Introduction
Esophageal cancer (EC) is a highly malignant
neoplasm. The 5-year survival rate of patients is only 10%
(1). Advanced EC is one of the
most refractory cancers and is associated with poor outcome.
Conventional chemotherapy and radiotherapy are widely used for EC.
However, more than 40% of EC cases eventually result in recurrence
and patients succumb to chemotherapy- and radiotherapy-resistant
disease (2). Mounting evidence
suggests that small populations of cells within tumors, known as
cancer stem cells (CSCs), contribute to tumor maintenance and
progression and are intrinsically resistant to therapies (3). CSCs have the ability to recreate the
full phenotypic heterogeneity of the parent tumor (4). These cells express distinct surface
markers allowing for reproducible and differential purification.
Several stem cell markers, such as Nanog and Oct4, have been used
successfully to identify CSCs in normal and tumor tissue (5). In addition, side population (SP)
cells found in various types of cancer have been reported to
exhibit CSC characteristics (6).
The anchorage-independent tumorsphere culture of
stem cells was instrumental in the study of adult CSCs (7–9).
Sphere formation is particularly useful to enrich the potential CSC
subpopulations as a functional approach (10,11).
CSCs form spheres in vitro in serum-free suspension culture.
In the suspension culture, tumorsphere-forming cells failed to
express cytokeratins (CK), but were found to express stem cell
markers (12). Thus, the
suspension culture system is thought to maintain CSCs in their
undifferentiated state, facilitating their enrichment.
However, few reports are currently available
regarding tumorspheres in EC. Therefore, the aim of the present
study was to enrich and identify EC cell subsets with CSC
properties. The tumorsphere of EC is considered to be a valuable
model for the further study of both CSCs and chemoresistance. To
select esophageal CSC markers, we performed comparative global gene
expression analyses between adherent and spheroid cells. We
compared profiles of adherent and spheroid cells and obtained one
representative differentially expressed gene, aldehyde
dehydrogenase (ALDH). We also verified that the cancer stem-like
cells of EC contained a high ALDH enzymatic activity.
Materials and methods
Cells and culture conditions
The Eca109 human esophageal cancer cell line was
purchased from the Shanghai Cell Biology Institute of the Chinese
Academy of Sciences, China. The cells were cultured in DMEM medium
(Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Hyclone) and 100 U/ml penicillin/streptomycin (Gibco,
Langley, OK, USA). Cultures were maintained in a humidified
incubator at 37°C in 5% CO2 air atmosphere.
Tumorsphere culture and
differentiation
Cells (1,000 cells/ml) were cultured in suspension
in serum-free Ham's F-12 medium (Gibco), supplemented with B27
(1:50; Gibco), 20 ng/ml EGF (Invitrogen, Grand Island, NY, USA) and
20 ng/ml FGF (Invitrogen). To propagate spheres in vitro,
spheres were collected by gentle centrifugation, dissociated to
single cells and then cultured to generate tumorspheres of the next
generation. To guide the differentiation of spheres in
vitro, spheroids were cultured in DMEM supplemented with 10%
FBS without growth factors.
Immunofluorescent staining
For immunofluorescent staining, adherent or
semi-differentiated spheroid cells were grown on the surface of
cover slides. Spheroid staining was performed in 96-well
microplates. The cells were fixed with 4% paraformaldehyde.
Following rehydration in PBS, cells were incubated with respective
primary antibodies at 37°C for 45 min. Mouse anti-Nanog, Oct4,
CK5/6 and ALDH1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA)
were used as primary antibodies. Slides or spheroids were then
washed with PBS for 15 min and secondary antibodies were incubated
at 37°C for 45 min. Alexa594-conjugated goat anti-mouse IgG
(against anti-Nanog, Oct4 and CK5/6; Invitrogen) or FITC-conjugated
goat anti-mouse IgG (against anti-ALDH1; Invitrogen) were used as
secondary antibodies. The nuclei were stained with DAPI. Sections
were examined with confocal microscopy (Olympus-FV1000, Japan).
Immunoblotting
Total protein was extracted from spheroid or
adherent Eca109 cells using cell lysis buffer. Proteins were run in
10% SDS-PAGE and transferred on a PVDF sheet. The blots were
incubated for 1–2 h in blocking solution (5% skimmed milk in
Tris-buffer), and then for 1 h using the following primary
antibodies: mouse anti-Nanog, Oct4, CK5/6, ABCG2, MDR1, ALDH1 and
GAPDH (Santa Cruz). The sheet was then incubated for 1 h with
HRP-conjugated secondary antibodies (Invitrogen) against mouse
immunoglobulins. The bands were visualized using the ECL-Plus
detection system (Bio-Rad, Hercules, CA, USA).
Drug sensitivity assay to antitumor
drug
Cells obtained from adherent or spheroid Eca109
cells were seeded in 96-well microplates at a density of 3,000
cells/well. The cells were treated with increasing concentrations
of cisplatin (Sigma-Aldrich, St. Louis, MO, USA) as indicated by
the manufacturer's instructions. MTT assay was performed to
determine the cell viability following exposure to cisplatin for 72
h. The number of living cells was directly proportional to the
absorbance at 490 nm.
Hoechst staining and SP cell assay
Cells obtained from adherent or spheroid Eca109
cells were suspended in DMEM/2% FBS at 1×106 cells/ml
and stained with Hoechst-33342 dye (5 μg/ml; Sigma-Aldrich) for 90
min at 37°C. Following this incubation, cells were washed with
ice-cold PBS and stained with propidium iodide (1 μg/ml;
Sigma-Aldrich) to label and exclude dead cells. The cells were
maintained at 4°C for the flow cytometric analysis and for sorting
of the SP fraction using a FACSAria flow cytometer (BD Biosciences,
San Jose, CA, USA).
RNA isolation and microarray
analysis
Eca109 spheroids were filtered by a cell strainer
(40 μm; BD Biosciences). Spheroids with a diameter of >40 μm
were selected. Total RNA was extracted separately from adherent and
spheroid Eca109 cells using TRIzol reagent (Invitrogen), according
to the manufacturer's instructions. RNA was subjected to
GeneChip_expression array analysis with two-cycle target labeling
(implemented by CapitalBio Corp., Beijing, China). Briefly, cDNA
was synthesized from total RNA using T7-Oligo (dT) primers, and
biotinylated cRNA was synthesized using cDNA. Labeled cRNA (2 μg)
was hybridized to the 22K Human Genome Array. The array image was
scanned and analyzed using LuxScan 10KA.
Aldefluor assay by FACS
The ALDEFLUOR kit (StemCell Technologies, Durham,
NC, USA) was used to analyze the population with a high ALDH
enzymatic activity. Cells obtained from adherent or spheroid Eca109
cells were suspended in ALDEFLUOR assay buffer containing ALDH
substrate and incubated during 40 min at 37°C. As a negative
control, for each sample of cells an aliquot was treated with 50
mmol/l diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor.
FACS was performed using a FACSAria flow cytometer (BD
Biosciences).
Statistical analyses
Data were analyzed using statistics soft SPSS 13.0
and were shown as the means ± SD. P<0.05 were considered
statistically significant.
Results
Esophageal cancer tumorsphere contains
cells with cancer stem-like properties
Ponti et al first reported that breast CSC
properties could be propagated in vitro as non-adherent
mammospheres under serum-free culture conditions (13). In the present study, we attempted
to enrich the CSC population from the Eca109 EC cell line. To
observe the differentiation of the tumorspheres, spheres were
cultivated in serum-driven culture. After 48 h of culture, floating
undifferentiated cells attached to the plastic, gradually migrating
from tumorspheres and differentiating into adherent cells (Fig. 1A). We detected two typical CSC
markers, Nanog and Oct4, that were spheroid-cultured under
differentiation conditions by immunofluorescence. In addition, the
expression of the marker which indicates EC surface epithelium,
CK5/6, was also observed. As shown in Fig. 1B, Nanog and Oct4 were expressed in
the center of the semi-differentiated spheroids. However, a
markedly decreased expression was observed at the edge of the
semi-differentiated spheroids. Inversely, CK5/6 expression was
almost absent in the center of the semi-differentiated spheroids,
but was markedly expressed at the edge of the semi-differentiated
spheroids.
Cancer stem-like properties were confirmed at the
protein level in EC spheroids by immunoblotting. As expected,
cancer cells cultured in the serum-free medium caused a CSC marker
shift in the cells, including a marked upregulation of the CSC
markers Nanog and Oct4, and the downregulation of the epithelium
marker CK5/6 (Fig. 1C). The
results indicated that EC tumorspheres demonstrated an increased
expression of stem cell markers.
EC tumorspheres exhibit an increased
expression of ABC-transporter and resistance to chemotherapeutic
drugs
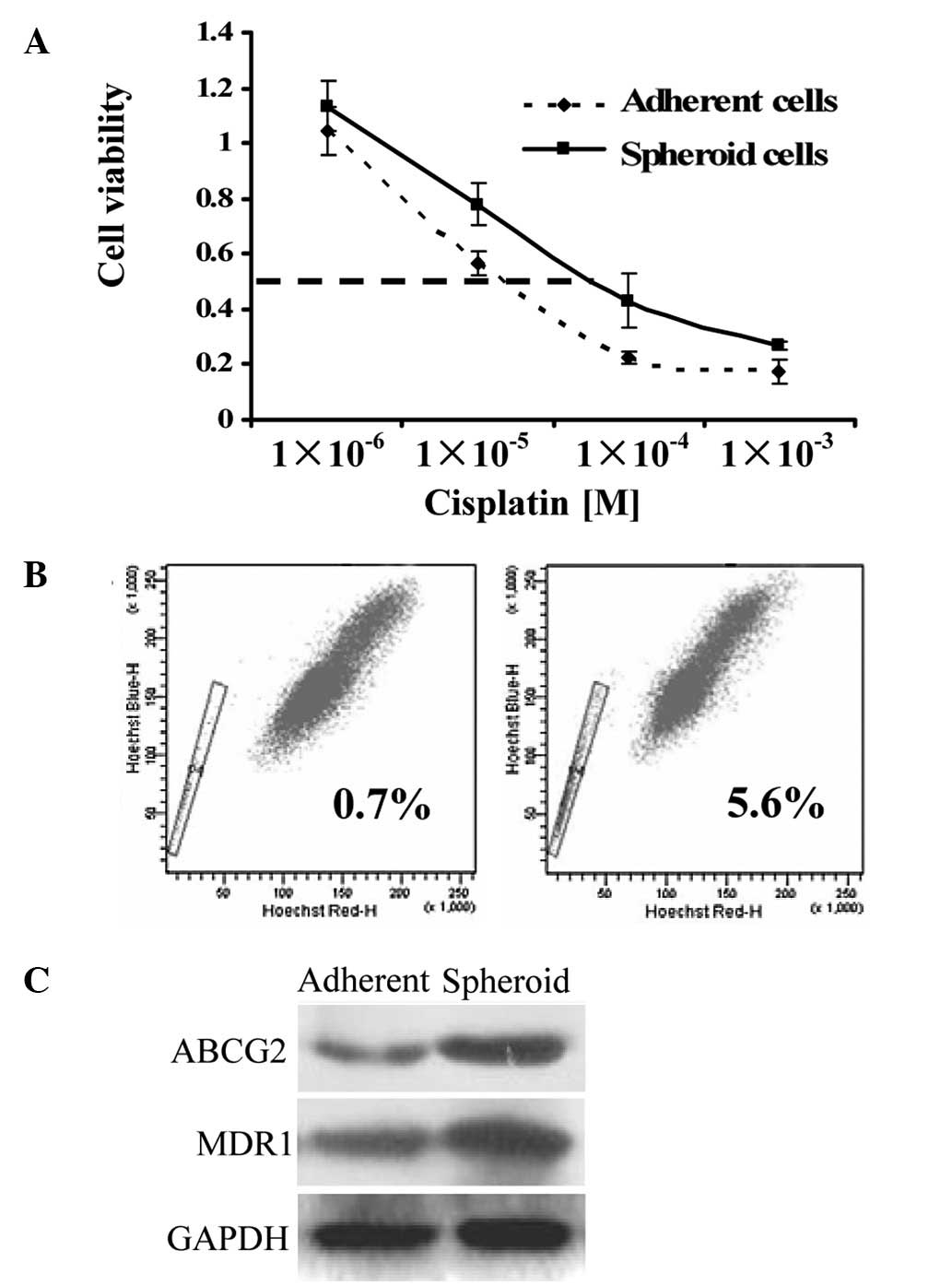
To examine whether EC tumorspheres possess a
hypothesized chemoresistant phenotype of the CSCs, we assessed the
sensitivity of the sphere-forming cells and the differentiated
cells to drugs commonly used in chemotherapy. The EC tumor cells
from the spheroids exhibited an increased IC50 (half
maximal inhibitory concentration) value (4- to 5-fold; 32.5 vs.
135.8 μM) to cisplatin compared to the control adherent cells
(Fig. 2A). Tumor cells resistant
to chemotherapy occur in part due to the overexpression of
ATP-binding cassette multidrug-resistance gene1 (MDR1) (14) and ATP-binding cassette sub-family G
member 2 (ABCG2) (15). This
property correlates with the ability to expel dyes, defined as a
flow cytometry SP (6). SP cells
have also been reported to exhibit CSC characteristics (16). In our study, EC cells cultured in
suspension cultures were found to contain an 8-fold increase in the
proportion of SP cells compared to the adherent controls (0.7 vs.
5.6%; Fig. 2B). ABCG2 and MDR1
were also confirmed at the protein level by immunoblotting. The
result indicated that ABCG2 and MDR1 were substantially increased
in tumorspheres compared to the adherent cells (Fig. 2C).
Gene expression profile analysis of EC
spheroids based on microarray data
To clarify differential gene expression profiles
between tumorsphere and the adherent cells of EC, microarray
analysis was performed. A previous study has verified that the more
serial passages in the spheroids, the more CSCs in spheroids
(12). To ensure the reliability
of microarray results, we achieved the 20th passage of EC
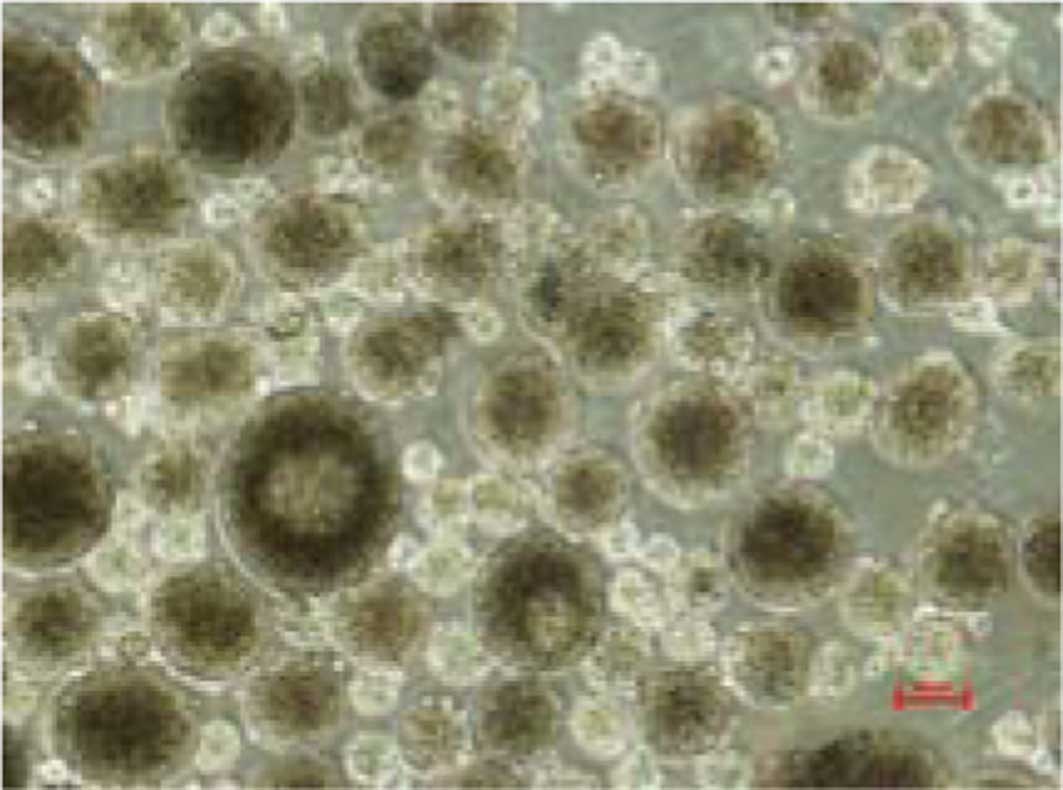
spheroids. The spheroids were filtered by a cell strainer.
Spheroids with a diameter of >40 μm were selected to perform the
microarray analysis (Fig. 3). The
mRNA expression profiles of the spheroid and adherent Eca109 cells
were analyzed by human cDNA microarray. Among the 21,522 probes
examined, 376 genes were upregulated (ratio >2.0) in the
spheroid cells compared to the adherent cells, whereas 325 genes
were downregulated in the spheroid cells. The upregulated genes
were then assigned to a functional class using a gene ontology
annotation tool by the Database for Annotation, Visualization and
Integrated Discovery (Bioresource for array genes; http://david.abcc.ncifcrf.gov/). Based on their
functions, the majority of these genes were classified into
‘polymorphism’, ‘extracellular matrix’, ‘phosphoprotein’, ‘cell
adhesion’ and ‘cell secretion’ groups. In addition, we found 39
genes that showed a >5-fold upregulation in the spheroid cells
compared to the adherent cells (Table
I). Among these genes, three upregulated genes of the ALDH
family exhibited a >5-fold difference in expression, including
ALDH1A1, ALDH1A3 and ALDH3A1 (Table
I, bold).
 | Table IDifferentially expressed genes (sphere
vs. adherent). |
Table I
Differentially expressed genes (sphere
vs. adherent).
| ID | Gene symbol | Ratio | Gene description |
|---|
| 12964 | EREG | 38.0760 | Epiregulin
precursor |
| 5324 | S100A2 | 36.6280 | S100 calcium-binding
protein A2 |
| 13689 | IL1B | 25.3600 | Interleukin-1 β
precursor |
| 9469 | DUSP6 | 19.1750 | Dual specificity
protein phosphatase 6 |
| 10355 | TNC | 17.8630 | Tenascin
precursor |
| 13167 | IGFBP7 | 16.8850 | Insulin-like growth
factor binding protein 7 precursor |
| 4767 | SAT | 11.5940 | Diamine
acetyltransferase 1 |
| 7765 | CLECSF2 | 11.5100 | C-type lectin
superfamily member 2 |
| 20212 | EGR1 | 10.4540 | Early growth response
protein 1 |
| 4340 | FOS | 9.6953 | Proto-oncogene
protein c-fos |
| 5981 | KLK11 | 8.8023 | Kallikrein 11
precursor |
| 16753 | FHL1 | 8.3500 | Skeletal muscle
LIM-protein 1 |
| 7978 | MMP1 | 8.3340 | Interstitial
collagenase precursor |
| 7871 | HAS3 | 8.1108 | Hyaluronan synthase
3 |
| 15869 | TNFAIP3 | 7.3406 | Tumor necrosis
factor, α-induced protein 3 |
| 12711 | COL17A1 | 7.2343 | Collagen α 1 (XVII)
chain |
| 1415 | IL1A | 7.2178 | Interleukin-1 α
precursor |
| 14111 | SERPINB7 | 7.0842 | Megsin |
| 8895 | GBP2 | 6.6438 | Interferon-induced
guanylate-binding protein 2 |
| 958 | LTB | 6.5935 | Lymphotoxin-β |
| 5021 | ALDH1A1 | 6.5078 | Aldehyde
dehydrogenase 1 family, member A1 |
| 1813 | TIMP1 | 6.5020 | Metalloproteinase
inhibitor 1 precursor |
| 6918 | SERPINB2 | 6.4078 | Plasminogen
activator inhibitor-2 precursor |
| 7661 | S100A4 | 6.3883 | S100
calcium-binding protein A4 |
| 6163 | GPR87 | 6.3117 | Probable G
protein-coupled receptor GPR8 |
| 6936 | ALDH1A3 | 6.0678 | Aldehyde
dehydrogenase 6 |
| 2523 | FST | 6.0256 | Follistatin
precursor |
| 5966 | LAMC2 | 5.9743 | Laminin γ-2 chain
precursor |
| 6517 | BF | 5.9716 | Complement factor B
precursor |
| 1393 | FGFBP1 | 5.8656 | Fibroblast growth
factor binding protein 1 |
| 5322 | TSN1 | 5.6896 | Tetraspanin 1 |
| 3284 | PLAG1 | 5.6665 | Pleiomorphic
adenoma gene 1 |
| 3883 | PHCA | 5.4134 | Alkaline
phytoceramidase |
| 6664 | C10orf116 | 5.4024 | Adipose most
abundant gene transcript 2 |
| 1512 | CXCL10 | 5.2340 | Small inducible
cytokine B10 precursor |
| 3028 | SNX8 | 5.1606 | Sorting nexin
8 |
| 968 | ALDH3A1 | 5.1533 | Aldehyde
dehydrogenase, dimeric NADP-preferring |
| 1336 | SEMA3A | 5.0699 | Semaphorin 3A
precursor |
| 17731 | MA17 | 5.0025 | 17 kDa membrane
associated protein |
EC spheroids contain high ALDH enzymatic
activity
In the different profiles, we found that three ALDH
family-related genes were significantly upregulated in the
tumorsphere. ALDH, which detoxifies intracellular aldehydes through
oxidation, may have a role in the differentiation of stem cells
through the oxidation of retinoic acid. (17) ALDH expression has been suggested as
a potential functional marker for CSCs (18). To confirm this finding, we utilized
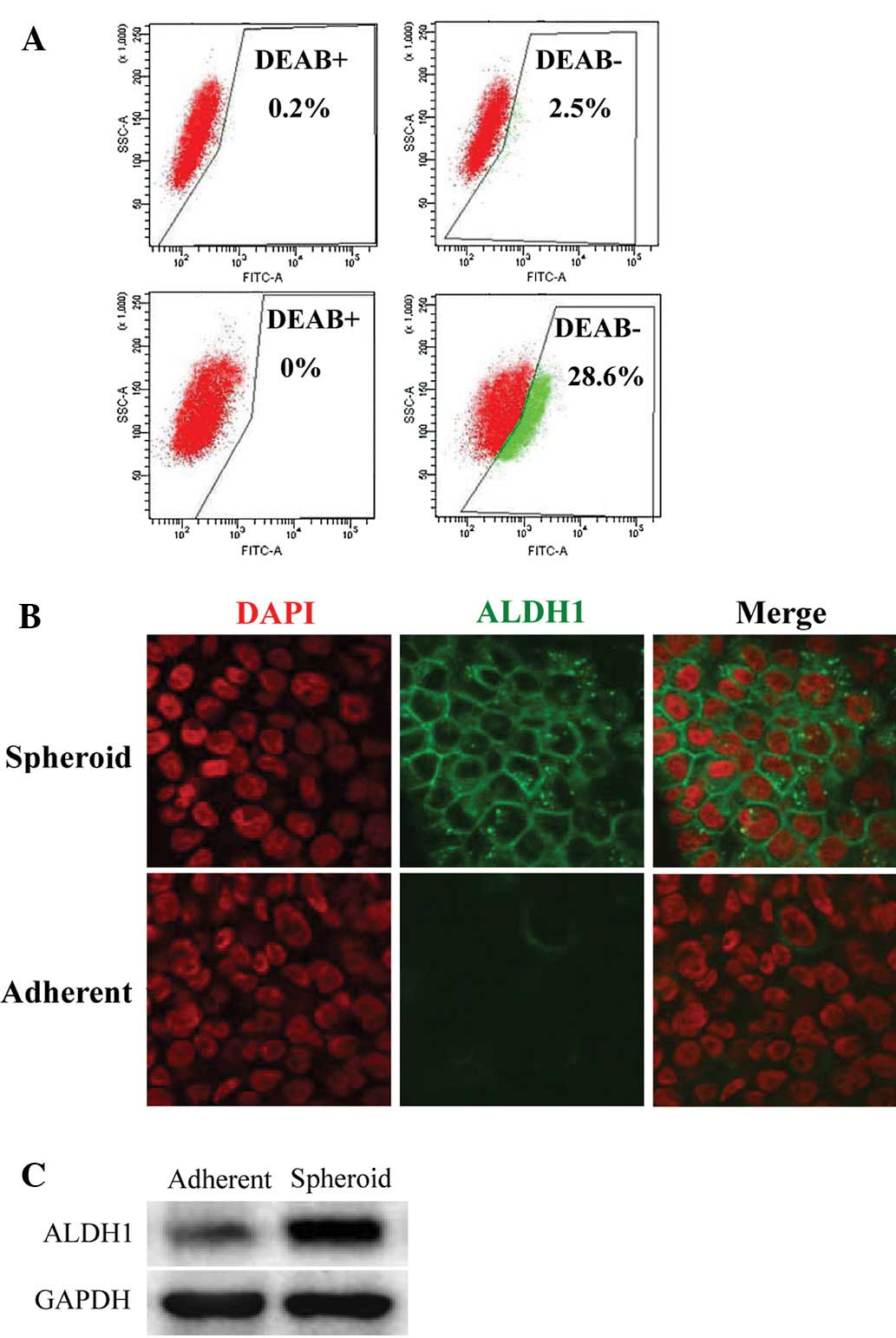
the ALDEFLUOR assay to assess the size of the population with ALDH
enzymatic activity in the Eca109 EC cell line. ALDEFLUOR-positive
cells were enriched by 11- to 12-fold in spheroids, compared to the
adherent cells (2.5 vs. 28.6%; Fig.
4A). The ALDH increase in the tumorsphere was further confirmed
at the protein level by immunofluorescence and immunoblotting.
Immunofluorescence indicated that ALDH1 expression was observed in
the tumorspheres, but was markedly decreased in the adherent cells
(Fig. 4B). Immunoblotting showed a
similar result; ALDH1 was found to be upregulated in tumorspheres
compared to the control adherent cells (Fig. 4C). These results suggest that
ALDH1-positive cells represent the stem/progenitor population of
EC.
Discussion
Current therapies for EC eliminate most cells within
a tumor. However, advanced EC still progresses to incurable,
androgen-independent metastatic disease (19). According to the CSC hypothesis,
current therapies fail to prevent cancer relapse and metastasis,
since the small population of tumor stem cells is not susceptible
to therapy (3). The tumorsphere,
SP cells and drug-resistant cells have cancer stem-like properties.
The SP technique is widely used to identify stem-like cells in
cancer cells (20). SP cells
derived from primary EC cells were more resistant to
chemotherapeutic reagents and formed more colonies in vitro
than non-SP cells; xenograft experiments revealed that SP cells
were more tumorigenic in vivo (21). Drug resistance-related gene ABCG2
expression is an independent unfavorable prognostic factor in
esophageal squamous cell carcinoma (22).
Previous studies have reported the application of
sphere culture to isolate, enrich, maintain or expand potential CSC
subpopulations from various types of cancer (23–27).
It is generally agreed that, as with all stem cells, the
tumorsphere-forming cells are capable of proliferation and
self-renewal and possess higher tumorigenicity. To the best of our
knowledge, few reports are available on the propagation of
esophageal CSCs using sphere culture. In the present study, we
provide a systematic investigation of sphere-propagating cells that
are derived from the Eca109 EC cell line.
The hypothesis that our tumorspheres exhibitied
stem-like properties was based on the following observations: i)
Nanog and Oct4 were expressed in the undifferentiated spheroid
cells, but the expression of CK5/6 was markedly decreased in
undifferentiated spheroid cells; ii) spheroid cells contain more
Nanog and Oct4 protein, but less CK5/6 protein than adherent cells;
iii) tumorspheres exhibited an increased resistance to cisplatin;
iv) spheroid cells had an increased prevalence of SP cells and v)
the ABC-transporter protein was enriched in spheroid cells compared
to adherent cells. Therefore, we suggest that the non-adherent
tumorspheres cultured in serum-free conditions possess esophageal
CSC properties. Thus, suspension culture may effectively be used to
enrich esophageal CSCs.
To understand the mechanisms underlying the
difference in spheroid and adherent cells in the Eca109 cell line,
we performed gene chip analysis and found that three genes from the
ALDH family were highly expressed in esophageal cancer stem-like
cells. This observation was further confirmed by immunoblotting.
ALDHs are a superfamily of 17 intracellular enzymes that protect
cells from the cytotoxic effects of peroxic aldehydes (28). Increased ALDH activity has also
been found in stem cell populations in various types of cancer
(18). ALDH activity may therefore
provide a marker for normal and malignant stem as well as
progenitor cells. Our study indicated that a high ALDH enzymatic
activity is a function of EC tumorsphere. ALDH1 expression has been
confirmed to associate with lymph node metastasis and poor survival
in EC (29). Thus, we believe that
ALDH is a putative CSC marker of EC.
In conclusion, our study outlines a condition for
long-term culture of EC stem-like cells. This system is likely to
be beneficial for the investigation of unique properties of EC
stem-like cells in terms of their biology and their specific cell
surface marker expression that distinguishes them from common EC
cells. Regarding specific surface markers that are associated with
stem-like cells, our current understanding is that ALDH is a
potential esophageal CSC surface marker. Nevertheless, esophageal
CSC cell surface markers remain to be identified. EC tumorsphere
and our tumorsphere microarray analysis data provide a unique
opportunity to find and identify such markers.
Acknowledgements
This study was supported by a Grant from the
Technology Program of the Science and Technology Department of
Guangdong Province (no. 2011B080701021).
References
|
1
|
Ekman S, Dreilich M, Lennartsson J,
Wallner B, Brattström D, Sundbom M and Bergqvist M: Esophageal
cancer: current and emerging therapy modalities. Expert Rev
Anticancer Ther. 8:1433–1448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aminian A, Panahi N, Mirsharifi R,
Karimian F, Meysamie A, Khorgami Z and Alibakhshi A: Predictors and
outcome of cervical anastomotic leakage after esophageal cancer
surgery. J Cancer Res Ther. 7:448–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalerba P and Clarke MF: Cancer stem cells
and tumor metastasis: first steps into uncharted territory. Cell
Stem Cell. 1:241–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathieu J, Zhang Z, Zhou W, et al: HIF
induces human embryonic stem cell markers in cancer cells. Cancer
Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greve B, Kelsch R, Spaniol K, Eich HT and
Götte M: Flow cytometry in cancer stem cell analysis and
separation. Cytometry A. 81:284–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha M: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bez A, Corsini E, Curti D, Biggiogera M,
Colombo A, Nicosia RF, Pagano SF and Parati EA: Neurosphere and
neurosphere-forming cells: morphological and ultrastructural
characterization. Brain Res. 993:18–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi X, Gipp J and Bushman W:
Anchorage-independent culture maintains prostate stem cells. Dev
Biol. 312:396–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Balch C, Chan MW, et al:
Identification and characterization of ovarian cancer-initiating
cells from primary human tumors. Cancer Res. 68:4311–4320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphereforming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
12
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Podolski-Renić A, Andelković T, Banković
J, Tanić N, Ruždijić S and Pešić M: The role of paclitaxel in the
development and treatment of multidrug resistant cancer cell lines.
Biomed Pharmacother. 65:345–353. 2011.PubMed/NCBI
|
|
15
|
Ding XW, Wu JH and Jiang CP: ABCG2: a
potential marker of stem cells and novel target in stem cell and
cancer therapy. Life Sci. 86:631–637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tabor MH, Clay MR, Owen JH, Bradford CR,
Carey TE, Wolf GT and Prince ME: Head and neck cancer stem cells:
the side population. Laryngoscope. 121:527–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stagos D, Chen Y, Brocker C, et al:
Aldehyde dehydrogenase 1B1: molecular cloning and characterization
of a novel mitochondrial acetaldehyde-metabolizing enzyme. Drug
Metab Dispos. 38:1679–1687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma I and Allan AL: The role of human
aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell
Rev. 7:292–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kunisaki C, Makino H, Kimura J, et al:
Therapeutic strategy for esophageal cancer based on solitary lymph
node metastasis. Hepatogastroenterology. 58:1561–1565.
2011.PubMed/NCBI
|
|
20
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar
|
|
21
|
Li H, Gao Q, Guo L and Lu SH: The
PTEN/PI3K/Akt pathway regulates stem-like cells in primary
esophageal carcinoma cells. Cancer Biol Ther. 11:950–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsunoda S, Okumura T, Ito T, et al: ABCG2
expression is an independent unfavorable prognostic factor in
esophageal squamous cell carcinoma. Oncology. 71:251–258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirschhaeuser F, Menne H, Dittfeld C, West
J, Mueller-Klieser W and Kunz-Schughart LA: Multicellular tumor
spheroids: an underestimated tool is catching up again. J
Biotechnol. 148:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks P: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
25
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang D, Nguyen TK, Leishear K, et al: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gou S, Liu T, Wang C, Yin T, Li K, Yang M
and Zhou J: Establishment of clonal colony-forming assay for
propagation of pancreatic cancer cells with stem cell properties.
Pancreas. 34:429–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma S, Chan KW, Lee TK, Tang KH, Wo JY,
Zheng BJ and Guan XY: Aldehyde dehydrogenase discriminates the
CD133 liver cancer stem cell populations. Mol Cancer Res.
6:1146–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Zhe H, Gao P, Zhang N, Li G and
Qin J: Cancer stem cell marker ALDH1 expression is associated with
lymph node metastasis and poor survival in esophageal squamous cell
carcinoma: a study from high incidence area of northern China. Dis
Esophagus. Nov;2011.(E-pub ahead of print).
|