently available to confirm this. Our study
also shows that it is unclear whether the use of AlloDerm permits
any conclusions about the incidence of other perioperative
complications. Further studies are required to establish the
optimal design and optimal outcome indicators.
Acknowledgements
This study was supported by grants from the
Foundation of Taihe Hospital of Shiyan City (2011QD01 and
2011QD05), without commercial or not-for-profit sectors.
References
|
1
|
MH AnsariSalivary gland tumors in an
Iranian population: a retrospective study of 130 casesJ Oral
Maxillofac Surg6521872194200710.1016/j.joms.2006.11.02517954313
|
|
2
|
EA VuhahulaSalivary gland tumors in
Uganda: clinical pathological studyAfr Health
Sci41523200415126188
|
|
3
|
JA PinkstonP ColeIncidence rates of
salivary gland tumors: results from a population-based
studyOtolaryngol Head Neck
Surg120834840199910.1016/S0194-5998(99)70323-210352436
|
|
4
|
LJ LiY LiYM WenH LiuHW ZhaoClinical
analysis of salivary gland tumor cases in West China in the past 50
yearsOral Oncol44187192200817418612
|
|
5
|
FG SmiddyTreatment of mixed parotid
tumoursBr Med J1322325195610.1136/bmj.1.4962.322
|
|
6
|
YC LimSY LeeK KimConservative
parotidectomy for the treatment of parotid cancersOral
Oncol4110211027200510.1016/j.oraloncology.2005.06.00416129655
|
|
7
|
MA ClaymanSM ClaymanMB SeagleA review of
the surgical and medical treatment of Frey syndromeAnn Plast
Surg57581584200610.1097/01.sap.0000237085.59782.6517060744
|
|
8
|
A HerxheimerGustatory sweating and
pilomotionBr Med J1688689195810.1136/bmj.1.5072.68813510771
|
|
9
|
DH PateyRisk of facial paralysis after
parotidectomyBr Med
J211001102196310.1136/bmj.2.5365.110014057712
|
|
10
|
N MohyuddinSR HoffM YaoThe use of
allograft dermis implants and the facelift incision in parotid
surgeryOperative Techniques in Otolaryngology-Head and Neck
Surgery20114119200910.1016/j.otot.2009.01.010
|
|
11
|
D NitzanJ KronenbergZ HorowitzQuality of
life following parotidectomy for malignant and benign diseasePlast
Reconstr
Surg11410601067200410.1097/01.PRS.0000135326.50939.C115457013
|
|
12
|
M KochJ ZenkH IroLong-term results of
morbidity after parotid gland surgery in benign
diseaseLaryngoscope120724730201010.1002/lary.2082220205175
|
|
13
|
K HarrisonI DonaldsonFrey’s syndromeJ R
Soc Med725035081979
|
|
14
|
R De BreeI van der WaalCR
LeemansManagement of Frey syndromeHead Neck297737782007
|
|
15
|
P DulguerovF MarchalC GysinFrey syndrome
before Frey: the correct
historyLaryngoscope10914711473199910.1097/00005537-199909000-0002110499057
|
|
16
|
P DulguerovF MarchalC GysinThe bullet that
hit a nerve: the history of Lucja Frey and her syndromeJ Laryngol
Otol12010872006
|
|
17
|
DH GlaisterJR HearnshawPF HeffronAW PeckDH
PateyThe mechanism of post-parotidectomy gustatory sweating (the
auriculo-temporal syndrome)Br Med
J2942946195810.1136/bmj.2.5102.94213584806
|
|
18
|
CH BaekMK ChungHS JeongQuestionnaire
evaluation of sequelae over 5 years after parotidectomy for benign
diseasesJ Plast Reconstr Aesthet Surg62633638200918314403
|
|
19
|
D BeutnerC WittekindtS DinhKB HuttenbrinkO
Guntinas-LichiusImpact of lateral parotidectomy for benign tumors
on quality of lifeActa
Otolaryngol12610911095200610.1080/0001648060060673116923716
|
|
20
|
SR BomeliSC DesaiJT JohnsonRR
WalvekarManagement of salivary flow in head and neck cancer
patients – a systematic reviewOral Oncol44100010082008
|
|
21
|
CC WangCP WangPreliminary experience with
botulinum toxin type A intracutaneous injection for Frey’s
syndromeJ Chin Med Assoc68463467200516265860
|
|
22
|
K AsalA KoybasiogluE
InalSternocleidomastoid muscle flap reconstruction during
parotidectomy to prevent Frey’s syndrome and facial contour
deformityEar Nose Throat J841731762005
|
|
23
|
RY RubinsteinA RosenD LeemanFrey syndrome:
treatment with temporoparietal fascia flap interpositionArch
Otolaryngol Head Neck
Surg125808811199910.1001/archotol.125.7.80810406323
|
|
24
|
R Moulton-BarrettG AllisonI
RappaportVariation’s in the use of SMAS (superficial
musculoaponeurotic system) to prevent Frey’s syndrome after
parotidectomyInt Surg811741761996
|
|
25
|
KA WallisT GibsonGustatory sweating
following parotidectomy: correction by a fascia lata graftBr J
Plast Surg316871197810.1016/0007-1226(78)90020-6623929
|
|
26
|
S ChandaranaK FungJH FranklinT KotylakDB
MaticJ YooEffect of autologous platelet adhesives on dermal fat
graft resorption following reconstruction of a superficial
parotidectomy defect: a double-blinded prospective trialHead
Neck31521530200910.1002/hed.20999
|
|
27
|
LJ ShemenExpanded polytef for
reconstructing postparotidectomy defects and preventing Frey’s
syndromeArch Otolaryngol Head Neck Surg1211307130919957576480
|
|
28
|
O Guntinas-LichiusB GabrielJP
KlussmannRisk of facial palsy and severe Frey’s syndrome after
conservative parotidectomy for benign disease: analysis of 610
operationsActa Otolaryngol126110411092006
|
|
29
|
P DulguerovD QuinodozG CosendaiP PilettaF
MarchalW LehmannPrevention of Frey syndrome during
parotidectomyArch Otolaryngol Head Neck
Surg125833839199910.1001/archotol.125.8.83310448728
|
|
30
|
BM AchauerVM Van der KamB CelikozDG
JacobsonAugmentation of facial soft-tissue defects with Alloderm
dermal graftAnn Plast
Surg41503507199810.1097/00000637-199811000-000099827953
|
|
31
|
J ShulmanClinical evaluation of an
acellular dermal allograft for increasing the zone of attached
gingivaPract Periodontics Aesthet Dent820120819969028293
|
|
32
|
DS ThomaGI BenicM ZwahlenCH HammerleRE
JungA systematic review assessing soft tissue augmentation
techniquesClin Oral Implants Res20Suppl
4146165200910.1111/j.1600-0501.2009.01784.x19663961
|
|
33
|
K WebsterEarly results using a porcine
dermal collagen implant as an interpositional barrier to prevent
recurrent Frey’s syndromeBr J Oral Maxillofac
Surg3510410619979146867
|
|
34
|
S GovindarajM CohenEM GendenPD
CostantinoML UrkenThe use of acellular dermis in the prevention of
Frey’s syndromeLaryngoscope111199319982001
|
|
35
|
DZ LiYH WuXL WangSY LiuZJ LiProspective
cohort study on prevention of Frey syndrome in parotid
surgeryZhonghua Wai Ke Za Zhi4410331035200617074239
|
|
36
|
UK SinhaD SaadatCM DohertyDH RiceUse of
AlloDerm implant to prevent Frey syndrome after parotidectomyArch
Facial Plast Surg5109112200310.1001/archfaci.5.1.10912533152
|
|
37
|
WM YeHG ZhuJW ZhengUse of allogenic
acellular dermal matrix in prevention of Frey’s syndrome after
parotidectomyBr J Oral Maxillofac Surg46649652200818547692
|
|
38
|
K YuJ YangMJ LiHB MaClinical application
of acellular dermal matrix to prevent gustatory sweating
syndromeZhonghua Kou Qiang Yi Xue Za Zhi42570571200718070440
|
|
39
|
W ChenJ LiZ YangW YongjieW ZhiquanY
WangSMAS fold flap and ADM repair of the parotid bed following
removal of parotid haemangiomas via pre- and retroauricular
incisions to improve cosmetic outcome and prevent Frey’s syndromeJ
Plast Reconstr Aesthet Surg61894900200818504166
|
|
40
|
MA ClaymanLZ ClaymanUse of AlloDerm as a
barrier to treat chronic Frey’s syndromeOtolaryngol Head Neck
Surg1246872001
|
|
41
|
N PapadogeorgakisV PetsinisP
ChristopoulosN MavrovouniotisC AlexandridisUse of a porcine dermal
collagen graft (Permacol) in parotid surgeryBr J Oral Maxillofac
Surg47378381200910.1016/j.bjoms.2008.09.00418963286
|
|
42
|
JPT HigginsS GreenCochrane Handbook for
Systematic Reviews of Interventions version 510 (updated March
2011)The Cochrane Collaboration2011Available from www.cochrane-handbook.org
|
|
43
|
AR JadadRA MooreD CarrollAssessing the
quality of reports of randomized clinical trials: is blinding
necessary?Control Clin
Trials17112199610.1016/0197-2456(95)00134-4
|
|
44
|
JP HigginsSG ThompsonQuantifying
heterogeneity in a meta-analysisStat
Med2115391558200210.1002/sim.118612111919
|
|
45
|
M EggerGD SmithBias in location and
selection of
studiesBMJ3166166199810.1136/bmj.316.7124.619451274
|
|
46
|
C MacKinnonM LovieAn alternative treatment
for Frey syndromePlast Reconstr
Surg103745746199910.1097/00006534-199902000-000769950578
|
|
47
|
JM CurryN KingD ReiterK FisherRN
HeffelfingerEA PribitkinMeta-analysis of surgical techniques for
preventing parotidectomy sequelaeArch Facial Plast
Surg11327331200910.1001/archfacial.2009.6219797095
|
|
48
|
SM SachsmanDH RiceUse of AlloDerm implant
to improve cosmesis after parotidectomyEar Nose Throat
J86512513200717915677
|
|
49
|
JC Lopez GutierrezThe SMAS fold flap and
ADM repair of the parotid bed following removal of parotid
haemangiomas via pre- and retroauricular incisions to improve
cosmetic outcome and prevent Frey’s syndromeJ Plast Reconstr
Aesthet Surg61900200818504166
|
|
50
|
JK WuThe SMAS fold flap and ADM repair of
the parotid bed following removal of parotid haemangiomas via pre-
and retroauricular incisions to improve cosmetic outcome and
prevent Frey’s syndromeJ Plast Reconstr Aesthet
Surg21889900200818504166
|
|
51
|
J RustemeyerH EufingerA BremerichThe
incidence of Frey’s syndromeJ Craniomaxillofac Surg3634372008
|
|
52
|
S MalatskeyI RabinovichM FradisM PeledFrey
syndrome – delayed clinical onset: a case reportOral Surg Oral Med
Oral Pathol Oral Radiol Endod943383402002
|
|
53
|
GI WenzelW DrafUnusually long latency
before the appearance of Frey’s syndrome after
parotidectomyHNO525545562004(In German).
|
|
54
|
AG ShumanCR BradfordEthics of Frey
syndrome: ensuring that consent is truly informedHead
Neck3211251128201010.1002/hed.2144320641103
|
|
55
|
KF SchulzDG AltmanD MoherC GroupCONSORT
2010 statement: updated guidelines for reporting parallel group
randomised trialsBMJ340c332201010.1136/bmj.c33220332509
|