Introduction
MicroRNAs (miRNAs) are short regulatory RNA
molecules with an average length of 22 nucleotides. When miRNAs are
integrated into RNA-induced silencing complexes, the molecules bind
to a partially complementary sequence in the 3′-untranslated
regions (3′-UTRs) of target mRNAs and regulate gene expression by
inhibiting translation and destabilizing transcripts (1). Beyond the involvement in diverse
biological processes, including cell growth, apoptosis (2), development (3), differentiation (4) and endocrine homeostasis (5), it has been well demonstrated that the
deregulation or dysfunction of miRNAs contributes to cancer
development. In recent years, large high-throughput studies in
patients revealed that miRNA profiling has the potential to
classify tumors with high accuracy. In addition, miRNA alterations
have been reported to be associated with specific clinical
phenotypes, including disease progression or recurrence,
development of metastases and post-operative outcome. The results
of functional studies, some of which involved animal models,
indicate that miRNAs act as tumor suppressors and oncogenes
(6). It has been proposed that
alterations in miRNA genes play a critical role in the
pathophysiology of various types of human cancer (7).
Oral squamous cell carcinomas (OSCCs) account for
over 40% of head and neck malignancies and rank sixth worldwide in
incidence. OSCCs have a particularly poor prognosis due to their
invasive nature. Despite advances in the fields of oncology and
surgery, the five-year survival rates of OSCC are less than 50% and
have remained unchanged in the last three decades (8). New molecular insights into the
mechanisms of cancer formation and progression are likely to aid
the improvement of diagnosis and prognosis and the development of
new therapies for treating oral cancer.
Using microarrays or quantitative reverse
transcription-polymerase chain reaction (qRT-PCR), a number of
studies have analyzed miRNA expression profiles in OSCC and head
and neck squamous cell carcinoma (HNSCC) (9). Certain functional studies have
revealed miRNA-regulated pathways in OSCC proliferation or
apoptosis, metastasis and chemoresistance. miRNA (miR)-21 is
overexpressed and exhibits oncogenic activity in various
carcinomas, including OSCC (10).
The high expression of miR-21 was found to be associated with low
levels of tropomyosin 1 (TPM1) and phosphatase tensin homolog
(PTEN) expression and reduced cell apoptosis (10). The overexpression of miR-184 was
confirmed in tongue squamous cell carcinoma (TSCC) tissues. The
inhibition of miR-184 was able to reduce TSCC cell proliferation
while suppressing miR-184 induced cell apoptosis. Plasma miR-184
levels were significantly higher in TSCC patients compared with
normal individuals and the levels were significantly reduced
following the surgical removal of the primary tumors (11). miR-133a, −133b, −137, −193a, −125b
and −100 are frequently downregulated in OSCC (12). In addition, a recent study showed
that reduced levels of miR-138 and miR-222 were correlated with the
enhanced metastatic potential of TSCC (13). These deregulated miRNAs have
potential as novel diagnostic, prognostic and therapeutic tools,
which are expected to advance the clinical management of OSCC in
the near future.
However, the role of miR-99a in OSCC development
remains unknown. In the present study, we investigated the roles of
miR-99a in OSCC development and the underlying mechanisms.
Materials and methods
Cell line
The human OSCC cell line Tca-8113 was routinely
maintained in the Institute of Immunology, Zhejiang University
School of Medicine (Hangzhou, China). Tca-8113 cells were cultured
in RPMI-1640 medium, supplemented with 10% (v/v) fetal bovine serum
(FBS), 100 U/ml penicillin and 100 U/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2.
Patients and samples
Surgical samples of 25 human primary OSCC tissues
and 25 tumor-free tissues were obtained during the surgical
resection of OSCC patients and immediately frozen in liquid
nitrogen and stored at −80°C until analysis. Patients with primary
OSCC treated between 2008 and 2010 in the Department of Oral and
Maxillofacial Surgery, Ningbo First Hospital (Ningbo, China), were
studied with the approval of the local Ethics Committee. The
patients included in the present study: i) were primary OSCC
patients confirmed by pathology following surgery; ii) did not
undergo radiotherapy, chemotherapy or immunotherapy prior to
surgery; iii) had complete clinicopathological data available. The
tissue samples were from the following sites: tongue (20), mouth floor (4) and gingiva (1). The histology of the tissues was
evaluated by the hospital's pathologist. Histological grading was
performed according to the World Health Organization
classification. Samples were collected only after receiving
informed consent from the patients.
RNA isolation and real-time qPCR
For RT-PCR, total RNA of tumor and tumor-free
tissues was isolated using TRIzol reagent (Invitrogen, Carlsbad,
CA, USA) according to the manufacturer's instructions. Real-time
qPCR, using SYBR-Green detection chemistry (Takara Bio Inc., Shiga,
Japan), was performed on a 7500 Real-Time PCR system (Applied
Biosystems, Carlsbad, CA, USA). For miRNA analysis, the RT primer
for miR-99a was
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACAAG-3′. The qPCR
primers were 5′-GGAACCCGTAGATCCGAT-3′ (forward) and 5′-GTGC
AGGGTCCGAGGT-3′ (reverse). U6 small nuclear RNA was quantified
using its reverse primer for the RT reaction and its forward and
reverse primers for qPCR, which were 5′-CTCGCTTCGGCAGCACA-3′
(forward) and 5′-AACG CTTCACGAATTTGCGT-3′ (reverse). Quantitative
measurements were determined using the ΔΔCt method and the
expression of U6 was used as the internal control.
Transfection of miR-99a mimics and MTT
assay
miR-99a mimics and controls obtained from GenePharma
(Shanghai, China) were used for the overexpression of miR-99a
activity in cells. The cells were transfected with RNAs at a final
concentration of 20 nM. JetSI-ENDO transfection reagents
(Polyplus-transfection, Illkirch, France) were used for the
transfection of RNAs, according to the manufacturer's
instructions.
The growth of Tca-8113 cells following miR-99a
treatment was examined using an MTT assay. The cells were
subcultured in 96-well plates at a density of 1×104
cells/well, transfected with miR-99a mimics or control mimics (at a
final concentration of 20 nM) and cultured for 24, 48 or 72 h. The
medium was then removed and 20 μl of MTT (5 mg/ml in PBS) was added
to the fresh medium. Following a 2-h incubation at 37°C, 100 μl
DMSO was added to each well and the plates were agitated for 1 min.
The spectrophotometric absorbance at 570 nm was measured. The
experiments were performed independently a minimum of three
times.
Detection of cell apoptosis by FACS
analysis
Following the treatment with miR-99a mimics or
control mimics for 48 or 72 h, the harvested cells were suspended
in 100 μl binding buffer (1X), including 1 μl Annexin V-FITC and 10
μl PI for 15 min in the dark at room temperature, and 400 μl
binding buffer (1X) was added to each sample. The FITC and PI
fluorescence were measured through FL-1 filter (530 nm) and FL-2
filter (585 nm), respectively, and 10,000 events were acquired.
3′-UTR luciferase reporter assays
The wild-type human mTOR 3′-UTR luciferase reporter
vectors were constructed as previously described (14) by amplifying the human mTOR mRNA
3′-UTR and cloning it into a PGL3-promoter vector (Promega,
Madison, WI, USA). HEK-293 cells were cotransfected with luciferase
reporter plasmid, thymidine kinase promoter-Renilla luciferase
reporter plasmid and the indicated miRNA mimics or controls (final
concentration, 20 nM). After 24 h, the cells were collected for
application in the Dual-Luciferase Reporter system (Promega)
according to the manufacturer's instructions. The dual-luciferase
reporter assays were performed in triplicate within each experiment
and three independent experiments were conducted.
Western blot analysis
Tca-8113 cells were washed with PBS and lysed in
lysis buffer (Cell Signaling Technology, Danvers, MA, USA) for 30
min on ice following the treatment (miR-99a mimics or control
mimics, 20 nM) for 24 h. The lysates were then centrifuged at
14,000 × g for 10 min to remove insoluble material. A BCA kit
(Pierce Biotechnology, Rockford, IL, USA) was used to determine the
concentration of the lysates. The cell extracts were separated by
10% sodium dodecylsulfate-polyacrylamide gel electrophoresis and
transferred to a polyvinylidene difluoride membrane blocked with 5%
non-fat milk. The blot was probed with antibodies against mTOR
(Cell Signaling Technology) and β-actin (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). The blot was then washed and exposed to
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature. The signals were detected as previously described
(15).
Statistical analysis
Experiments were repeated three times. Data were
presented as the mean ± SD. Statistical analysis was carried out
using Student's t-test, analysis of variance (ANOVA) and Wilcoxon
matched pair test. P<0.05 was considered to indicate a
statistically significant result.
Results
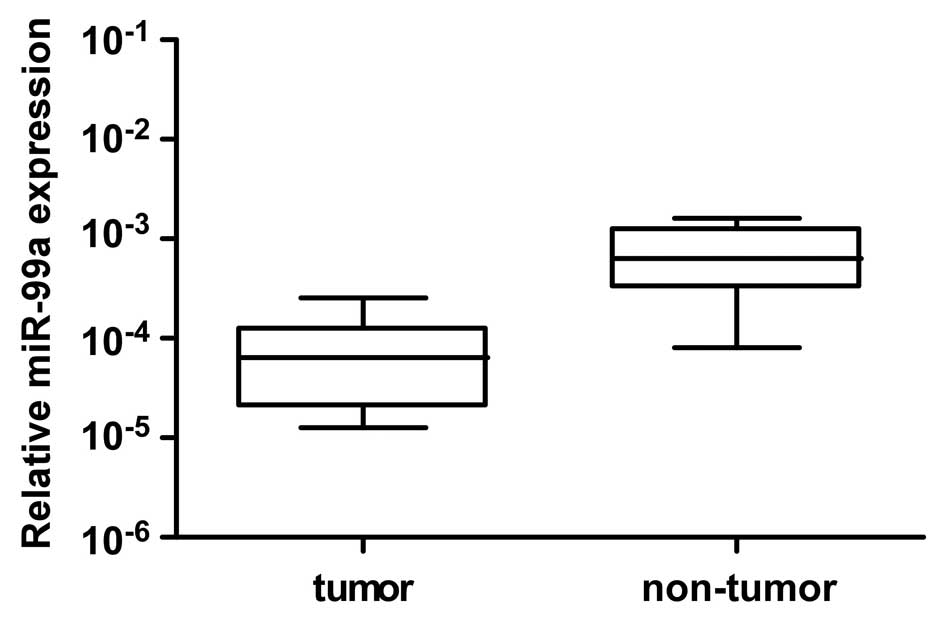
The expression of miR-99a was
significantly downregulated in OSCC specimens
To investigate the possible role of miR-99a in OSCC
carcinogenesis, the expression level of miR-99a was determined in
25 cases of primary OSCC tissues together with paired adjacent
non-tumor tissues by qRT-PCR assay. As shown in Fig. 1, miR-99a expression was
significantly reduced in OSCC tissues compared with their
corresponding adjacent non-tumor tissues (n=25, P<0.01, Wilcoxon
matched pair test). This result indicates that miR-99a is involved
in the development of OSCC.
miR-99a inhibited the proliferation of
Tca-8113 TSCC cells
To confirm the function of miR-99a on OSCC
development, we transfected Tca-8113, a TSCC cell line, with
miR-99a mimics or control small RNAs and analyzed the cell growth
rate by MTT assay at different time points (24, 48 and 72 h). The
results show that miR-99a mimics significantly inhibited the cell
growth of Tca-8113 compared with that of the blank control cells or
control mimics (NC)-transfected cells at the 48 (P<0.05) and 72
h (P<0.05) time points (Fig.
2). This result suggests that miR-99a plays a significant role
in regulating the growth of TSCC cells.
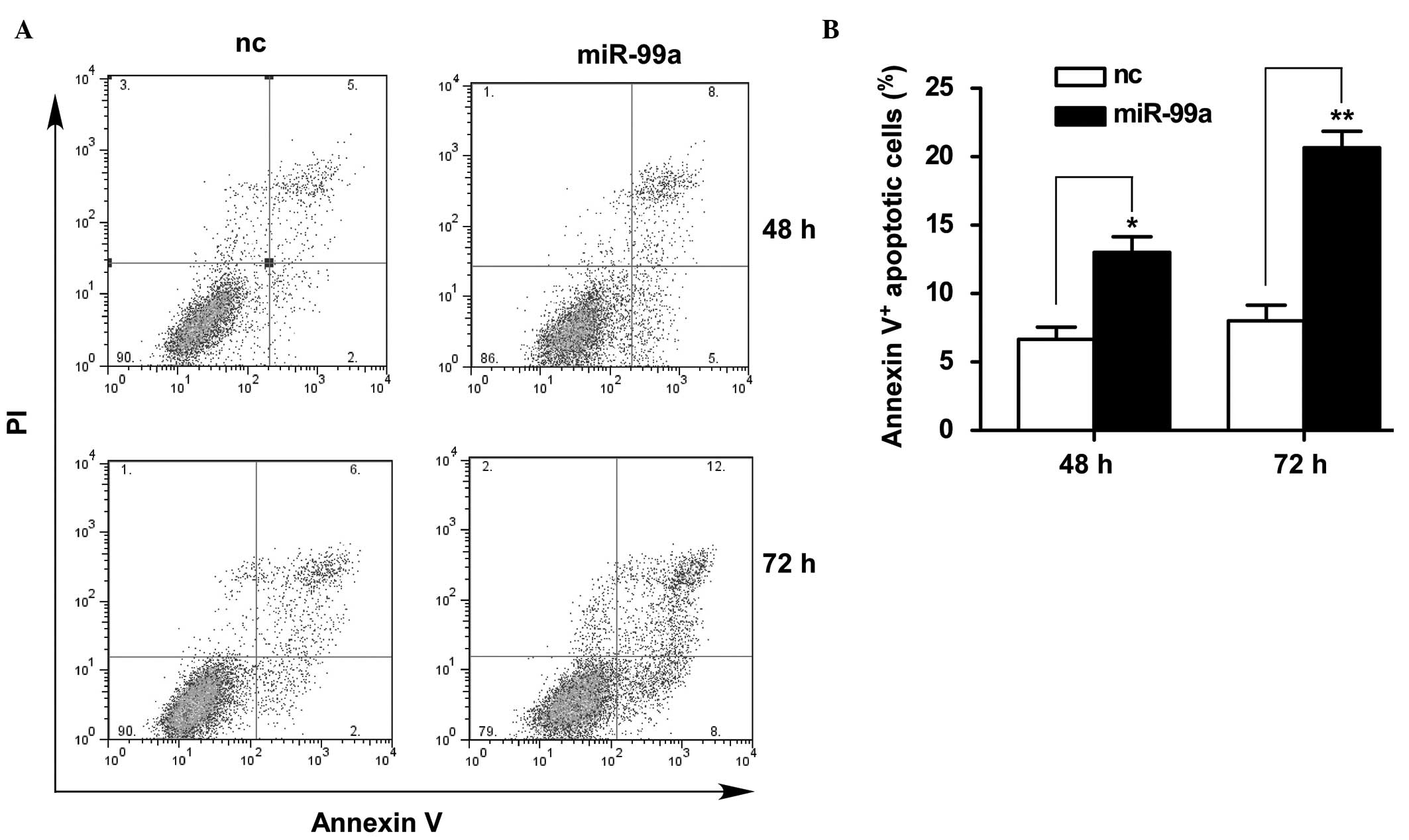
miR-99a mimics induced the apoptosis of
Tca-8113 cells
To investigate the possible mechanisms by which
miR-99a inhibits the cell growth of Tca-8113, the apoptosis of
Tca-8113 cells following treatment with miR-99a mimics was detected
by PI/Annexin V staining with FACS analysis. The percentage of
Annexin V+ cells was significantly increased in Tca-8113
cells transfected with miR-99a mimics compared with the cells
transfected with control mimics at the 48 (P<0.05) and 72 h
(P<0.01) time points, indicating that miR-99a overexpression in
Tca-8113 cells markedly induced apoptosis (Fig. 3A and B). This result suggests that
the inhibitory effect of miR-99a on the cell growth of Tca-8113
occurred at least partially through the induction of cell
apoptosis, indicating that the downregulation of miR-99a in OSCC
tissues was correlated with the decreased apoptosis of cancer
cells, thus promoting cancer progression.
mTOR is a functional target of miR-99a in
oral cancer cells
We investigated possible targets by which miR-99a
may act through to regulate the growth and survival of oral cancer
cells. Computational prediction via TargetScan (www.targetscan.org) revealed that miR-99a is a broadly
conserved miRNA that putatively targets one conserved site of human
mTOR 3′-UTR (Fig. 4A). mTOR
belongs to the phosphatidylinositol 3-kinase-related kinase (PI3K)
protein family and is a significant downstream target signal of the
PI3K pathway. Previous studies have reported that mTOR is a
serine/threonine protein kinase that is critical in the regulation
of cell growth, cell proliferation, cell motility, cell survival,
protein synthesis and transcription (16,17).
mTOR plays a significant role in the complex carcinogenesis of
HNSCC, predicts survival and is a potential biomarker used to
identify candidate patients for mTOR inhibition-based adjuvant
therapy. In vivo and in vitro studies have reported
that mTOR blockade has antitumor activity, exhibits radio- or
chemosensitization and overcomes epidermal growth factor receptor
(EGFR) resistance (18,19).
miR-99a directly targeted mTOR
To investigate the possibility that mTOR is
regulated post-transcriptionally by miR-99a in OSCC cells, reporter
plasmids were constructed by amplifying the human mTOR mRNA 3′-UTR
and cloning into the pGL3-luciferase vector. By cotransfection of
the reporter plasmids and internal control
pRL-TK-Renilla-luciferase plasmids with miR-99a mimics or the
controls (scrambled oligonucleotide) in HEK-293 cells, we observed
that miR-99a mimics markedly decreased the luciferase activity in
cells transfected with the mTOR 3′-UTR vector compared with the
cells treated with controls (P<0.05). No change in luciferase
activity was observed in the cells transfected with the empty PGL3
control vector (Fig. 4B). We also
examined the luciferase activity of mTOR mRNA 3′-UTR reporter
vector in Tca-8113 TSCC cells. As shown in Fig. 4C, miR-99a mimics also markedly
decreased the luciferase activity in Tca-8113 cells
(P<0.05).
Protein level of mTOR was inhibited by
miR-99a overexpression in Tca-8113 cells
We further determined the protein expression level
of mTOR in Tca-8113 cells following treatment with miR-99a mimics.
As shown in Fig. 5, the protein
level of mTOR was significantly decreased following miR-99a mimics
treatment compared with that in the cells transfected with control
mimics (P<0.05). The results show that mTOR is a functional
target of miR-99a and that endogenous mTOR is directly regulated by
miR-99a in TSCC cells.
Discussion
To date, certain miRNAs have been suggested to play
significant roles in OSCC development (9,20–23),
which may function alone or in a cooperative manner for OSCC
development. The overexpression of oncogenic miRNA may reduce the
levels of the protein products of tumor-suppressor genes. However,
the loss of tumor-suppressor miRNA expression may result in
elevated levels of the oncogenic protein. One or both of these
alterations may be new targets for cancer diagnosis and therapeutic
intervention (11,24–26).
Exploring and understanding the more aberrantly expressed miRNAs
may provide new insights to reveal the mechanisms underlying OSCC
carcinogenesis and progression.
In the present study, we found that miR-99a
expression was markedly decreased in OSCC tissues compared with the
adjacent non-tumor tissues from 25 cases of OSCC patients. The
downregulation of miR-99a in OSCC tissues has been identified by
qPCR (11), but its function in
regulating oral cancer cell growth and survival has not been
reported. The suppressive effect of miR-99a on OSCC cell growth was
demonstrated with in vitro experiments performed in the TSCC
cell line Tca-8113. miR-99a mimics significantly inhibited the
proliferation of Tca-8113 cells and miR-99a overexpression markedly
induced the apoptosis of Tca-8113 cells, suggesting that the
downregulation of miR-99a in OSCC tissues is associated with tumor
development. A number of aberrantly expressed miRNAs have been
verified in OSCC, including downregulated miR-15a, 133a, 133b, 137,
138, 193a, 222 and 503 and upregulated miR-21, 23a, 24, 31, 98,
184, 211 and 214 (9). We suggest
that the combined detection of the levels of miR-99a and other
OSCC-associated miRNAs may aid the more precise identification of
OSCC. As shown in the present study, miR-99a has notable antitumor
effects following restoration in TSCC cells, thus miR-99a has the
potential to be applied in OSCC therapy.
Furthermore, we demonstrated that mTOR was
identified as a direct target of miR-99a and that the
overexpression of miR-99a in Tca-8113 cells downregulated the
protein expression level of mTOR. mTOR is crucial in tumor
development, invasion, metastasis and angiogenesis that impacts
local recurrence and survival (27). The PI3K/AKT/mTOR pathway is an
intracellular signaling pathway composed of different kinases and
is responsible for the dysregulation of cell growth, proliferation,
survival and angiogenesis. mTOR may also be a biomarker for
personalized adjuvant therapy. Rapamycin and its analogs
temsirolimus and everolimus are specific inhibitors of mTOR that
exert suppressive effects on the proliferation, invasion and
metastasis and induce the apoptosis of tumor cells (28). A number of studies have shown that
mTOR plays significant roles in the carcinogenesis of HNSCC
(18). In vivo and in
vitro studies have revealed that an mTOR inhibitor suppresses
tumor growth and sensitizes HNSCC to radiation, cytotoxic agents
and epidermoid growth factor receptor inhibitors (19,29).
Either enhanced upstream signals or the overexpression of mTOR
itself may strengthen the signals passed down by mTOR, which cause
the overphosphorylation of the downstream molecules p70S6K and
4E-BP1 (30,31). Phosphorylated p70S6K and 4E-BP1
promote protein synthesis, thus cell cycle-related proteins,
including cyclin D and cyclin E, are upregulated and lead to the
promotion of tumor growth (31). A
recent study reported that mTOR was identified as a target of
miR-99a in prostate cancer (32)
and liver cancer (33). The
present study demonstrated that mTOR is a functional target of
miR-99a in TSCC cells. MicroRNAs have been thought to target
multiple mRNAs and a single mRNA may be regulated by several
miRNAs. It is probable that there are other molecules or signaling
pathways that are targeted by miR-99a that also modulate OSCC
pathogenesis. Future studies are needed to reveal the functions of
miR-99a in OSCC carcinogenesis and progression.
Previous studies have shown that the expression of
miR-99a is downregulated in various types of tumors, including
liver (33), lung (34) and prostate cancer (32), serous ovarian carcinoma (35) and bladder cancer (36). However, the mechanisms responsible
for its repression remain unclear. The deregulated expression of
microRNAs may result from epigenetic mechanisms (DNA methylation
and histone modification), chromosome deficiency or duplication,
abnormal transcription factors and disordered microRNA maturation
(7,21,37,38).
The methylation of miR-9-1 and miR-9-3 was higher in oral and
oropharyngeal carcinomas than that in laryngeal carcinoma. Reduced
miR-9 expression was associated with the methylation of miR-9 in
tumor tissues. The methylations of miR-9-1 and miR-9-3 are
sensitive and specific biomarkers for HNSCC, particularly for oral
and oropharyngeal squamous cell carcinomas (39). The mechanisms for the
downregulation of miR-99a in OSCC remain to be elucidated.
In conclusion, we explored the potential role of
miR-99a in controlling OSCC cell growth and survival, which may
provide new insights in understanding the molecular events involved
in OSCC carcinogenesis and identifying miR-99a as a biomarker for
OSCC. We found that a lower miR-99a expression was detected in OSCC
tissues compared with adjacent non-tumor tissues derived from OSCC
patients. Furthermore, restored miR-99a expression in Tca-8113 TSCC
cells suppressed cell growth and induced cell apoptosis. Mammalian
target of rapamycin (mTOR) was found to be involved as a direct
target of miR-99a. Therefore, our findings demonstrate that miR-99a
acts as a suppressor of OSCC and may be a new potential therapeutic
target for OSCC. Our findings also suggest that the restoration of
miR-99a may be a prospective therapeutic strategy for HNSCC
intervention.
Acknowledgements
This study was supported by grants from the Ningbo
Municipal Bureau of Science and Technology (2010A610058), the
National Natural Science Foundation of China (81072405), the
Program for New Century Excellent Talents in University from the
Ministry of Education of PRC (NCET-08-0486) and the Zhejiang
Provincial Natural Science Foundation (R2100528) and was also
sponsored by the Zhejiang Provincial Program for the Cultivation of
High-level Innovative Health talents and for the Innovative
Research Team in Zhejiang Province (2010R50046).
References
|
1
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009.
|
|
2
|
Xu P, Guo M and Hay BA: MicroRNAs and the
regulation of cell death. Trends Genet. 20:617–624. 2004.
|
|
3
|
Karp X and Ambros V: Developmental
biology. Encountering microRNAs in cell fate signaling. Science.
310:1288–1289. 2005.
|
|
4
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004.
|
|
5
|
Poy MN, Eliasson L, Krutzfeldt J, et al: A
pancreatic islet-specific microRNA regulates insulin secretion.
Nature. 432:226–230. 2004.
|
|
6
|
Garzon R, Fabbri M, Cimmino A, Calin GA
and Croce CM: MicroRNA expression and function in cancer. Trends
Mol Med. 12:580–587. 2006.
|
|
7
|
Hermeking H: p53 enters the microRNA
world. Cancer Cell. 12:414–418. 2007.
|
|
8
|
Massano J, Regateiro FS, Januário G and
Ferreira A: Oral squamous cell carcinoma: review of prognostic and
predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 102:67–76. 2006.
|
|
9
|
Wu BH, Xiong XP, Jia J and Zhang WF:
MicroRNAs: new actors in the oral cancer scene. Oral Oncol.
47:314–319. 2011.
|
|
10
|
Li J, Huang H, Sun L, et al: MiR-21
indicates poor prognosis in tongue squamous cell carcinomas as an
apoptosis inhibitor. Clin Cancer Res. 15:3998–4008. 2009.
|
|
11
|
Wong TS, Liu XB, Wong BY, et al: Mature
miR-184 as potential oncogenic microRNA of squamous cell carcinoma
of tongue. Clin Cancer Res. 14:2588–2592. 2008.
|
|
12
|
Wong TS, Liu XB, Chung-Wai Ho A, et al:
Identification of pyruvate kinase type M2 as potential oncoprotein
in squamous cell carcinoma of tongue through microRNA profiling.
Int J Cancer. 123:251–257. 2008.
|
|
13
|
Jiang L, Liu X, Kolokythas A, et al:
Downregulation of the Rho GTPase signaling pathway is involved in
the microRNA-138-mediated inhibition of cell migration and invasion
in tongue squamous cell carcinoma. Int J Cancer. 127:505–512.
2010.
|
|
14
|
Liu Y, Chen Q, Song Y, et al: MicroRNA-98
negatively regulates IL-10 production and endotoxin tolerance in
macrophages after LPS stimulation. FEBS Lett. 585:1963–1968.
2011.
|
|
15
|
Wang QQ, Li H, Oliver T, et al: Integrin
beta 1 regulates phagosome maturation in macrophages through Rac
expression. J Immunol. 180:2419–2428. 2008.
|
|
16
|
Yellen P, Saqcena M, Salloum D, et al:
High-dose rapamycin induces apoptosis in human cancer cells by
dissociating mTOR complex 1 and suppressing phosphorylation of
4E-BP1. Cell Cycle. 10:3948–3956. 2011.
|
|
17
|
Chen CH and Sarbassov dos D: The mTOR
(mammalian target of rapamycin) kinase maintains integrity of mTOR
complex 2. J Biol Chem. 286:40386–40394. 2011.
|
|
18
|
Liao YM, Kim C and Yen Y: Mammalian target
of rapamycin and head and neck squamous cell carcinoma. Head Neck
Oncol. 3:222011.
|
|
19
|
Chang KY, Tsai SY, Wu CM, et al: Novel
phosphoinositide 3-kinase/mTOR dual inhibitor, NVP-BGT226, displays
potent growth-inhibitory activity against human head and neck
cancer cells in vitro and in vivo. Clin Cancer Res. 17:7116–7126.
2011.
|
|
20
|
Yang CJ, Shen WG, Liu CJ, et al: miR-221
and miR-222 expression increased the growth and tumorigenesis of
oral carcinoma cells. J Oral Pathol Med. 40:560–566. 2011.
|
|
21
|
Wiklund ED, Gao S, Hulf T, et al: MicroRNA
alterations and associated aberrant DNA methylation patterns across
multiple sample types in oral squamous cell carcinoma. PLoS One.
6:e278402011.
|
|
22
|
Yang CC, Hung PS, Wang PW, et al: miR-181
as a putative biomarker for lymph-node metastasis of oral squamous
cell carcinoma. J Oral Pathol Med. 40:397–404. 2011.
|
|
23
|
Yu CC, Chen YW, Chiou GY, et al: MicroRNA
let-7a represses chemoresistance and tumourigenicity in head and
neck cancer via stem-like properties ablation. Oral Oncol.
47:202–210. 2011.
|
|
24
|
Yu ZW, Zhong LP, Ji T, et al: MicroRNAs
contribute to the chemoresistance of cisplatin in tongue squamous
cell carcinoma lines. Oral Oncol. 46:317–322. 2010.
|
|
25
|
Lo WL, Yu CC, Chiou GY, et al:
MicroRNA-200c attenuates tumour growth and metastasis of
presumptive head and neck squamous cell carcinoma stem cells. J
Pathol. 223:482–495. 2010.
|
|
26
|
Gomes CC and Gomez RS: MicroRNA and oral
cancer: future perspectives. Oral Oncol. 44:910–914. 2008.
|
|
27
|
Coutte L, Dreyer C, Sablin MP, Faivre S
and Raymond E: PI3K-AKT-mTOR pathway and cancer. Bull Cancer.
99:173–180. 2012.(In French).
|
|
28
|
Seeliger H, Guba M, Kleespies A, Jauch KW
and Bruns CJ: Role of mTOR in solid tumor systems: a therapeutical
target against primary tumor growth, metastases, and angiogenesis.
Cancer Metastasis Rev. 26:611–621. 2007.
|
|
29
|
Aissat N, Le Tourneau C, Ghoul A, et al:
Antiproliferative effects of rapamycin as a single agent and in
combination with carboplatin and paclitaxel in head and neck cancer
cell lines. Cancer Chemother Pharmacol. 62:305–313. 2008.
|
|
30
|
Fingar DC, Richardson CJ, Tee AR, et al:
mTOR controls cell cycle progression through its cell growth
effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor
4E. Mol Cell Biol. 24:200–216. 2004.
|
|
31
|
Grewe M, Gansauge F, Schmid RM, Adler G
and Seufferlein T: Regulation of cell growth and cyclin D1
expression by the constitutively active FRAP-p70s6K pathway in
human pancreatic cancer cells. Cancer Res. 59:3581–3587. 1999.
|
|
32
|
Sun D, Lee YS, Malhotra A, et al: miR-99
family of MicroRNAs suppresses the expression of prostate-specific
antigen and prostate cancer cell proliferation. Cancer Res.
71:1313–1324. 2011.
|
|
33
|
Li D, Liu X, Lin L, et al: MicroRNA-99a
inhibits hepatocellular carcinoma growth and correlates with
prognosis of patients with hepatocellular carcinoma. J Biol Chem.
286:36677–36685. 2011.
|
|
34
|
Yamada H, Yanagisawa K, Tokumaru S, et al:
Detailed characterization of a homozygously deleted region
corresponding to a candidate tumor suppressor locus at 21q11-21 in
human lung cancer. Genes Chromosomes Cancer. 47:810–818. 2008.
|
|
35
|
Nam EJ, Yoon H, Kim SW, et al: MicroRNA
expression profiles in serous ovarian carcinoma. Clin Cancer Res.
14:2690–2695. 2008.
|
|
36
|
Catto JW, Miah S, Owen HC, et al: Distinct
microRNA alterations characterize high- and low-grade bladder
cancer. Cancer Res. 69:8472–8481. 2009.
|
|
37
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010.
|
|
38
|
He L, Thomson JM, Hemann MT, et al: A
microRNA polycistron as a potential human oncogene. Nature.
435:828–833. 2005.
|
|
39
|
Minor J, Wang X, Zhang F, et al:
Methylation of microRNA-9 is a specific and sensitive biomarker for
oral and oropharyngeal squamous cell carcinomas. Oral Oncol.
48:73–78. 2012.
|