Introduction
Acute cerebrovascular disease (ACVD) is a common
disorder of the nervous system that typically affects blood vessels
in the brain and mainly includes cerebral infarction (CI),
intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH),
which can result in severe neurological impairment or death
(1). ACVD frequently affects older
individuals, resulting in an increase in morbidity and mortality
rates each year in China. However, changes to the living
environment and diet structures are also key factors that should be
considered (2). Notably, early
diagnosis and treatment are able to reduce the degree of
neurological impairment and mortality resulting from ACVD events
(3).
ACVD diagnosis currently relies heavily on
neuroimaging (2). However, studies
conducted in China and worldwide have focused on the identification
of biochemical markers to improve early diagnosis and to
distinguish between ischemic and hemorrhagic stroke, similar to the
way in which troponin is used to diagnose acute myocardial
infarction (2). One biochemical
marker, ischemia-modified albumin (IMA), has been widely studied in
tissue ischemia in recent years. IMA appears to be an early
indicator of myocardial ischemia, which is detectable before the
occurrence of myocardial infarction (4–6).
Moreover, serum IMA increases in mesenteric thrombosis, pulmonary
embolism, stroke and other ischemic diseases (7–11).
Thus, in addition to being a predictor of myocardial infarction,
IMA may be a useful marker for ACVD.
Increased IMA also appears to be associated with
altered lipid profiles. Individuals with hypercholesterolemia
demonstrate increased IMA in addition to increased low-density
lipoproteins (LDL) (12).
Similarly, in cases of obesity or metabolic syndrome, an altered
lipid metabolism and an increased IMA are often observed together
(13,14). Notably, ACVD patients have been
known to exhibit complications of abnormal lipid metabolism
(15). Hypercholesterolemia has
been identified as an independent risk factor for ACVD (16). While findings of an earlier study
demonstrated that IMA could be used to distinguish between certain
types of ACVD (11), whether or
not IMA levels are associated with lipid profiles in ACVD and
whether these markers may be combined to improve diagnosis or
treatment of ACVD remain to be determined.
To determine whether IMA and lipid levels are
associated with ACVD, serum IMA was measured using the albumin
cobalt binding test in 62 subjects with CI, 40 with ICH, 18 with
SAH and 100 healthy individuals. In addition, total cholesterol
(TC), LDL cholesterol (LDL-C), high-density lipoprotein cholesterol
(HDL-C) and triglyceride (TG) levels were determined to in order to
explore correlations between serum IMA and lipid levels in ACVD
patients.
Subjects and methods
Subjects
A total of 120 ACVD patients who were hospitalized
at the First Affiliated Hospital of Liaoning Medical College
(Jinzhou, Liaoning, China) between January 2009 and December 2011
were included in the study. Diagnoses met the criteria established
by the 4th National Cerebrovascular Disease Conference in 1995
(17), and were confirmed by CT or
MRI. Complete biochemical data were available for all participants.
Participants were divided into groups according to the type of
ACVD. The CI group included 62 patients, 38 males and 24 females,
aged 40–74 years; the ICH group included 40 patients, 24 males and
16 females, aged 44–74 years; the SAH group included 18 patients,
10 males and 8 females, aged 42–75 years. The exclusion criteria
for patients with ACVD were: severe liver or renal failure, major
surgery one month prior to disease onset or trauma history,
coronary artery thrombosis within six months, chronic inflammatory
or infectious diseases, previous history of cerebral apoplexy,
pulmonary embolism or thrombotic diseases related to systemic
circulation or pregnancy. One hundred healthy individuals who
received physical examination at our hospital during the same
period were selected as the control group. Participants in the
control group had not taken any medicine within two weeks. The
study was approved by the Ethics Committee of our hospital, and all
enrolled participants or their families provided informed
consent.
Biochemistry
Blood samples for the detection of IMA were
collected 3 h after ACVD. Blood samples for the lipid profiles were
collected following a 12-h fasting period. Serum was separated and
stored at −70°C. IMA levels were detected by enzyme-linked
immunosorbent assay (ELISA) using a kit from R&D Systems
(Minneapolis, MN, USA). TC, TG, LDL-C and HDL-C levels were
measured by enzymatic method (Roche Diagnostics, Indianapolis, IN,
USA) using the Beckman LX-20 Automatic Biochemical Analyzer
according to the manufacturer’s instructions.
Statistical analysis
SPSS 17.0 statistical software was used for
statistical analysis. Measurements were presented as the mean ±
standard deviation. The χ2 test was used to compare
gender distribution between the groups, and the single-factor ANOVA
test was used to compare general characteristics, serum IMA and
lipid levels, as well as to perform a paired comparison (Student
Newman-Keuls test). Pearson’s correlation method was used to
analyze the correlation between serum IMA and lipid levels in the
ACVD patients. Analyses were two-sided, with an α level of 0.05.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ACVD demographics
No statistical difference was found between the CI,
ICH and SAH and control groups with regard to variables such as
gender, age, body mass index and blood albumin (Table I). However, blood glucose, systolic
blood pressure and diastolic pressure were significantly higher in
all of the ACVD groups compared to the control group
(P<0.05).
 | Table IComparison of characteristics of
patients with ACVD vs. healthy individuals. |
Table I
Comparison of characteristics of
patients with ACVD vs. healthy individuals.
| ACVD type | | | |
|---|
|
| | | |
|---|
| Characteristics | CI | ICH | SAH | Control | χ2 or
F-value | P-value |
|---|
| No. of patients | 62 | 40 | 18 | 100 | | |
| Gender (M/F) | 38/24 | 24/16 | 10/8 | 53/47 | 1.276 | 0.735 |
| Age (years) | 59.18±8.60 | 60.93±8.13 | 60.44±9.52 | 60.83±8.58 | 0.55 | 0.648 |
| Body mass index
(kg/m2) | 24.74±3.38 | 24.68±2.90 | 24.45±1.81 | 24.12±3.00 | 0.663 | 0.576 |
| Blood glucose
(mmol/l) | 5.78±0.57a | 5.62±0.43a | 5.65±0.47a | 5.25±0.44 | 17.69 | 0.001 |
| Systolic pressure
(mmHg) | 142.10±11.70a | 147.85±12.12a | 147.44±10.04a | 133.52±7.24 | 27.155 | 0.001 |
| Diastolic pressure
(mmHg) | 85.85±4.45a | 87.75±3.95a | 86.67±3.50a | 82.01±7.52 | 11.708 | 0.001 |
| Serum albumin
(g/l) | 39.40±2.85 | 39.48±2.89 | 39.01±3.15 | 38.67±3.48 | 0.965 | 0.41 |
Serum IMA and lipid levels
Upon measuring the serum IMA levels as well as the
lipid profiles of participants experiencing an ACVD event, we found
that serum IMA, TC and LDL-C (‘bad’ cholesterol) levels were
significantly higher in the patients of each ACVD group compared to
the control group (P<0.05). However, HDL-C (‘good’ cholesterol)
levels significantly decreased in individuals with ACVD compared to
the controls (P<0.05) (Table
II).
 | Table IIComparison of serum IMA and blood
lipid levels among types of ACVD. |
Table II
Comparison of serum IMA and blood
lipid levels among types of ACVD.
| AVCD type | | | |
|---|
|
| | | |
|---|
| CI | ICH | SAH | Control | F-value | P-value |
|---|
| No. of patients | 62 | 40 | 18 | 100 | | |
| IMA (U/ml) | 80.81±11.97a | 80.25±10.91a | 74.43±11.39a | 41.08±5.10 | 324.176 | 0.001 |
| TC (mmol/l) | 5.74±0.19a | 5.42±0.26a | 5.23±0.29a | 4.91±0.06 | 312.241 | 0.001 |
| TG (mmol/l) | 1.75±0.21 | 1.65±0.20 | 1.55±0.17 | 1.44±0.15 | 40.801 | 0.001 |
| LDL-C (mmol/l) | 3.52±0.36a | 3.17±0.34a | 3.02±0.27a | 2.64±0.23 | 117.767 | 0.001 |
| HDL-C (mmol/l) | 1.07±0.16a | 1.16±0.16a | 1.19±0.12a | 1.30±0.17 | 24.849 | 0.001 |
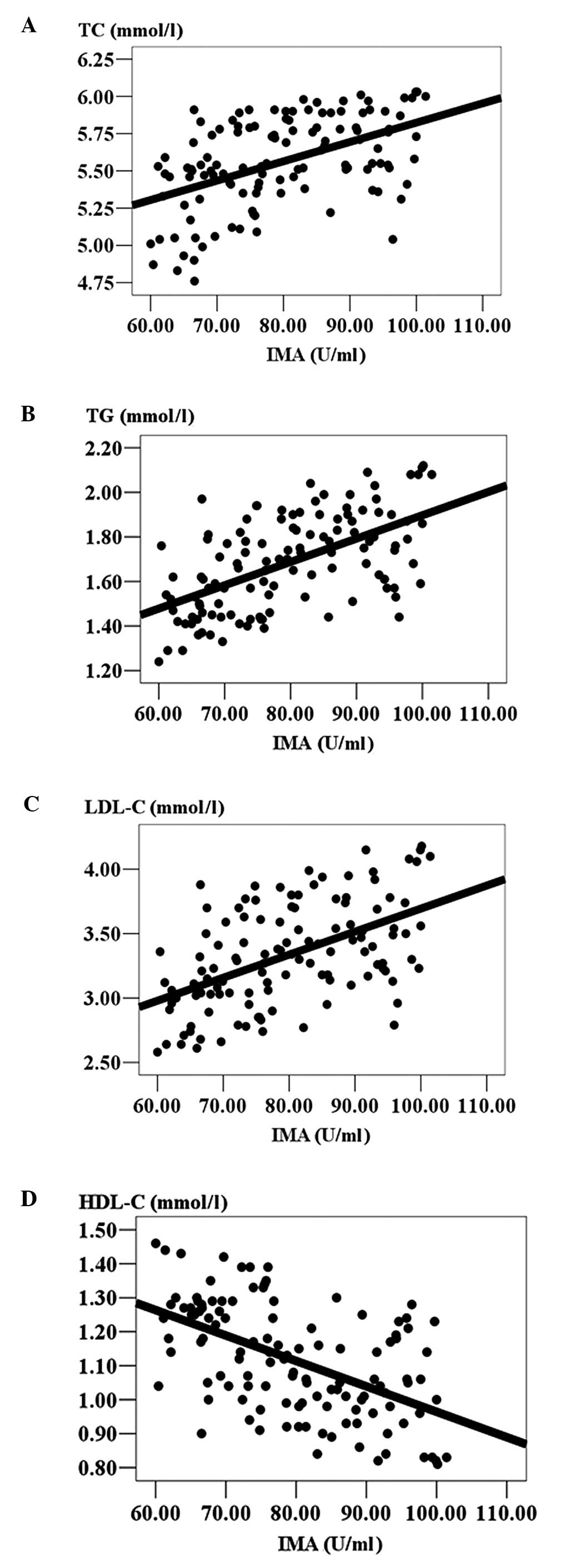
Correlation between serum IMA and lipid
levels
To determine whether serum IMA levels in ACVD
patients are associated with the observed changes in lipid levels,
the Pearson’s correlation was used. Serum IMA levels positively
correlated with patients’ TC, TG and LDL-C levels, but negatively
correlated with HDL-C levels (P<0.05; Table III). Serum TC, TG and LDL-C
levels significantly increased, while HDL-C levels significantly
decreased, with increasing serum IMA levels (Fig. 1).
 | Table IIICorrelation between serum IMA and
blood lipid levels in ACVD patients. |
Table III
Correlation between serum IMA and
blood lipid levels in ACVD patients.
| Correlation | TC | TG | LDL-C | HDL-C |
|---|
| r-value | 0.496 | 0.575 | 0.523 | −0.536 |
| P-value | 0.001 | 0.001 | 0.001 | 0.001 |
Discussion
ACVD accounts for 10% of mortality among all
diseases and is one of the three major causes of mortality in
humans (18). Additionally, ACVD
events result in high rates of disability due to lasting
neurological damage (2). The key
to reducing morbidity and mortality associated with these events is
early diagnosis and treatment. However, ACVD diagnosis currently
relies on clinical symptoms and neuroimaging (2). Molecular markers that are able to
improve diagnosis are required to reduce ACVD mortality.
IMA, derived from human serum albumin (HSA), is an
important molecular marker in cardiac care due to its association
with ischemic events (19). The
amino-terminal sequence of HSA, which is unique to humans, is the
major binding site of transition metals, including Co, Cu and Ni.
When HSA is transported through ischemic tissues, free radicals
released following ischemia/re-perfusion disrupt the amino-terminal
sequence of HSA, reducing its binding capacity with transition
metals; the altered HSA is termed IMA (20). IMA increases during acute
myocardial ischemia, but may also increase during non-cardiac
ischemic events, including infection, cerebrovascular disease,
end-stage renal failure and tumor diseases. A recent study
demonstrated that serum IMA levels differed according to the type
of ACVD event (11). In this
study, we found that serum IMA levels in early-stage (<3 h
following event) ACVD patients were significantly higher compared
to the healthy individuals, which is likely to be due to the fact
that serum IMA concentrations increased in relation to the ischemic
event. Additionally, IMA reflects the degree of ischemia, as
demonstrated by results obtained by Abboud et al (21). Thus, IMA may be a useful marker for
detecting ischemia correlated with ACVD.
Serum IMA concentrations are affected by a number of
factors, particularly serum albumin and serum lipid levels
(19). Since other diseases that
are closely associated with changes in serum lipids also appear to
demonstrate increased IMA (e.g., metabolic syndrome and obesity),
there may be a specific link between these features in causing
disease (13,14). This observation is emphasized by
the fact that patients with pure hypercholesterolemia have
increased IMA (12). We observed
that IMA levels in ACVD patients were positively correlated with
TC, TG and LDL-C concentrations, supporting findings for other
diseases. A potential reason for the correlation between IMA and
lipid changes in ACVD is that, during ACVD attack, high
concentrations of blood lipids increase the local blood viscosity,
causing slow blood flow. In turn, platelets aggregate and form
blood clots more easily, promoting the progression of ACVD. In
addition, high concentrations of serum lipids are able to increase
the instability of vulnerable plaques, aggravating artery stenosis
or promoting the formation of blood clots, thereby aggravating
ischemia and resulting in increased IMA (22). A second possibility is that highly
concentrated serum combines with albumin to reduce binding sites of
albumin and cobalt ions, causing a decrease in the binding capacity
of albumin cobalt, which is manifested as increased levels of serum
IMA (23).
In conclusion, an increase in the levels of serum
IMA of ACVD patients was observed. This increase renders IMA a
useful marker for the early diagnosis of ACVD. Additionally, serum
IMA levels in ACVD are closely correlated with lipid levels. The
molecular mechanisms behind the correlation between IMA and lipids
should be investigated. However, it is possible these mechanisms
may be developed into a more sensitive diagnostic tool for
ACVD.
References
|
1
|
Martinez-Gonzãlez NA and Sudlow CL:
Effects of apolipoprotein E genotype on outcome after ischaemic
stroke, intracerebral haemorrhage and subarachnoid haemorrhage. J
Neurol Neurosurg Pshychiatry. 77:1329–1335. 2006.
|
|
2
|
Gong X, Fang M, Wang J, et al:
Three-dimensional reconstruction of brain surface anatomy based on
magnetic resonance imaging diffusion-weighted imaging: a new
approach. J Biomed Sci. 11:711–716. 2004.
|
|
3
|
Salvarani C, Brown RD Jr, Calamia KT, et
al: Primary central nervous system vasculitis: analysis of 101
patients. Ann Neurol. 62:442–451. 2007.
|
|
4
|
Wu AH: The ischemia modified albumin
biomarker for myocardial ischemia. MLO Med Lab Obs. 35:36–38.
2003.
|
|
5
|
Sinha MK, Roy D, Gaze DC, Collinson PO and
Kaski JC: Role of ‘ischemia modified albumin’, a new biochemical
marker of myocardial ischemia, in the early diagnosis of acute
coronary syndrome. Emerg Med J. 21:29–34. 2004.
|
|
6
|
Sacchetti A: ‘Ischemia modified albumin’:
a new biochemical marker of myocardial ischemia. Emerg Med J.
21:3–4. 2004.
|
|
7
|
Keating L, Benger JR, Beetham R, et al:
The PRIM A study: presentation of ischemia modified albumin in the
emergency department. Emerg Med J. 23:764–768. 2006.
|
|
8
|
Refaai MA, Wright RW, Parvin CA, et al:
Ischemia-modified albumin increases after skeletal muscle ischemia
during arthroscopic knee surgery. Clin Chim Acta. 366:264–268.
2006.
|
|
9
|
Turedi S, Gunduz A, Mentese, et al: The
value of ischemia-modified albumin in the diagnosis of pulmonary
embolism. AM J Emerg Med. 25:770–773. 2007.
|
|
10
|
Gunduz A, Turedi S, Mentese A, et al:
Ischemia-modified albumin in the diagnosis of acute mesenteric
ischemia: a preliminary study. AM J Emerg Med. 26:202–205.
2009.
|
|
11
|
Gunduz A, Turedi S, Mentese A, et al:
Ischemia-modified albumin levels in cerebrovascular accidents. Am J
Emerg Med. 26:874–878. 2008.
|
|
12
|
Duarte MM, Rocha JB, Moresco RN, et al:
Association between ischemia-modified albumin, lipids and
inflammation biomarkers in patients with hypercholesterolemia. Clin
Biochem. 42:666–671. 2009.
|
|
13
|
Valle Gottlieb MG, da Cruz IB, Duarte MM,
et al: Associations among metabolic syndrome, ischemia,
inflammatory, oxidatives, and lipids biomarkers. J Clin Endocrinol
Metab. 95:586–591. 2010.
|
|
14
|
Piva SJ, Duarte MM, Da Cruz IB, et al:
Ischemia-modified albumin as an oxidative stress biomarker in
obesity. Clin Biochem. 44:345–347. 2011.
|
|
15
|
Velcheva I, Antonova N, Dimitrova V, et
al: Plasma lipids and blood viscosity in patients with
cerebrovascular disease. Clin Hemorheol Microcirc. 35:155–157.
2006.
|
|
16
|
Kimura Y and Uchiyama S: Hyperlipidemia
and cerebrovascular disease. Nihon Rinsho. 23:685–689. 2001.
|
|
17
|
Moore WS, Barnett HJ, Beebe HG, et al:
Guidelines for carotid endarterectomy. A multidisciplinary
consensus statement from the Ad Hoc Committee, American Heart
Association. Circulation. 91:566–579. 1995.
|
|
18
|
Pappachan J and Kirkham FJ:
Cerebrovascular disease and stroke. Arch Dis Child. 93:890–898.
2008.
|
|
19
|
Roy D, Quiles J, Sharma R, et al:
Ischemia-modified albumin concentrations in patients with
peripheral vascular disease and exercise-induced skeletal muscle
ischemia. Clin Chem. 50:1656–1680. 2004.
|
|
20
|
Lippi G, Montagnana M and Guidi GC:
Albumin cobalt binding and ischemia modified albumin generation: an
endogenous response to ischemia. Int J Cardiol. 108:410–411.
2006.
|
|
21
|
Abboud H, Labreuche J, Meseguer E, et al:
Ischemia-modified albumin in acute stroke. Cerebrovasc Dis.
23:216–220. 2007.
|
|
22
|
Wei LX, Tang QH, Sun L, et al:
Relationship between oxidized lipoprotein, angiogenesis and human
coronary atherosclerotic plaque stabilization. Zhonghua Bing Li Xue
Za Zhi. 35:138–141. 2006.
|
|
23
|
Bhagavan NV, Ha JS, Park JH, et al:
Utility of serum fatty acid concentrations as a marker for acute
myocardial infarction and their potential role in the formation of
ischemia-modified albumin: a pilot study. Clin Chem. 55:1588–1590.
2009.
|