Introduction
Obesity is closely associated with a low-grade state
of inflammation, resulting from enlargement of adipocytes and
increased macrophage infiltration into the adipose tissue (1,2).
Obese adipose tissue is characterized by abnormal production and
secretion of adipokines as well as activation of inflammatory
signaling in adipocytes (1,2). As
obesity-induced inflammation in adipocytes develops, secretion of
pro-inflammatory adipokines, including monocyte chemoattractant
protein-1 (MCP-1) and interleukin-6 (IL-6), increases, which in
turn leads to a decrease in insulin sensitivity (1,2).
Furthermore, it has been suggested that the nuclear factor-κB
(NF-κB) pathway plays an important role in facilitating adipocyte
inflammation. The NF-κB signal is related to the up-regulation of
pro-inflammatory adipokines and the down-regulation of adiponectin
which has anti-inflammatory and insulin-sensitizing properties
(3–5).
Tumor necrosis factor-α (TNF-α) is one of the most
important molecules in obesity. TNF-α released from adipose tissue
and macrophages is markedly increased in obese or diabetic subjects
(6,7). It induces insulin resistance by
modulating the secretion of pro-inflammatory adipokines, such as
MCP-1 and IL-6, and directly contributing to the inhibition of
adiponectin production (6–9). It has been shown that TNF-α activates
the NF-κB pathway and suppresses the expression of peroxisome
proliferator-activated receptor-γ (PPARγ), which is a strong
transcriptional inducer of adiponectin (10).
Vitamin E exists in nature as eight vitamers that
are subdivided into two subgroups called tocopherols and
tocotrienols, each including the α-, β-, γ- and δ-forms (11,12).
Tocopherols possess a saturated phytyl chain, whereas tocotrienols
contain an unsaturated side chain. Vitamin E isomers differ from
each other by the number of methyl groups in the chroman ring.
Although tocopherols and tocotrienols exhibit strong anti-oxidant
activities (11,12), most vitamin E studies have focused
on tocopherols, and very little is known about tocotrienols.
Tocotrienols are primarily found in oat, wheat germ, rice bran and
palm oil (11). However, previous
studies have found that tocotrienols have various physiological
activities, including anticancer, cardiovascular-protective,
hypocholesterolemic and neuroprotective activities (12,13).
Among the tocotrienol isomers, γ-tocotrienol, the most common
tocotrienol isomer, has been well documented for its physiological
availability. It has been shown that γ-tocotrienol suppresses
adipocyte differentiation (14),
and oral administration of γ-tocotrienol significantly decreases
body fat in rats (15).
Tocotrienols have been shown to possess
anti-inflammatory effects in certain cell types, which are mediated
by inhibition of the NF-κB activation pathway (16,17).
It remains unknown whether γ-tocotrienol exerts such effects in
adipocytes. Although administered γ-tocotrienol can be accumulated
in adipose tissue (18), there
have been no reports on its effects on adipokine regulation.
Therefore, in the present study, we examined the effects of
γ-tocotrienol on the TNF-α-induced changes in secretion and gene
expression of inflammatory-related adipokines, and activation of
the NF-κB pathway in 3T3-L1 adipocytes.
Materials and methods
Reagents
Recombinant TNF-α was purchased from R&D Systems
(Minneapolis, MN, USA). γ-tocotrienol was from Cayman Chemical (Ann
Arbor, MI, USA). Dulbecco's modified Eagle's medium (DMEM) and
insulin were from Sigma-Aldrich (St. Louis, MO, USA).
Isobutylmethylxanthine (IBMX), sodium pyruvate and dexamethasone
(DEX) were from Nacalai Tesque (Kyoto, Japan). Fetal bovine serum
(FBS) was from Gibco-BRL (Rockville, MD, USA).
Cell culture and treatment
3T3-L1 cells (Health Science Research Resources
Bank, Osaka, Japan), were maintained in DMEM containing 25 mM
glucose, 1 mM sodium pyruvate and 10% FBS, in a 5% CO2
atmosphere at 37˚C. Differentiation was induced by replacing the
medium with FBS-supplemented DMEM containing 200 nM insulin, 0.5 mM
IBMX and 1 μM DEX for 2 days. After another 2 days of incubation in
10% FBS/DMEM medium with 200 nM insulin, the medium was replaced
every 2 days with 10% FBS/DMEM medium until >90% of cells were
demonstrating the adipocyte phenotype. On days 6–8 of
differentiation, 3T3-L1 adipocytes were pre-treated with 0.024–2.4
μM γ-tocotrienol for 6 h and then stimulated for 24 h with 10 ng/ml
TNF-α. Following the 24-h incubation, the conditioned medium was
collected for measurement of adipokines by ELISA. Total RNA and
protein were isolated and the expression levels of the genes and
proteins of interest were evaluated by quantitative real-time
RT-PCR and Western blotting, respectively.
Real-time RT-PCR analysis
Total RNA was extracted from 3T3-L1 adipocytes using
Sepasol-RNA I (Nacalai Tesque, Kyoto, Japan). For complementary DNA
synthesis, 1 μg of total RNA was reverse-transcribed using the
reverse transcription system (PrimeScript RT reagent kit, Takara
Bio, Shiga, Japan). Real-time RT-PCR was performed by the ABI Prism
7000 sequence detection system (Applied Biosystems, Foster City,
CA, USA) using SYBR Green fluorescence signals (SYBR Premix Ex Taq
II; Takara Bio). The following oligonucleotide primer pairs were
used: mouse MCP-1, 5′-CCA CTC ACC TGC TGC TAC TCA T-3′
(forward) and 5′-TGG TGA TCC TCT TGT AGC TCT CC-3′ (reverse); mouse
IL-6, 5′-GCT ACC AAA CTG GAT ATA ATC AGG A-3′ (forward) and
5′-CCA GGT AGC TAT GGT ACT CCG AA-3′ (reverse); mouse
adiponectin, 5′-GTT CTA CTG CAA CAT TCC GG-3′ (forward) and
5′-TAC ACC TGG AGC CAG ACT TG-3′ (reverse); mouse PPARγ,
5′-GGC GAT CTT GAC AGG AAA GAC-3′ (forward) and 5′-CCC TTG AAA AAT
TCG GAT GG-3′ (reverse); mouse 36B4, 5′-CCG GAT GTG AGG CAG
CAG-3′ (forward) and 5′-GCT CCA AGC AGA TGC AGC A-3′ (reverse).
Expression levels of RNA, expressed as relative mRNA levels
compared to control, were calculated after normalization to
36B4.
Adipokine ELISA
The culture medium from 3T3-L1 adipocytes was
collected from each sample 24 h after TNF-α treatment. The
concentrations of MCP-1, IL-6 and adiponectin were assayed using a
mouse MCP-1 ELISA kit (R&D systems), a mouse IL-6 ELISA kit
(R&D systems) and a mouse adiponectin ELISA kit (R&D
systems).
Protein isolation and Western
blotting
Whole cell lysates were prepared using RIPA buffer
(50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5%
Na-deoxycholate and 0.1% SDS) containing a protease inhibitor
cocktail (Nacalai Tesque) and complete phosphatase inhibitors
(Nacalai Tesque). Lysates were centrifuged at 12,000 g for 10 min
at 4˚C and the supernatants were boiled in SDS loading buffer. The
boiled samples were separated by an SDS-PAGE gradient gel (10–20%)
and transferred to PVDF membranes (Bio-Rad Laboratories, CA, USA).
Membranes were blocked with blocking reagent (Blocking One-P;
Nacalai Tesque) and incubated with anti-IκB-α and
anti-phospho-IκB-α (Ser 32) antibodies (Cell Signaling Technology,
Beverly, MA, USA), followed by incubation with horseradish
peroxidase-conjugated secondary antibodies. Western blot analysis
was conducted using an enhanced chemiluminescence detection system
(ECL-Plus; Amersham Pharmacia, Arlington, IL, USA).
Quantification of NF-κB activation
To quantify NF-κB activity, nuclear extracts were
prepared using Nuclear Extract kit (Active Motif, Carlsbad, CA,
USA) and analyzed with a sensitive ELISA-based kit (PathScan Total
NF-κB p65 Sandwich ELISA kit; Cell Signaling Technology) to
quantify NF-κB activity, according to the manufacturer's
instructions.
Statistical analysis
Results are expressed as the means ± SEM. Data were
analyzed using one-way analysis of variance (ANOVA) between groups
with the Dunnett post-hoc test. Statistical analyses were
performed using the SPSS 11.0 software (SPSS Inc., Chicago, IL,
USA). Significant differences were considered to be present at
P<0.05.
Results
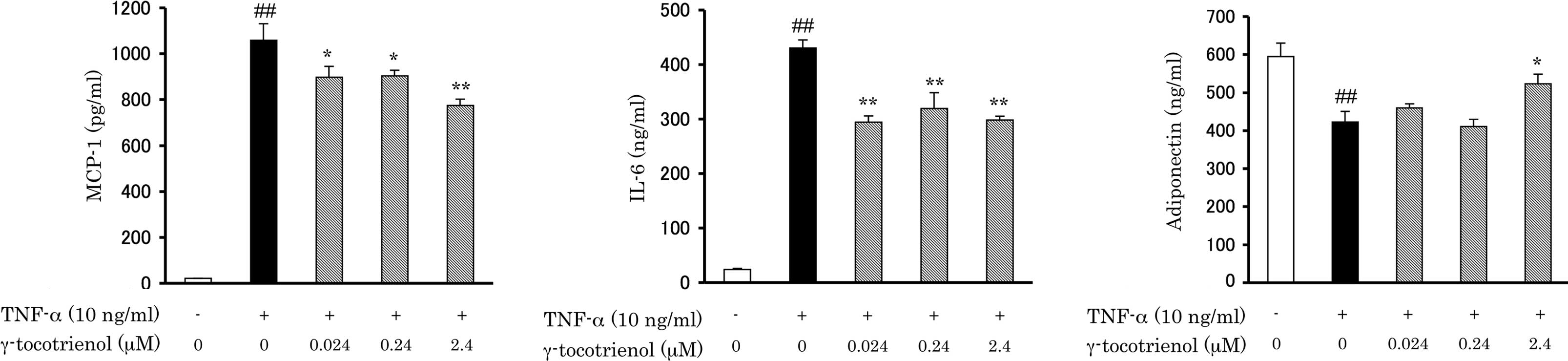
Effects of γ-tocotrienol on adipokine
secretion in TNF-α-treated 3T3-L1 adipocytes
To investigate whether γ-tocotrienol affects the
TNF-α-induced secretion of adipokines, 3T3-L1 adipocytes were
pre-treated with various concentrations of γ-tocotrienol for 6 h
and then incubated with 10 ng/ml TNF-α for 24 h. Adipokines
secreted into the conditioned medium were measured by an ELISA
assay. TNF-α-induced increases in MCP-1 and IL-6 secretion were
significantly inhibited by γ-tocotrienol treatment (Fig. 1A and B). At the γ-tocotrienol
concentration of 2.4 μM, the secretions of MCP-1 and IL-6 were
decreased by 27.7 and 36.5%, respectively. By contrast, adiponectin
secretion, which was decreased by TNF-α stimulation, was restored
by γ-tocotrienol treatment (Fig.
1C). In the presence of 2.4 μM γ-tocotrienol, adiponectin
levels were 1.24-fold higher than with TNF-α alone. Thus, treatment
with γ-tocotrienol attenuated the effects of TNF-α on the
secretions of three adipokines.
Effects of γ-tocotrienol on adipokine
gene expression in TNF-α-treated 3T3-L1 adipocytes
The gene expression of MCP-1, IL-6 and
adiponectin tested by real-time quantitative RT-PCR analysis
is shown in Fig. 2. The enhanced
expression of MCP-1 and IL-6 mRNA by
TNF-α-stimulation was effectively inhibited by γ-tocotrienol
treatment (Fig. 2A and B). At 2.4
μM γ-tocotrienol, the gene expression of MCP-1 and
IL-6 was suppressed by 55.6 and 62.8%, respectively.
γ-tocotrienol also attenuated the inhibiting effect of TNF-α on
adiponectin gene expression (Fig. 2C). The expression of
adiponectin mRNA was restored to 87.2% of control by
γ-tocotrienol (2.4 μM) treatment. Furthermore, PPARγ mRNA
expression, which was suppressed by TNF-α, was restored to the
control level by treatment with γ-tocotrienol at all concentrations
tested (Fig. 2D). Thus,
TNF-α-induced changes in the mRNA transcription levels of
adipokines were also effectively suppressed by γ-tocotrienol.
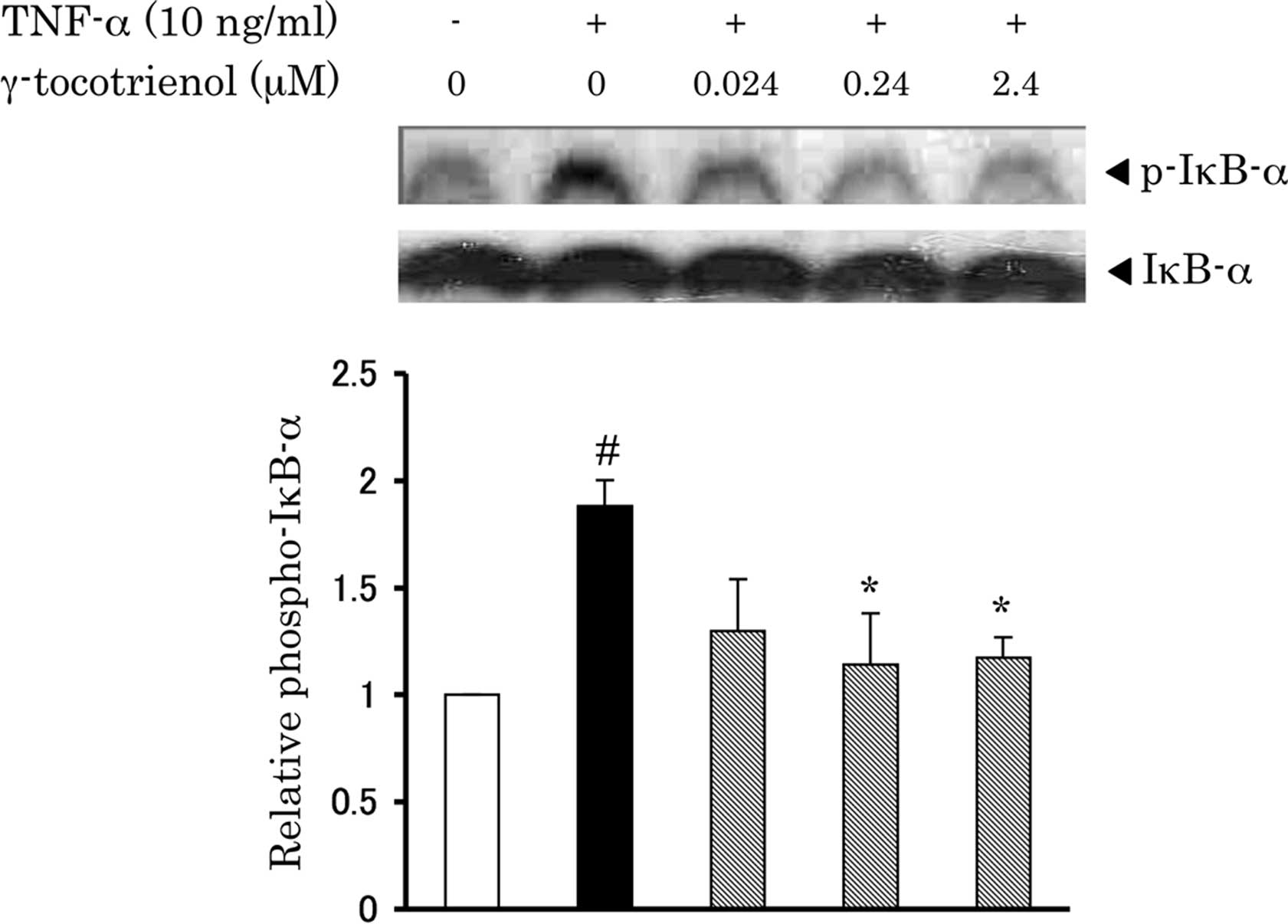
γ-tocotrienol inhibits TNF-α-induced
activation of NF-κB in 3T3-L1 adipocytes
Activation of transcription factor NF-κB plays an
important role in the TNF-α-mediated inflammation progress, the
down-regulation of adiponectin and the up-regulation of
inflammatory molecules, including MCP-1 and IL-6 (3–5). The
release and nuclear translocation of active NF-κB are regulated by
phosphorylation of IκB-α (19). To
further evaluate whether the anti-inflammatory function of
γ-tocotrienol is mediated by NF-κB, the effects of γ-tocotrienol on
IκB-α phosphorylation and NF-κB (p65) nuclear translocation were
examined by Western blot analysis and ELISA assay. As shown in
Fig. 3, TNF-α increased the
phosphorylation level of IκB-α, which was attenuated by treatment
with γ-tocotrienol. Furthermore, γ-tocotrienol effectively
suppressed the TNF-α-enhanced nuclear translocation of NF-κB
(Fig. 4).
Discussion
Obesity is considered to be a state of low-grade
inflammation in adipose tissues, which is closely associated with
the development of insulin resistance (1,2).
This inflammatory condition is partly caused by macrophage
infiltration into the adipose tissue and, subsequently, the
inflamed adipocyte itself enhances the production of various
pro-inflammatory cytokines, including TNF-α, MCP-1 and IL-6
(1,2). It has been reported that TNF-α levels
are increased in obese subjects, and that they induce the
inflammation of adipocytes through the elevation of inflammatory
adipokines (6,7). Furthermore, it is known that TNF-α is
one of the negative regulators of adiponectin, attenuating its
beneficial effects, such as anti-inflammation and facilitation of
insulin sensitivity (8,9). Therefore, anti-inflammatory treatment
could be an effective way to prevent or treat insulin resistance
and type 2 diabetes. Several reports have suggested that dietary
tocotrienols accumulate in adipose tissue and skin (18); however, not much is known about
their physiological effects on adipocytes. It has also been
reported that treatment with γ-tocotrienol reduced body fat mass in
rats (15). A more recent study by
Uto-Kondo et al showed that γ-tocotrienol suppressed
adipocyte differentiation in 3T3-L1 preadipocytes (14). In the present study, we
demonstrated for the first time that γ-tocotrienol effectively
attenuated the TNF-α-mediated increase in MCP-1 and IL-6 secretion
and decrease in adiponectin secretion in 3T3-L1 adipocytes.
Furthermore, the TNF-α-induced changes in the mRNA expression of
each adipokine were also inhibited by γ-tocotrienol. These results
indicate that γ-tocotrienol affected the TNF-α-mediated changes in
the secretion of adipokines at the transcription level.
Activation of the transcription factor NF-κB is
considered to play a major role in TNF-α-induced inflammatory
responses, including down-regulation of adiponectin and
up-regulation of MCP-1 and IL-6 in adipocytes (3–5).
NF-κB is activated by TNF-α via phosphorylation and removal of
IκB-α, resulting in its translocation to the nucleus and
up-regulation of gene expression of pro-inflammatory adipokines,
such as MCP-1 and IL-6 (3–5).
Adiponectin suppression mediated by TNF-α is also regulated by
NF-κB activation. Indeed, Kamon et al showed that
TNF-α-induced down-regulation of adiponectin secretion was
cancelled by IκB kinase β inhibitor in 3T3-L1 adipocytes (20). Consistent with these studies, we
observed that TNF-α enhanced the phosphorylation of IκB-α and the
nuclear translocation of NF-κB.
Recent studies have demonstrated in different cell
types that the anti-inflammatory effects of tocotrienols are
mediated by suppression of the NF-κB pathway (16). A tocotrienol-rich fraction of palm
oil showed anti-inflammatory activity by inhibiting NF-κB
expression in human monocytic cells (17). Moreover, treatment of
streptozotocin-induced diabetic rats with tocotrienols
significantly suppressed the activation of the NF-κB pathway in the
kidney and improved the renal function (21). In this study, our results show that
γ-tocotrienol inhibits the TNF-α-induced activation of NF-κB in
3T3-L1 adipocytes. These observations indicate that γ-tocotrienol
possesses anti-inflammatory properties, such as attenuation of
MCP-1 and IL-6 expression, through the suppression of
NF-κB activation in adipocytes.
It is well known that adiponectin improves insulin
sensitivity, partly through its anti-inflammatory effects (9,22,23).
Adiponectin is highly expressed in adipocytes, and is partly
transcriptionally activated by PPARγ, which is negatively regulated
by TNF-α-induced inflammation (10). Previous studies have found that
adiponectin treatment suppressed inflammation-mediated increase in
MCP-1 and IL-6 production in 3T3-L1 cells, through attenuation of
NF-κB activation and increased PPARγ expression (23). Thus, MCP-1 and IL-6 counteract
adiponectin production in adipocytes. In our experiments,
γ-tocotrienol reversed the TNF-α-induced decrease in both
adiponectin secretion and PPARγ expression. These results
suggest that γ-tocotrienol regulates adiponectin production via
PPARγ, which may be involved in its anti-inflammatory effects in
adipocytes. Moreover, it has been demonstrated that the
transcriptional activity of NF-κB is inhibited by PPARγ in other
cell types (24). Thus, our
results suggest that γ-tocotrienol down-regulates the activation of
NF-κB in part by increasing PPARγ expression in adipocytes.
In summary, γ-tocotrienol inhibits the TNF-α-induced
inflammatory effects in 3T3-L1 adipocytes, and this action is
mediated by suppression of NF-κB activation. These findings provide
novel insight into the prevention and treatment of obesity-related
pathologies.
References
|
1
|
P DandonaA AljadaA
BandyopadhyayInflammation: the link between insulin resistance,
obesity and diabetesTrends
Immunol2547200410.1016/j.it.2003.10.01314698276
|
|
2
|
GS HotamisligilInflammation and metabolic
disordersNature444860867200610.1038/nature0548517167474
|
|
3
|
KY KimJK KimJH JeonSR YoonI ChoiY
Yangc-Jun N-terminal kinase is involved in the suppression of
adiponectin expression by TNF-alpha in 3T3-L1 adipocytesBiochem
Biophys Res
Commun327460467200510.1016/j.bbrc.2004.12.02615629137
|
|
4
|
PP TakGS FiresteinNF-kappaB: a key role in
inflammatory diseasesJ Clin
Invest107711200110.1172/JCI1183011134171
|
|
5
|
B TeferedegneMR GreenZ GuoJM BossMechanism
of action of a distal NF-kappaB-dependent enhancerMol Cell
Biol2657595770200610.1128/MCB.00271-0616847329
|
|
6
|
GS HotamisligilP ArnerJF CaroRL AtkinsonBM
SpiegelmanIncreased adipose tissue expression of tumor necrosis
factor-alpha in human obesity and insulin resistanceJ Clin
Invest9524092415199510.1172/JCI1179367738205
|
|
7
|
GS HotamisligilNS ShargillBM
SpiegelmanAdipose expression of tumor necrosis factor-alpha: direct
role in obesity-linked insulin
resistanceScience2598791199310.1126/science.76781837678183
|
|
8
|
WP CawthornJK SethiTNF-alpha and adipocyte
biologyFEBS
Lett582117131200810.1016/j.febslet.2007.11.05118037376
|
|
9
|
N MaedaM TakahashiT FunahashiPPARgamma
ligands increase expression and plasma concentrations of
adiponectin, an adipose-derived
proteinDiabetes5020942099200110.2337/diabetes.50.9.209411522676
|
|
10
|
B ZhangJ BergerE HuNegative regulation of
peroxisome proliferator-activated receptor-gamma gene expression
contributes to the antiadipogenic effects of tumor necrosis
factor-alphaMol Endocrinol10145714661996
|
|
11
|
A Kamal-EldinLA AppelqvistThe chemistry
and antioxidant properties of tocopherols and
tocotrienolsLipids31671701199610.1007/BF025228848827691
|
|
12
|
CK SenS KhannaS RoyTocotrienols in health
and disease: the other half of the natural vitamin E familyMol
Aspects Med28692728200710.1016/j.mam.2007.03.00117507086
|
|
13
|
S DasK NesaretnamDK DasTocotrienols in
cardioprotectionVitam
Horm76419433200710.1016/S0083-6729(07)76016-8
|
|
14
|
H Uto-KondoR OhmoriC KiyoseTocotrienol
suppresses adipocyte differentiation and Akt phosphorylation in
3T3-L1 preadipocytesJ
Nutr1395157200910.3945/jn.108.09613119056650
|
|
15
|
S Ima-NirwanaS SuhanizaEffects of
tocopherols and tocotrienols on body composition and bone calcium
content in adrenalectomized rats replaced with dexamethasoneJ Med
Food74551200410.1089/10966200432298469915117552
|
|
16
|
M KailehR SenRole of NF-kappaB in the
anti-inflammatory effects of tocotrienolsJ Am Coll
Nutr29S334S339201010.1080/07315724.2010.1071984820823493
|
|
17
|
SJ WuPL LiuLT NgTocotrienol-rich fraction
of palm oil exhibits anti-inflammatory property by suppressing the
expression of inflammatory mediators in human monocytic cellsMol
Nutr Food Res52921929200810.1002/mnfr.20070041818481320
|
|
18
|
S IkedaK ToyoshimaK YamashitaDietary
sesame seeds elevate alpha- and gamma-tocotrienol concentrations in
skin and adipose tissue of rats fed the tocotrienol-rich fraction
extracted from palm oilJ Nutr13128922897200111694614
|
|
19
|
M KarinY YamamotoQM WangThe IKK NF-kappa B
system: a treasure trove for drug developmentNat Rev Drug
Discov31726200410.1038/nrd127914708018
|
|
20
|
J KamonT YamauchiS MutoA novel IKKbeta
inhibitor stimulates adiponectin levels and ameliorates
obesity-linked insulin resistanceBiochem Biophys Res
Commun323242248200410.1016/j.bbrc.2004.08.08315351728
|
|
21
|
A KuhadK ChopraAttenuation of diabetic
nephropathy by tocotrienol: involvement of NF-κB signaling
pathwayLife Sci84296301200919162042
|
|
22
|
T YamauchiJ KamonH WakiThe fat-derived
hormone adiponectin reverses insulin resistance associated with
both lipoatrophy and obesityNat
Med7941946200110.1038/9098411479627
|
|
23
|
E ZoicoU GarbinD OliosoThe effects of
adiponectin on interleukin-6 and MCP-1 secretion in
lipopolysaccharide-treated 3T3-L1 adipocytes: role of the NF-kappaB
pathwayInt J Mol Med24847851200910.3892/ijmm_0000030219885628
|
|
24
|
P WangPO AndersonS ChenKM PaulssonHO
SjogrenS LiInhibition of the transcription factors AP-1 and
NF-kappaB in CD4 T cells by peroxisome proliferator-activated
receptor gamma ligandsInt
Immunopharmacol1803812200110.1016/S1567-5769(01)00015-711357893
|