Introduction
Aquaporins (AQPs) are small, integral membrane
proteins that selectively transport water across cell plasma
membranes. AQPs are expressed in a number of fluid-transporting
tissues, including kidney tubules and glandular epithelia, as well
as in non-fluid-transporting tissues, such as epidermis, adipose
tissue and astroglia. AQPs are strongly expressed in tumor cells of
different origins, particularly aggressive tumors (1). AQP expression in tumors has been
suggested to be of diagnostic and prognostic value. Recent
discoveries of AQP involvement in cell migration and proliferation
suggest that AQPs play key roles in tumor biology. AQP-dependent
cell migration has been found in a variety of cell types in
vitro and in mice in vivo. AQP3 has been found to be
expressed in cultured fibroblasts, with AQP3 knockdown by RNA
inhibition reducing fibroblast migration (2). Impaired cell migration has also been
observed in AQP1-deficient proximal tubule epithelial cells,
AQP3-deficient corneal epithelial cells and enterocytes (3).
The epidermal growth factor (EGF) family of growth
factors exert their roles and elicit a series of physiological and
pathological actions by binding to the EGF receptor (EGFR) family.
Activation of the EGFR family has been identified as a key event
that initiates the cascade of intracellular signaling pathways
leading to proliferation, cell survival, angiogenesis and
metastasis (4). A number of the
most common human epithelial cancers express relatively high levels
of EGFR at advanced stages of malignancy and an increased
metastatic potential of the disease (5). Elevated expression or activity of
EGFR is common in human cancer and is associated with poor patient
prognosis (6). Therefore, we
hypothesized that AQPs in MPC-83 pancreatic cancer cells also
mediate EGF-induced cell migration and thus their metastatic
potential.
In the present study, we investigated whether EGF
induced the phosphorylation of EGFR and ERK and if so, whether AQP3
expression was upregulated by EGF. We also observed whether EGF
facilitated MPC-83 pancreatic cancer cell migration. Using western
blot analysis and in vitro cell migration, we found that EGF
phosphorylates EGFR and ERK and that AQP3 expression is upregulated
by EGF. EGF, via the EGFR signaling pathway, facilitated AQP3
expression, which is involved in cell migration in MPC-83
pancreatic cancer cells. Our findings provide an explanation as to
the molecular mechanisms of MPC-83 pancreatic cancer cell migration
and may contribute to potential therapeutic strategies for the
treatment of pancreatic cancer.
Materials and methods
Reagents
Rabbit anti-aquaporin3 was obtained from Chemicon
(Temecula, CA, USA). Rabbit anti-phospho-EGFR (Tyr1068),
phospho-ERK and ERK were obtained from Cell Signaling Technology
(Beverly, MA, USA). Rabbit anti-mouse IgG-HRP antibody was obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Monoclonal mouse anti-β-actin was obtained from Sigma (St. Louis,
MO, USA). PD153035 and U0126 were obtained from CalbioChem (San
Diego, CA, USA). CuSO4 was obtained from Sigma.
Cell culture
Cultured pancreatic cancer cell line MPC-83 cells
were maintained in DMEM (Sigma) supplemented with 10% fetal bovine
serum (FBS), penicillin/streptomycin (1:100, Sigma) and 4 mM
L-glutamine (1:100, Sigma), in a CO2 incubator at 37°C.
For western blot analysis, cells were reseeded in 6-well plates at
a density of 0.2×106 cells/ml with fresh complete
culture medium. Morphological changes were observed under a phase
contrast microscope.
Western blot analysis
Cultured MPC-83 pancreatic cancer cells with or
without treatment were washed with cold PBS and harvested by
scraping into 150 μl of RIPA buffer (containing 50 mM Tris-HCl, pH
7.4, 150 mM NaCl, 1% NP40, 1 mM EDTA, 0.25% sodium deoxycholate)
with fluoride and protease inhibitor cocktail (10 μl/ml leupeptin,
10 μl/ml aprotinin and 1 μM pepstatin). Cell lysates were incubated
at 4°C for 30 min. After centrifugation at 14,000 rpm for 10 min at
4°C, the protein concentration was determined by a Bio-Rad protein
assay (Bio-Rad, Hercules, CA, USA). Proteins (20 μg for AQP3,
phospho-EGFR and EGFR, phospho-ERK and ERK; 10 μg for β-actin) were
denatured in 5X SDS-PAGE sample buffer for 5 min at 95°C. The
proteins were separated by 10 or 7.5% SDS-PAGE and transferred onto
PVDE membranes (Millipore, Bedford, MA, USA) for 2 h at 4°C.
Non-specific binding was blocked with 10% dry milk in TBST (20 mM
Tris-HCl, 137 mM NaCl, 0.01% Tween-20, pH 7.4) for 1 h at room
temperature. After blocking, the membranes were incubated with
specific antibodies against AQP3 (1:1,000), EGFR (1:1,000),
phospho-EGFR (1:1,000), phospho-ERK and ERK (1:1,000) and β-actin
(1:20,000) in dilution buffer (2% BSA in TBS) overnight at 4°C. The
blots were incubated with HRP-conjugated anti-rabbit or anti-mouse
IgG at appropriate dilutions and room temperature for 1 h. Antibody
binding was detected using an enhanced chemiluminescence (ECL)
detection system following the manufacturer’s instructions and
visualized by autoradiography with Hyperfilm.
Phagokinetic track motility assay
Twelve-well plates were coated with coating medium
of 20 μg/ml fibronectin (Sigma) in PBS and placed in a
CO2 incubator at 37°C for at least 2 h. After removing
the coating medium gently with a pasteur pipette, the wells were
washed with PBS and 2.4 ml of microsphere suspension (86 μl of
stock microbeads solution in 30 ml PBS) was added to each well. The
plates were then centrifuged at 1,200 rpm at 4°C for 20 min and
carefully transferred to a CO2 incubator and incubated
at 37°C for at least 1 h. From each well, 1.8 ml of supernatant was
removed and 1,500 freshly trypsinized cells in 2 ml assay medium
(DMEM supplemented with a 0.05% FBS) were seeded per well. The
cells were cultured for 24 h and photographed under a phase
contrast microscope.
Statistical analysis
Experiments were performed in triplicate. Data were
presented as the mean ± standard error (SE). Comparisons among
groups were performed using analysis of variance (ANOVA). P<0.05
was considered to indicate statistically significant
differences.
Results
Phosphorylation of EGFR and ERK was
induced by EGF in MPC-83 pancreatic cancer cells
MPC-83 pancreatic cancer cells were pre-treated with
EGF at different time points, then p-EGFR and p-ERK were detected
by western blotting. The results showed that EGFR phosphorylation
was induced by EGF. This phosphorylation peaked at 5 min and lasted
1 h (Fig. 1A). Similar results
were observed in ERK phosphorylation, which peaked at 5 min after
EGF treatment and lasted 40 min (Fig.
1B). The phosphorylation of EGFR and ERK induced by EGF was
significantly inhibited in MPC-83 pancreatic cancer cells which
were pre-treated by PD153035 (PD) or U0126 (U0) (Fig. 1C and D). This finding confirms that
EGF induces the phosphorylation of EGFR and further activates its
downstream signal pathway.
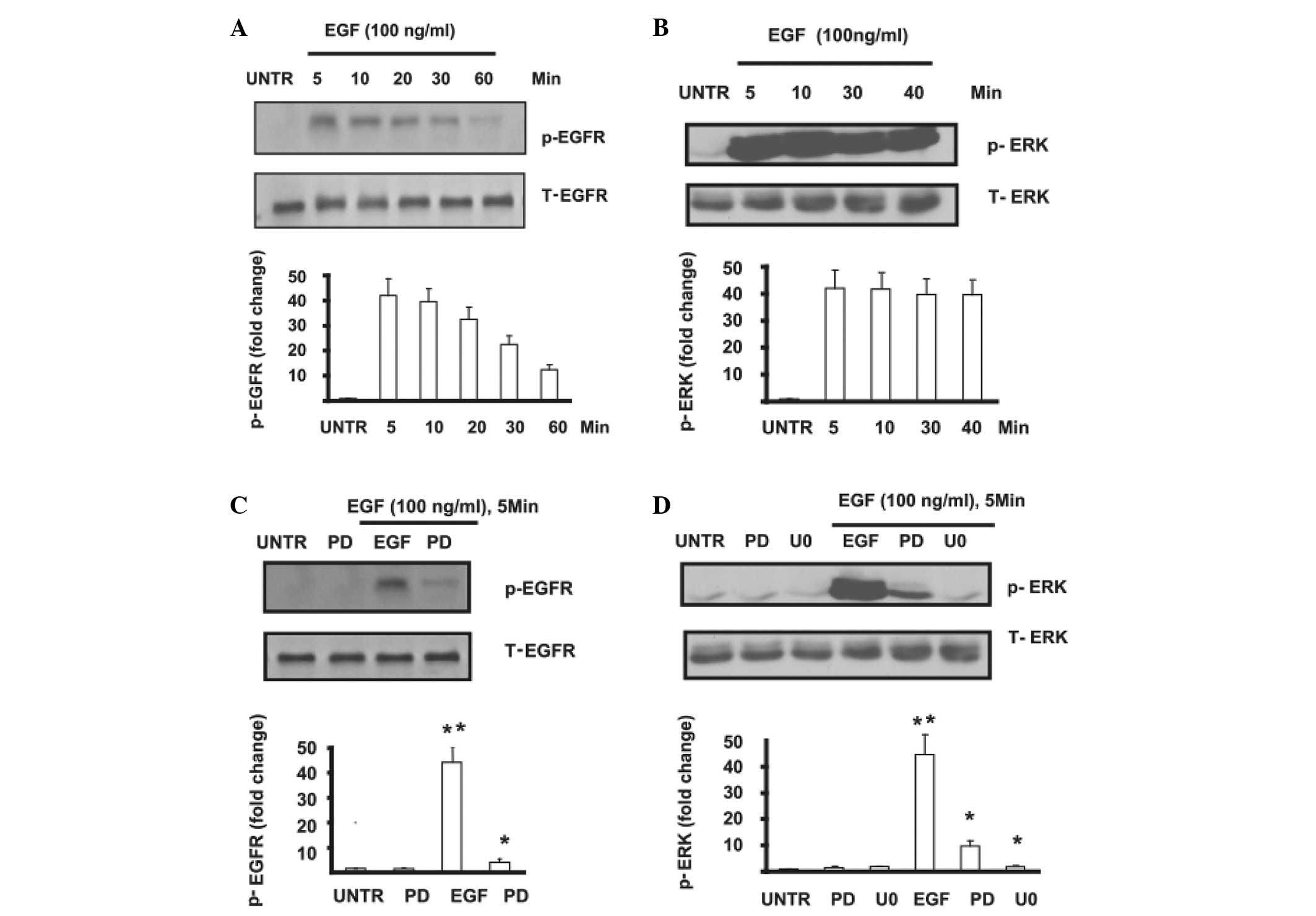 | Figure 1(A) Cells were treated with EGF (100
ng/ml) and collected at different time points (5, 15, 30 and 60
min). EGFR phosphorylation was analyzed by western blotting and
quantified. (B) Cells were treated with EGF (100 ng/ml) and
collected at different time points (5, 10, 30 and 40 min), cell
lysates were collected and ERK phosphorylation was analyzed by
western blotting and quantified. (C) Cells were pre-treated with or
without PD (PD153035) followed by EGF (100 ng/ml) for 5 min, and
EGFR phosphorylation was analyzed by western blotting and
quantified. (D) Cells were pre-treated with or without PD or U0
(U0126) followed by EGF (100 ng/ml) for 5 min, and ERK
phosphorylation was analyzed by western blotting and quantified.
The data are presented as the mean ± SE of triplicate experiments.
*P<0.05. EGF, epidermal growth factor; EGFR, EGF
receptor; ERK, extracellular signal-regulated kinase. |
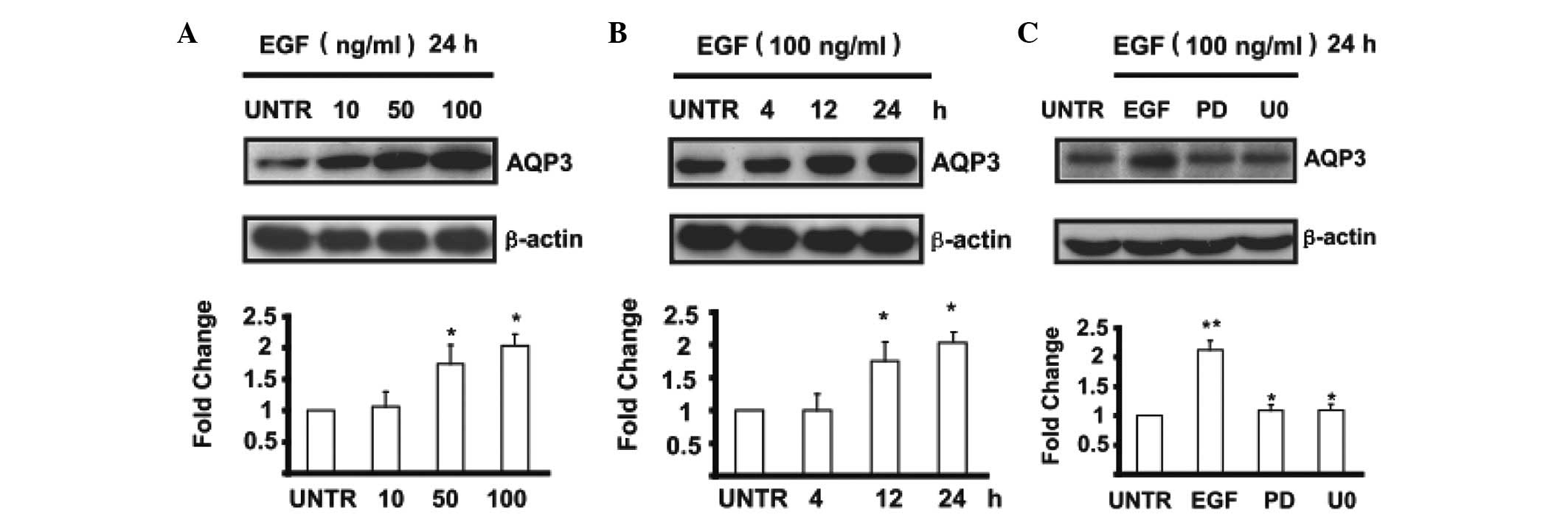
Expression of AQP3 was upregulated by EGF
in MPC-83 pancreatic cancer cells
Cells were treated with EGF at different
concentrations of 10, 50 and 100 ng/ml and cell lysates were
analyzed for AQP3 by western blotting. The results showed that EGF
induced the upregulation of AQP3 in a dose-dependent manner in
MPC-83 pancreatic cancer cells (Fig.
2A). AQP3 expression begins to increase after cells are treated
with 50 ng/ml of EGF and is highest at 100 ng/ml of EGF treatment.
The expression of AQP3 was upregulated by EGF in a time-dependent
manner (Fig. 2B). AQP3 begins to
increase at 12 h and is most obvious at 24 h after being treated
with 100 ng/ml of EGF. The expression of AQP3 was inhibited in
MPC-83 pancreatic cancer cells which were pre-treated by PD or U0
followed with EGF treatment (Fig.
2C). The data show the mean ± SE of triplicate experiments.
Migration of MPC-83 pancreatic cancer
cells was inhibited by PD, U0 and CuSO4 (water and
glycerol transport inhibitors of AQP3)
The migration of MPC-83 pancreatic cancer cells
which were pre-treated with PD, U0 and CuSO4 followed by
EGF treatment was significantly inhibited (Fig. 3).
Discussion
Pancreatic cancer remains a potentially fatal
malignancy giving rise to continuing concern for a number of
reasons. Pancreatic cancer is a major unsolved health problem, with
conventional cancer treatments having little impact on disease
course. Almost all patients who have pancreatic cancer develop
metastases and succumb to the disease. The main risk factors are
smoking, age and certain genetic disorders, although the primary
causes are poorly understood.
Tumor-committed cells generally have an aggressive
metabolic energy profile, allowing them to compete with surrounding
cells, proliferate and form characteristic structures. At least 12
different tumor cell types have been reported to express AQPs in
vivo in humans and rodents. For certain tumors, positive
correlations have been established between histological tumor grade
and the level of AQP expression. AQP3, which is found in normal
epidermis and becomes upregulated in basal cell carcinoma,
facilitates cell proliferation in different cell types. AQP3-null
mice are resistant to skin tumorigenesis by a mechanism that may
involve reduced tumor cell glycerol metabolism and ATP generation
(7). AQP expression in tumors is
known to facilitate tumor cell migration and spread, suggesting a
novel function for AQP expression in high-grade tumors. One
consequence of AQP involvement in tumor cell migration is the
possibility of AQP inhibition to limit tumor spread, although
testing of this possibility requires the development of suitable
AQP-selective inhibitors (8). We
have found that AQP3 is expressed in cultured MPC-83 pancreatic
cancer cells. Using specific AQP3 water transport inhibitors, it is
confirmed that AQP3 is involved in EGF-induced pancreatic cancer
cell migration in vitro.
Growth factors control cell growth, proliferation,
differentiation, survival and migration by activating receptors on
specific target cells. Aberrant activation of EGFR and the EGF
signal pathway is associated with neoplastic cell proliferation,
migration, stromal invasion, resistance to apoptosis and
angiogenesis (9). Aberrant EGFR
expression and signaling contribute to the development of multiple
epithelial malignancies in humans, including squamous cell
carcinomas of the skin and breast cancer (10). In particular, EGFR expression is
upregulated in 33–50% of human epithelial tumors (11). In this study, we confirm that AQP3
upregulation is involved in MPC-83 pancreatic cancer cell migration
and this effect can be induced by EGF. We also provide evidence
that an EGFR-mediated MEK/ERK pathway is involved in EGF-induced
AQP3 expression and cell migration in MPC-83 pancreatic cancer
cells.
EGFR-mediated signaling pathways have been shown to
contribute to the regulation of angiogenesis and metastasis
(12). To demonstrate whether one
of these pathways was involved in MPC-83 pancreatic cancer cell
migration, EGFR and ERK inhibitors were used. We found that these
two inhibitors suppressed AQP3 expression and cell migration
induced by EGF in MPC-83 pancreatic cancer cells. The above results
clearly demonstrate that an EGF/EGFR/ERK signaling pathway is at
least partly involved in EGF-induced AQP3 expression and cell
migration in MPC-83 pancreatic cancer cells. First, EGF induces the
phosphorylation of EGFR and ERK, then AQP3 expression is
up-regulated and finally, MPC-83 pancreatic cancer cell migration
is enhanced.
We conclude that AQP3 is expressed in MPC-83
pancreatic cancer cells and AQP3 facilitates MPC-83 pancreatic
cancer cell migration. EGF upregulates AQP3 expression and cell
migration via the EGFR/ERK signal transduction pathway in MPC-83
pancreatic cancer cells. This is likely to aid in future efforts to
target appropriate EGFR-mediated signals as a rational cancer
therapy.
References
|
1
|
Jeyaseelan K, Sepramaniam S, Armugam A, et
al: Aquaporins: a promising target for drug development. Expert
Opin Ther Targets. 10:889–909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walz T, Fujiyoshi Y and Engel A: The AQP
structure and functional implications. Handb Exp Pharmacol. 31–56.
2009.
|
|
3
|
Verkman AS, Hara-Chikuma M and
Papadopoulos MC: Aquaporins–new players in cancer biology. J Mol
Med (Berl). 86:523–529. 2008.
|
|
4
|
Sartore-Bianchi A, Bencardino K, Di
Nicolantonio F, et al: Integrated molecular dissection of the
epidermal growth factor receptor (EFGR) oncogenic pathway to
predict response to EGFR-targeted monoclonal antibodies in
metastatic colorectal cancer. Target Oncol. 5:19–28. 2010.
View Article : Google Scholar
|
|
5
|
Perol M and Arpin D: Tyrosine kinase
inhibitors in the management of non-small cell lung cancer. Rev Mal
Respir. 24:6S188–6S197. 2007.PubMed/NCBI
|
|
6
|
Valentini AM, Pirrelli M and Caruso ML:
EGFR-targeted therapy in colorectal cancer: does
immunohistochemistry deserve a role in predicting the response to
cetuximab? Curr Opin Mol Ther. 10:124–131. 2008.PubMed/NCBI
|
|
7
|
Nico B and Ribatti D: Role of aquaporins
in cell migration and edema formation in human brain tumors. Exp
Cell Res. 317:2391–2396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papadopoulos MC, Saadoun S and Verkman AS:
Aquaporins and cell migration. Pflugers Arch. 456:693–700. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giaccone G and Wang Y: Strategies for
overcoming resistance to EGFR family tyrosine kinase inhibitors.
Cancer Treat Rev. 37:456–464. 2011.PubMed/NCBI
|
|
10
|
Mahipal A, McDonald MJ, Witkiewicz A, et
al: Cell membrane and cytoplasmic epidermal growth factor receptor
expression in pancreatic ductal adenocarcinoma. Med Oncol.
29:134–139
|
|
11
|
Steelman LS, Franklin RA, Abrams SL, et
al: Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy.
Leukemia. 25:1080–1094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dragovich T and Campen C:
Anti-EGFR-targeted therapy for esophageal and gastric cancers: an
evolving concept. J Oncol. 8041082009.PubMed/NCBI
|