Introduction
The serious side-effects of chemotherapeutic agents
have greatly limited their applications in the treatment of tumors
(1). For instance,
cyclophosphamide is one of the most frequently used
chemotherapeutics with serious side-effects, such as
immunodepression and water intoxication, which can result in
infections and hyponatremia, respectively. However, organic
water-soluble antitumor agents, which have minor toxicity and fewer
side-effects, are entering the spotlight of tumor therapy research,
and are currently under investigation.
Chitooligosaccharides (COS) and
N-acetyl-D-glucosamine (NAG) are two favorable organic agents,
which have potential antitumor effects, with the advantages of
being abundant in nature, having good biological properties and
particular physiochemical characteristics. COS, composed of 3–10
N-acetyl-D-glucosamine molecules or glucosamine molecules, is the
enzymolysis lytic or chemical hydrolytic product of chitosan
(2). NAG, a type of special
monosaccharide, is also degraded from chitosan. COS and NAG have
much better solubility compared with chitosan or chitin, and can be
absorbed easily. They also have various beneficial biological
functions, such as the enhancement of immune responses,
anti-bacterial functions and the promotion of wound healing
(2).
It has previously been demonstrated that COS and NAG
have significant antitumor functions, through various mechanisms,
such as inhibiting the proliferation of tumor cells, inducing
necrosis and apoptosis, influencing angiogenesis and enhancing host
immunity. It has been suggested that the bio-activity of COS and
NAG is mainly due to their cationic properties which are attributed
to the amino groups. The immunostimulatory property of COS is
attributed to its N-acetyl-chitohexaose group, though it has no
cationic property (3). In
addition, the molecular weight and deacylation degree also play an
important role in the antitumor activities of COS (4,5).
It has been reported that the growth and
transformation of tumor cells is closely related to the
angiogenesis of tumors (6). The
vascular endothelial growth factor (VEGF) is one of the most
important cytokines that have effects on angiogenesis. The
VEGF-VEGF receptor (VEGFR), angiopoietin (Ang)-Tie2 and delta-like
ligand4 (Dll4)-Notch have been found to be significant signaling
pathways of angiogenesis. VEGFR2 is one of the most important
molecules. Therefore, the inhibition of the VEGF signaling pathway
may be a good strategy for treating malignancies.
It has been well documented that COS, especially
hexaacetyl-chitohexaose, has good antitumor activity. However, the
antitumor effect of NAG has not been extensively studied. In the
current study, the inhibitory effect of COS and NAG on cell
proliferation in sarcoma 180 tumor cells was investigated in
vitro and in vivo. Moreover, the natural killer (NK)
cell activity, as well as serum interleukin-2 (IL-2) and
interferon-γ (IFN-γ) levels were measured, in order to elucidate
the mechanisms involved in the tumor inhibitory effect of NAG.
Materials and methods
Reagents
COS and NAG were supplied by Qingdao Biotemed
Biomaterial Co., Ltd. (Qingdao, China). Polyclonal anti-mouse VEGF
primary antibody and polyclonal anti-mouse fibroblast growth
factor-2 (FGF-2) primary antibody were purchased from Wuhan Boshide
Experimental Equipment Co., Ltd. (Wuhan, China). The mouse IL-2
ELISA kit and mouse IFN-γ ELISA kit were purchased from the R&D
Systems (Minneapolis, MN, USA). The sarcoma 180-bearing mice were
obtained from Jinan DingGuo Biotech. Co. Ltd. (Jinan, China). All
animal experiments were performed according to the rules approved
by the Animal Protection Committee of Qingdao University, Qingdao,
China.
Peripheral blood mononuclear cell (PBMC)
preparation
Human venous blood (50 ml) was obtained and then
anti-coagulated with sodium citrate. PBMCs were collected by
density gradient centrifugation. Cells were cultured with GT-T551
medium containing autologous serum at 37°C (5% CO2) as
described previously (7,8).
Cell treatments with COS or NAG
The PBMCs were placed in 96-well plates at a
concentration of 106 cells/ml (100 μl per well). COS or
NAG were added to the medium to obtain concentrations of 500, 300,
100 or 50 μg/ml. The PBMCs cultured with normal medium were the
positive controls. The cells were observed under a microscope and
imaged. To assess the effect of various concentrations of COS and
NAG on the proliferation and viability of the PBMCs, the samples
were analyzed with the MTT method on the 2nd and the 4th day from
the beginning of the treatments.
Apoptosis detection
The PBMCs were treated with COS or NAG in 96-well
plates as described above. On the 8th day after cell seeding, 8 μl
of acridine orange (AO)/ethidium bromide (EB) dyes were added into
200 μl of cell suspensions. Fluorescence microscopy was performed
and apoptotic rates were calculated.
Flow cytometry
PBMCs (106 cells) treated with 500, 100,
50, or 10 COS or NAG were collected. Cells were washed with PBS
buffer, and subsequently incubated with anti-human-CD3-FITC and
CD4-PI, CD8-PI or CD56-PI monoclonal antibody. The cells were
analyzed using a flow cytometer to detect the subgroups of
CD3+CD4+ lymphocytes,
CD3+CD8+ lymphocytes and
CD3−CD56+ NK cells as described previously
(9).
Animal experiments
Sarcoma 180 cells were injected into the peritoneal
cavity of the sarcoma 180-bearing mice. They were harvested from
the peritoneal cavity of the tumor-bearing mice 7–9 days after
inoculation, and suspended in an air-saturated phosphate buffer
solution (10). The cells were
then collected by centrifugation and resuspended in PBS at a
concentration of 107 cells/ml and were subcutaneously
transplanted into the right oxter region of the mice to obtain
solid tumors. At day 1 post-inoculation, 80 mice were randomly
divided into 8 groups. COS and NAG solutions at the doses of 100,
300 and 500 mg/kg, were administered by gavage every 2 days over a
period of 15 days. The negative control group received 0.9% normal
saline. The positive control group received cyclophosphamide (300
mg/kg).
At day 16 after subcutaneous transplantation, the
mice were weighed and sacrificed. The solid tumor, thymus and
spleen were weighed. The rate of inhibition of the growth of tumors
was calculated as follows: inhibitory rate (%) = [(A−B)/A] ×100%,
where A is the mean tumor weight of the negative control group and
B is the mean tumor weight of the COS-, NAG-treated or positive
control group. The thymus and spleen indexes were expressed as the
thymus or spleen weight relative to the body weight as described
previously (11,12).
The spleens were aseptically collected from each
mouse and placed onto a 200-micron steel mesh immersed in chilled
Iscove’s modified Dulbecco’s medium (IMDM). The spleen was passed
through the steel sieve and collected in IMDM. Splenic lymphocytes
(100 μl) from each group were cultured in 96-well plates at
1×107 cells/ml in IMDM as effector cells. YAC-1 cells
(100 μl) were used as the target cells, and added at
2×105 cells/ml to obtain an effector/target (E/T) ratio
of 0:1. The plates were then incubated for 12 h at 37°C in a 5%
CO2 atmosphere. NK cell activity was analyzed by
measuring mitochondrial activity using the MTT assay and the
absorbance was measured at 490 nm on an automated plate reader.
Three types of control measurements were performed: target cell
control, effector cell control and blank control. NK cell activity
was calculated as follows: NK activity (%) = [1−
(ODE+T−ODE)/ODT] ×100%, where ODT
is the optical density value of the target cell control,
ODE+T is the optical density value of the test samples
and ODE is the optical density value of effector cell
control.
Blood was collected on day 16 after the
administration of COS and NAG by gavage and maintained at room
temperature for 4 h with natural coagulation, followed by
centrifugation at 1,500 rpm, and the supernatants were used for the
ELISA determination of IL-2 and IFN-γ levels.
Immunohistochemistry
Slides from the paraffin blocks of mice were
deparaffinized and treated in Tris-EDTA solution. After peroxidase
blocking reagent treatment, the anti-VEGF (1:100) primary
antibodies were applied to the slides. Secondary goat anti-mouse
antibody was used in this experiment. Samples were viewed under a
microscope and imaged.
ELISA
The microtiter plates were pre-coated with an
antibody specific to IL-2 or IFN-γ. Standards or samples were then
added to the appropriate microtiter plate wells with a
biotin-conjugated polyclonal antibody preparation specific for IL-2
or IFN-γ. Subsequenlty, avidin conjugated to horseradish peroxidase
(HRP) was added to each microplate well and incubated. A TMB
substrate solution was then added to each well. The color change
was measured at a wavelength of 450 nm.
Results
COS and NAG induce PBMC
proliferation
To examine whether COS and NAG can affect the
proliferation of PBMCs, we cultured the PBMCs with COS and NAG at
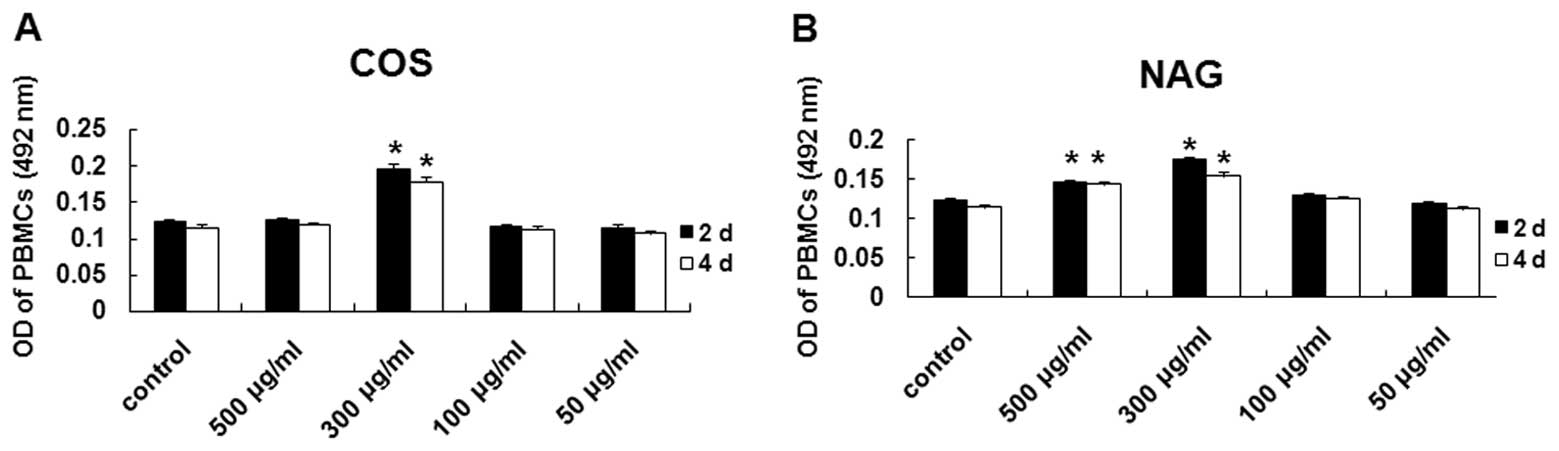
various concentrations. As shown in Fig. 1A and B, COS (300 μg/ml) and NAG
(300 μg/ml) induced the proliferation of the PBMCs in comparison
with the control (P<0.05). NAG at a concentration of 500 μg/ml
also induced the proliferation of the PBMCs, but not so much as COS
and NAG at the concentration of 300 μg/ml, possibly due to the low
molecule weight of NAG. These results suggest that COS and NAG
induce PBMC proliferation in vitro.
COS and NAG inhibit apoptosis of
PBMCs
To determine the effect of COS and NAG on the
apoptosis of PBMCs, AO/EB staining was performed and the cells with
a different redox status were counted. As shown in Table I, the apoptotic rates of the PBMCs
treated with COS at the concentrations of 500, 300, 100 and 50
μg/ml were 10.0±1.5, 1.5±0.5, 6.0±1.5 and 11.5±2.0%, respectively.
The apoptotic rates of the PBMCs treated with NAG at the
concentrations of 500, 300, 100 and 50 μg/ml were 9.0±1.5, 2.0±0.5,
4.5±1 and 13.0±2.5%, respectively. The apoptotic rates of the PBMCs
treated with COS or NAG were significantly lower than the apoptotic
rate of 38.5±3.5% for the PBMCs treated with the culture medium
without COS or NAG. These results suggest that COS and NAG inhibit
the apoptosis of PBMCs, with the most effective inhibition at the
concentration of 300 μg/ml.
 | Table IEffect of COS and NAG on PBMC
apoptosis. |
Table I
Effect of COS and NAG on PBMC
apoptosis.
| Group | Apoptotic rate
(%) |
|---|
| Negative control | 38.5±3.5 |
| COS 500 μμg/ml | 10.0±1.5a |
| COS 300 μg/ml | 1.5±0.5b |
| COS 100 μg/ml | 6.0±1.5b |
| COS 50 μg/ml | 11.5±2.0a |
| NAG 500 μg/ml | 9.0±1.5a |
| NAG 300 μg/ml | 2.0±0.5b |
| NAG 100 μg/ml | 4.5±1.0b |
| NAG 50 μg/ml | 13.0±2.5a |
COS and NAG affect antibody expression in
leukomonocytes
To determine the effect of COS and NAG on the
antibody expression in leukomonocytes, PBMCs were treated with COS
or NAG and flow cytometry was performed. As shown in Fig. 2, following treatment with COS or
NAG, at the concentrations of 300, 100 and 50 μg/ml, the proportion
of the PBMCs that expressed CD3-CD4, CD3-CD8 and CD3-CD56
antibodies increased.
The percentage of CD3+ cells was more
than 90%, suggesting that PBMCs cultured in vitro were
mostly T lymphocytes. COS at the dose of 300 μg/ml and NAG at the
dose of 100 and 300 μg/ml induced the differentiation of T
leukomonocytes that express the CD4 antibody (P<0.05), but not
the CD8 antibody. COS (300 or 100 μg/ml) and NAG (300 μg/ml)
significantly increased the amount of the
CD3−CD56+ subgroup of NK cells
(P<0.05).
Antitumor activity, thymus and spleen
indexes
The thymus and spleen are important immune organs
and are closely related with immune functions and the antitumor
activity. The change in weight of the animals is also an important
indication of immune function. The results of the changes in
weight, and thymus and spleen indexes are shown in Table II. The changes in weight of the
group of 300 mg/kg COS, 500 mg/kg COS, 300 mg/kg NAG and 500 mg/kg
NAG were similar to those of the normal control group, while the
increased weights of the positive control group and the negative
control group were significantly less than those of the normal
control group (P<0.05). The tumor inhibition rates are shown in
Table III. The tumor inhibition
rates of the groups administered with 100 mg/kg COS and 100 or 300
mg/kg NAG were significantly higher than those of the normal
control group (P<0.05).
 | Table IIChanges in weight, and thymus and
spleen indexes in each group after 15 days of COS or NAG
administration by gavage. |
Table II
Changes in weight, and thymus and
spleen indexes in each group after 15 days of COS or NAG
administration by gavage.
| Group | Change in weight
(g) | Thymus index (%) | Spleen index (%) | Tumor weight (g) |
|---|
| Normal control | 3.64±0.75 | 0.257±0.012 | 1.013±0.071 | - |
| Negative control | 0.89±0.09a | 0.109±0.006a | 0.615±0.037a | 2.64±0.38 |
| Positive control | 1.48±0.33a | 0.128±0.009a | 0.677±0.082a | 1.27±0.13a |
| COS 100 μg/ml | 1.97±0.45a | 0.158±0.011a | 0.984±0.053 | 1.24±0.26a |
| COS 300 μg/ml | 3.52±0.91 | 0.242±0.014 | 1.016±0.079 | 0.76±0.21b |
| COS 500 μg/ml | 3.58±0.86 | 0.239±0.015 | 1.247±0.085a | 1.05±0.37a |
| NAG 100 μg/ml | 2.386±0.71a | 0.134±0.012a | 0.893±0.068a | 1.36±0.38a |
| NAG 300 μg/ml | 3.77±0.79 | 0.243±0.007 | 1.098±0.064 | 0.77±0.23a |
| NAG 500 μg/ml | 3.85±0.92 | 0.185±0.008a | 1.214±0.057a | 0.74±0.25b |
 | Table IIITumor inhibition rate in each group
after 15 days of COS or NAG administration by gavage. |
Table III
Tumor inhibition rate in each group
after 15 days of COS or NAG administration by gavage.
| Group | Inhibition rate
(%) |
|---|
| Positive
control | 51.89±3.32 |
| COS 100 μg/ml | 53.03±3.16 |
| COS 300 μg/ml | 71.21±2.96a |
| COS 500 μg/ml | 60.22±2.74 |
| NAG 100 μg/ml | 48.48±2.52 |
| NAG 300 μg/ml | 70.83±2.59a |
| NAG 500 μg/ml | 71.97±3.63a |
The results relating to the thymus index were
similar to those relating to weight. The results relating to the
spleen index showed that the spleen index of the group of 500 mg/kg
COS, 100 mg/kg NAG and 500 μg/ml NAG was significantly higher than
that of the normal control group ( P<0.05), while the spleen
index of the positive and negative control group was significantly
lower than that of the normal control group (P<0.05). The
obvious inhibitory effect on tumor growth was observed in the
positive group, the groups of 300 μg/ml COS, 500 mg/kg COS, 300
mg/kg NAG and 500 mg/kg NAG (P<0.05).
COS and NAG upregulate NK cell activity
and IL-2 and IFN-γ levels
To investigate the effect of COS and NAG on the
immunological activity of the animals, NK activity was evaluated.
As shown in Fig. 3A, the NK
activity of the group of 500 mg/kg COS and 300 or 500 mg/kg NAG was
much higher than that of the positive control group (P<0.05),
while the NK activity of the negative control group was much lower
than that of the positive control group (P<0.05).
The secretory concentration of IL-2 and IFN-γ was
examined by ELISA assays; the results of IL-2 are shown in Fig. 3B and those of IFN-γ are shown in
Fig. 3C. The secretory
concentration of IL-2 in the serum of the COS group (all doses) and
the 300 and 500 mg/kg NAG groups was much higher than that of the
normal, positive control and negative groups (P<0.05). The
secretory concentration of IFN-γ in the serum in the positive group
and the group of 300 mg/kg COS, 500 mg/kg COS, 300 mg/kg NAG and
500 mg/kg NAG was much higher than that of the normal and negative
groups (P<0.05).
Immunohistochemistry for detection of
VEGF expression
The immunohistochemistry staining of the tumors from
each group is shown in Fig. 4. The
negative control and positive control groups were both stained
positively for VEGF, which is the marker of angiogenesis. No
obvious positive staining was observed in the experimental groups
of both COS and NAG (all doses). The negative staining in the COS
and NAG groups revealed that COS and NAG inhibited the expression
of VEGF in the tumors. VEGF is a very important molecule in the
pathway of angiogenesis. The inhibition of the expression of VEGF
can affect tumor angiogenesis. Therefore, it is suggested that COS
and NAG reduce tumor growth through the inhibition of VEGF
expression in tumors.
Discussion
Certain studies have reported on the
apoptosis-inducing effects of COS and NAG. Lee et al
reported the cytotoxicity of amino derivatized chitosan derivatives
in certain cancer cell lines, such as HepG2 and HeLa. However, the
exact mechanisms by which these molecules exert their anticancer
properties have yet to be identified (13). Previously, it was revealed that COS
exerted anticancer activity by inducing apoptosis in the HL-60 cell
line derived from a patient with acute promyelocytic leukemia
(14). However, even though this
mechanism of apoptosis induction has been suggested by some
studies, other studies have presented DNA fragmentation results
showing cells that have undergone necrosis but not apoptosis
(15,16). In this study, the results revealed
that COS and NAG inhibited the growth of sarcoma 180 tumors and
significantly improved the thymus and spleen indexes. This
indicates that COS and NAG can protect the thymus and spleen.
According to these results, it can be deduced that the antitumor
activity of COS and NAG is possibly not due to the cytotoxicity of
amino acids in COS or NAG, but due to their immunoregulation
(17).
Tumor angiogenesis is a process in which tumors
undergo microvascular growth and grow new blood vessels. Therefore,
the inhibition of tumor angiogenesis is necessary to control the
growth and migration of tumors. VEGF is an important moleculer
marker of microvascular growth. It has been reported that sonic
hedgehog homolog (SHH) signaling pathway plays an imprortant role
in embryo development and angiogenesis. There would be an obvious
decrease in both angiogenesis and in VEGF gene expression if the
SHH signaling pathwas was inhibited. There are certain clinical
data demonstrating the correlation between the SHH signaling
pathway and VEGF expression (18);
however, the mechanisms involved remain unknown. Other studies have
reported that the PI3K signaling pathway is also closely correlated
with VEGF expression in tumor cells (19). Therefore, the modulation of VEGF
expression is a very complex process. There are several signaling
pathways that paticipate in the modulation of VEGF expression. In
our study, COS and NAG incorporated with the receptor as a ligand
and played an important role in the modulation of one or several
signaling pathways. Consequently, the signaling pathway and VEGF
expression were inhibited, and tumor angiogenesis was restrained.
In this study, the negative control and positive control groups
were both positively stained for VEGF. The experimental group of
both COS and NAG (all doses) had no obvious positive staining.
Therefore, both COS and NAG inhibited tumor growth by inhibiting
tumor angiogenesis.
The thymus, the main repository for differentiating
and maturing T cells, plays an important role in the immune system.
The spleen, the largest peripheral immune organ, is the main
repository for immune response. The number of B cells in the spleen
accounts for 55% of the total leukomonocytes, and the number of T
cells in the spleen accounts for 40%. In this study, COS and NAG at
the dose of 300 and 500 μg/ml increased the weight of the spleen
and thymus, and improved the body’s immune system.
COS and NAG can activate macrophages, NK cells,
neutrophile granulocytes, leukomonocytes and complementary systems,
and can induce the secretion of a number of cytokines, which play
an important role in the antitumor process. Certain studies ahve
found that the antitumor effects of COS and NAG are due to the
increased activity of NK lymphocytes, as observed in sarcoma
180-bearing mice (20). These
results are in agreement with those from our study. In this study,
COS and NAG promoted the differentiation of NK cells by the
expression of the CD3-CD56 subgroup; NAG was more effective than
COS, however. COS and NAG also promoted the differentiation of T
cells by the expression of the CD4 subgroup but not the CD8
subgroup.
IL-2 is the principal growth factor for T and NK
lymphocytes and is responsible for regulating the magnitude and
duration of the immune response (21). The importance of IL-2 as a key
cytokine for T cell activation and immune function has extensive
experimental support (22). The
appropriate production of IL-2 is an important determinant of the
magnitude of T cell-dependent immune responses (23). IFN-γ, generally secreted by T cells
and NK cells, is a pleiotropic cytokine endowed with potent
immunomodulatory effects on a variety of immune cells in
vitro and in vivo (1,24).
This cytokine exerts its multiple biological activities by
controlling either positively or negatively the expression of many
genes and proteins (25). In this
study, COS and NAG activated both NK cells and T cells with special
subgroups, and induced the secretion of IL-2 and IFN-γ, thus
improving the antitumor activity. These results are in agreement
with those from the study by Maeda et al (20,26).
In conclusion, both COS and NAG can inhibit tumor
growth by promoting the secretion of IL-2 and IFN-γ, compared with
cyclophosphamide. COS and NAG can promote the proliferation,
differentiation and cytokine secretion of leukomonocytes. The
antitumor and immunoregulatory effect is dose-dependent. The
antitumor effect is achieved by the indirect improvement of
immunoregulation. In medical surgery, COS and NAG may be applied
together as a anticancer drug and immunomodulator to promote the
clinical application.
Acknowledgements
This study was supported by Ocean University of
China. We thank Professor Wanshun Liu and Professor Baoqin Han from
the Ocean University of China. We are also thankful to the
colleagues of Affiliated Hospital of Qingdao University for their
technical assistance.
References
|
1
|
Billiau A: Interferon-γ: biology and role
in pathogenesis. Advances Immunol. 62:61–130. 1996.
|
|
2
|
Han YP, Zhao LH and Wu HM: Mechanism of ol
igochitosan-induced macrophage activation. Zhejiang Da Xue Xue Bao
Yi Xue Ban. 35:265–272. 2006.(In Chinese).
|
|
3
|
Ko S, Takeshi M, Yoshio O, Akio T, Shigeo
S and Masuko S: Antitumor effect of hexa-N-acetylchitohexaose and
chitohexaose. Carbohydr Res. 151:403–408. 1986. View Article : Google Scholar
|
|
4
|
Muzzarelli RAA: Chitin. Oxford Pergamon
Press; London: pp. 262–270. 1977
|
|
5
|
Qin CQ, Du YM, Xiao L and Li Z: Enzymic
preparation of water-soluble chitosan and their antitumor activity.
Int J Biol Macromol. 31:111–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao BG, Ma CG, Xu LY, Hans L and Lu CZ:
IL-12/IFN-γ/NO axis plays critical role in development of
Th1-mediated experimental autoimmune encephalomyelitis. Mol Immnol.
45:1191–1196. 2008.
|
|
8
|
Caroline B, Derek D, Fran B and Frances B:
Involvement of both intrinsic and extrinsic pathways in
IFN-γ-induced apoptosis that are enhanced with cisplatin. Eur J
Cancer. 41:1474–1486. 2005.
|
|
9
|
Tsiavou A, Hatziagelaki E, Chaidaroglou A,
Koniavitou K, Degiannis D and Raptis SA: Correlation between
intracellular interferon-γ ( IFN-γ) production by CD4+
and CD8+ lymphocytes and IFN-γ gene polymorphism in
patients with type 2 diabetes mellitus and latent autoimmune
diabetes of adults (LADA). Cytokine. 31:135–141. 2005.
|
|
10
|
Wang XB, Liu QH, Wang P, Tang W and Hao Q:
Study of cell killing effect on S180 by ultrasound activating
protoporphyrin IX. Ultrasonics. 48:135–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao L, Liu XZ, Qian TX, et al: Antitumor
and immunomodulatory activity of arabinoxylans: a major constituent
of wheat bran. Int J Biol Macromol. 48:160–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nie XH, Shi BJ, Ding YT and Tao WY:
Antitumor and immunomodulatory effects Weikangfu granule compound
in tumor-bearing mice. Curr Ther Res. 67:138–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JK, Lim HS and Kim JH: Cytotoxic
activity of aminoderivatized cationic chitosan derivatives. Bioorg
Med Chem Lett. 12:2949–2951. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pae HO, Seo WG, Kim NY, et al: Induction
of granulocytic differentiation in acute promyelocytic leukemia
cells (HL-60) by watersoluble chitosan oligomer. Leuk Res.
25:339–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prashanth KVH and Tharanathan RN:
Depolymerized products of chitosan as potent inhibitors of
tumor-induced angiogenesis. Biochim Biophys Acta. 1722:22–29. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang RH, Mendis E, Rajapakse N and Kim
SK: Strong electronic charge as an important factor for anticancer
activity of chitooligosaccharides (COS). Life Sci. 78:2399–2408.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao XM, Xu HJ, Shang M and Liu WS:
Experimental study on antitumor activities and immunity regulation
of chitooligosaccharide. J Harbin Univ Commerce (Natural Sciences
Edition). 22:8–10. 2006.
|
|
18
|
He H, Zhang H, Li B, et al: Blockade of
the sonic hedgehog signalling pathway inhibits choroidal
neovascularization in a laser-induced rat model. J Huazhong Univ
Sci Technolog Med Sci. 30:659–665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Hua K, Zhou X, et al: Activation
of the PI3K/AKT pathway mediates FSH-stimulated VEGF expression in
ovarian serous cystadenocarcinoma. Cell Res. 18:780–791. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeda Y and Kimura Y: Antitumor effects of
various low-molecular weight chitosans are due to increased natural
killer activity of intestinal intraepithelial lymphocytes in
sarcoma 180-bearing mice. J Nutr. 134:945–950. 2004.
|
|
21
|
Lindholm CK: IL-2 receptor signaling
through the Shb adaptor protein in T and NK cells. Biochem Biophys
Res Commun. 296:929–936. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma A, Koka R and Burkett P: Diverse
functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Ann Rev
Immunol. 24:657–679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JM, Kim HJ, Lee KY, Choi HJ, Lee IS
and Kang BY: Increased IL-2 production in T cells by xanthohumol
through enhanced NF-AT and AP-1 activity. Int Immunopharmacol.
9:103–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yong HA and Hardy KJ: Role of
interferon-gamma in immune cell regulation. J Leukoc Biol.
58:373–378. 1995.
|
|
25
|
Sandra G and Filippo B: IFN-γ Expression
in macrophages and its possible biological significance. Cytokine
Growth Factor Rev. 9:117–123. 1998.
|
|
26
|
Xu QS, Bai XF and Du YG: Progress on
anti-tumor activity of oligochitosan and its derivates. Food and
Drug. 10:60–62. 2008.
|