Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
disease in which almost all the organ systems, including the skin,
kidneys, lungs, brain, heart and joints, are damaged (1). However, the precise mechanism of SLE
has not been fully elucidated. Previous studies show that SLE is
primarily caused by high levels of autoantibodies which are
generated by enhanced apoptosis in conjunction with defective
clearance of apoptotic cells (2)
and immune complex deposition. Although the pathogenesis of SLE
remains unclear, many studies have suggested that cytokines or
growth factors are considered to play an important role in SLE.
Deregulated cytokine production is known to contribute to immune
dysfunction as well as to mediate tissue inflammation and organ
damage. Cytokines produced by abnormal T helper (TH) cells have
been shown to be implicated in the pathogenesis of SLE (3).
IL-9 is a T cell-derived cytokine that is initially
designated a Th2 cytokine (4).
Recently, Tregs, Th1, Th17 and Th9 subsets of T cells were also
found to produce IL-9 (5–8). It has been reported that IL-9 causes
pleiotropic effects in these subsets (7), and that it plays different roles in
different situations in regulating immune responses. IL-9 targets
cells of the lymphoid, myeloid and mast cell lineages, and is
likely to contribute to the development of allergic (9) and autoimmune diseases (10) such as asthma, arthritis, multiple
sclerosis and experimental autoimmune encephalomyelitis (EAE).
However, whether abnormal expression and secretion
of IL-9 are present in SLE patients still remains unknown, and it
is also unclear whether IL-9 exerts main proinflammatory or
anti-inflammatory activities in SLE. Therefore, in the present
study, the expression of IL-9 levels and the percentages of
CD4+IL-9+ T cells in SLE patients as well as
their association with disease manifestations and activity were
investigated, in order to provide a better understanding of the
interrelationship and immunopathological roles of IL-9 in SLE.
Materials and methods
Subjects
Peripheral blood samples were obtained from 28 SLE
patients from the Second Affiliated Hospital of Soochow University
(Suzhou, China), including 13 active (12 females, 1 male; mean age,
36±12 years) and 15 inactive SLE patients (15 females, 0 male; mean
age, 34±11 years), excluding those with a current infection. Eight
patients were not administered any immuno-modulating medication at
the time of analysis. Control samples were collected from 12
healthy volunteers (10 females, 2 males; mean age, 31±7 years),
none of which suffered from any rheumatologic diseases. The
diagnosis of SLE was established by the presence of ≥4 American
College of Rheumatology (ACR) diagnostic criteria (11). Active SLE was defined as a SLE
disease activity index (SLEDAI) score ≥10, while patients with
SLEDAI <10 were evaluated as inactive (12). Patient clinical and demographic
characteristics are presented in Table
I. The processes involved in the present study as well as
informed consent forms were approved by the respective
institutions.
 | Table IBaseline characteristics and
medication of SLE patients. |
Table I
Baseline characteristics and
medication of SLE patients.
| Categories | SLE patients | HC |
|---|
| General
conditions |
| No. of cases
(active/inactive) | 13/15 | 12 |
| Age (mean ±
SD) | 36±12/34±11 | 31±7 |
| Male:female | 1:12/0:15 | 2:10 |
| SLEDAI [median
(range)] | 9 (2–21) | |
| Clinical features,
n |
| Arthritis | 4 | |
| Alopecia | 5 | |
| Anemia | 16 | |
| Butterfly
erythema | 3 | |
| Oral ulcer | 7 | |
|
Photosensitivity | 6 | |
| Renal
involvemet | 15 | |
| Serositis | 11 | |
| Nervous system
disorder | 6 | |
| Laboratory
parameters, n |
| Leukopenia | 8 | |
|
Thrombocytopenia | 10 | |
| Anti-dsDNA(+) | 7 | |
| Anti-Sm(+) | 12 | |
| Decreased | C3/C4 13/18 | |
| Increased | IgA/IgM/IgG
13/11/14 | |
| Increased ANA
(>1:100) | 16 | |
| Elevated ESR | 14 | |
| Proteinuria
>0.5 g/24 h | 12 | |
| Medications, n |
| None | 8 | |
|
GC:methylprednisolone | 17 | |
| Prednisone | 3 | |
|
MMF/LEF/CTX/FK-506 | 5/3/8/2 | |
Sample preparation and cell cultures
Blood samples were collected in vacutainer tubes
containing ethylenediaminetetraacetic acid (EDTA). Following the
collection of peripheral blood samples, reserve serum and
peripheral blood mononuclear cells (PBMCs) were isolated by
centrifugation against a density gradient, and the enriched cells
were removed from the density medium:plasma interface. The samples
were washed two times with phosphate-buffered saline (PBS); part of
the collected cells was used for RNA extraction. Isolated PBMCs
(1×106 in 1.5 ml of RPMI-1640) for intracellular
staining were stimulated for 6 h with PMA (50 ng/ml)
(Sigma-Aldrich, St. Louis, MO, USA) and ionomycin (500 ng/ml;
Sigma-Aldrich). Four hours prior to collection, brefeldin A
(GolgiPlug™, 1 μl/ml; BD Pharmingen, Franklin Lakes, NJ, USA) was
added to the cell culture, along with PMA and ionomycin. The cells
were fixed and permeabilized (Fix/Perm; eBioscience, San Diego, CA,
USA) according to the manufacturer’s instructions, and were
incubated at room temperature with formaldehyde.
RNA extraction and real-time PCR
analysis
RNA was isolated using TRIzol reagent, followed by
reverse transcription using M-MLV reverse transcriptase and
oligo(dT) primer (Invitrogen, Carlsbad, CA, USA). Approximately 1.5
μg RNA was converted to cDNA. For measuring the mRNA levels of IL-9
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 1 μl cDNA in
triplicate was used for amplification by the TaqMan RT-PCR system
(ABI Prism 7900HT Sequence Detection System; Applied Biosystems,
Foster City, CA, USA) with specific TaqMan primers. Amplification
was performed using standard conditions and calculations of fold
induction were performed. Gene expression was normalized to GAPDH
and the values were expressed relative to control using the ΔΔCT
method. The primers used included: IL-9 sense,
5′-GTGCCACTGCAGTGCTAATGT-3′ and anti-sense,
5′CTCTCACTAAGCATGGTCTGG-3′; GAPDH sense, 5′-AT
CCCATCACCATCTTCCAG-3′ and anti-sense, 5′-GAGTC
CTTCCACGATACCAA-3′.
Measurement of serum IL-9 levels
Venous blood samples were collected into into
pyogen-free blood collection tubes, immediately immersed in melting
ice, and allowed to clot 1 h prior to centrifugation. All the serum
samples were stored at −80°C before use. All the procedures used
were standardized. Serum IL-9 levels were measured using specific
ELISA kits (R&D Systems, Minneapolis, MN, USA). Each sample was
tested in duplicate. The results were expressed as pg/ml and the
detection limit of this assay was 0.5 pg/ml.
Flow cytometric analysis
The enriched cells were stained with the anti-CD4
Alexa Fluor 488 antibody (eBioscience) for purity checking.
Intracellular staining was performed using anti-IL-9-PE
(eBioscience). The percentages of cytokine-secreting
CD4+IL-9+ T cells were determined by flow
cytometry using a FACSCalibur instrument (Becton-Dickinson,
Franklin Lakes, NJ, USA).
Clinical and laboratory parameters
Part of clinical manifestations of SLE patients were
defined as follows: renal involvement (proteinuria >0.5 g/24 h,
presence of cellular casts, hematuria with >10 red blood
cells/hpf excluding infection or stone, >5 leukocytes/hpf
excluding infection, or plasma creatinine >1.4 mg%), arthritis
(non-erosive arthritis affecting ≥2 peripheral joints), nervous
system disorder (psychosis, seizure, depression and peripheral
neuropathy). Laboratory abnormalities were recorded, including
leukopenia (white blood cell count <4,000/mm3),
thrombocytopenia (platelet count <100,000/mm3),
elevated erythrocyte sedimentation rate (>20 mm/h), the presence
of anti-dsDNA, antinuclear, anti-Sm (by indirect
immunofluorescence), IgG, IgA, IgM and serum levels of C3, C4 and
24-hour urinary protein (by immunoturbidimetry).
Statistical analysis
Results were expressed as the means ± standard
deviation (SD). Statistical analysis of the data was performed
using the GraphPad Prism (Version 4.0) statistical program. The
nonparametric Mann-Whitney U test was used to compare data between
SLE patients and healthy controls. Analysis of covariance was used
to compare data among three groups. The association of the data
with clinical and laboratory parameters of SLE patients was
analyzed using the independent samples t-test. For the correlation
analysis with SLEDAI, Pearson correlation coefficient was used.
Repeated measurement ANOVA was used to compare the parameters
before and after treatment. Two-sided P-values <0.05 were
considered to indicate a statistically significant difference.
Results
IL-9 mRNA expression is increased in SLE
patients
In order to examine whether the expression of IL-9
is altered in SLE patients, real-time PCR was used to detect the
mRNA levels of IL-9 from single nuclear PBMCs from the peripheral
blood of SLE patients (n=28) and healthy controls (n=12). The
results showed that IL-9 mRNA in SLE patients was significantly
higher compared with that in healthy controls (P<0.01, two
groups of comparisons) (Fig.
1).
Serum IL-9 levels are increased in SLE
patients
Next, to define the abnormality of IL-9 protein
expression in the peripheral blood of SLE patients, we tested the
serum IL-9 levels in the fresh serum of the peripheral blood
samples of SLE patients. It was found that serum IL-9 levels were
significantly higher in active and inactive SLE patients compared
with the healthy controls (72.3±16.7 vs. 26.3±6.4 pg/ml, P<0.01;
56.7±11.5 vs. 26.3±6.4 pg/ml, P<0.05) (Fig. 2). However, there was no significant
difference between 8 untreated SLE patients and 5 active SLE
patients, to whom treatment with a small amount of immunodepressant
was administered prior to relapse (P>0.05).
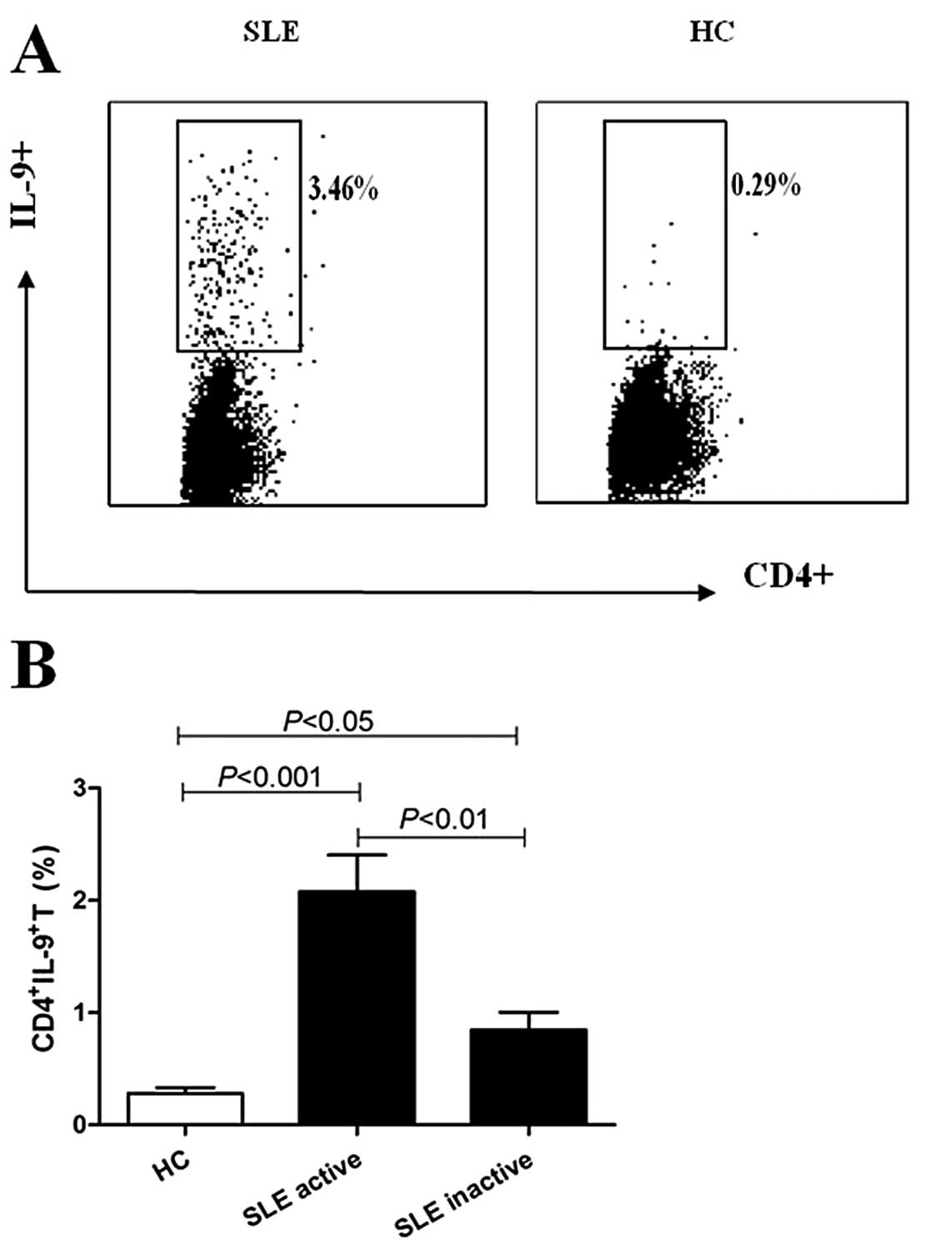
Increased percentages of
CD4+IL-9+ T cells in SLE patients
The percentages of CD4+IL-9+ T
cells in SLE patients were analyzed using flow cytometry. Results
showed that the percentages of CD4+IL-9+ T
cells were increased in active and inactive SLE patients compared
with healthy controls (2.11±0.26 vs. 0.28±0.05%, P<0.001;
0.86±0.16 vs. 0.28±0.05%, P<0.05), while it was higher in active
compared with inactive SLE patients (2.11±0.26 vs. 0.86±0.16%,
P<0.01) (Fig. 3). The
percentages of CD4+IL-9+ T cells in 8
untreated active SLE patients were moderately higher compared with
5 active SLE patients who were administered treatment with
immunodepressant (P>0.05, two groups of comparisons).
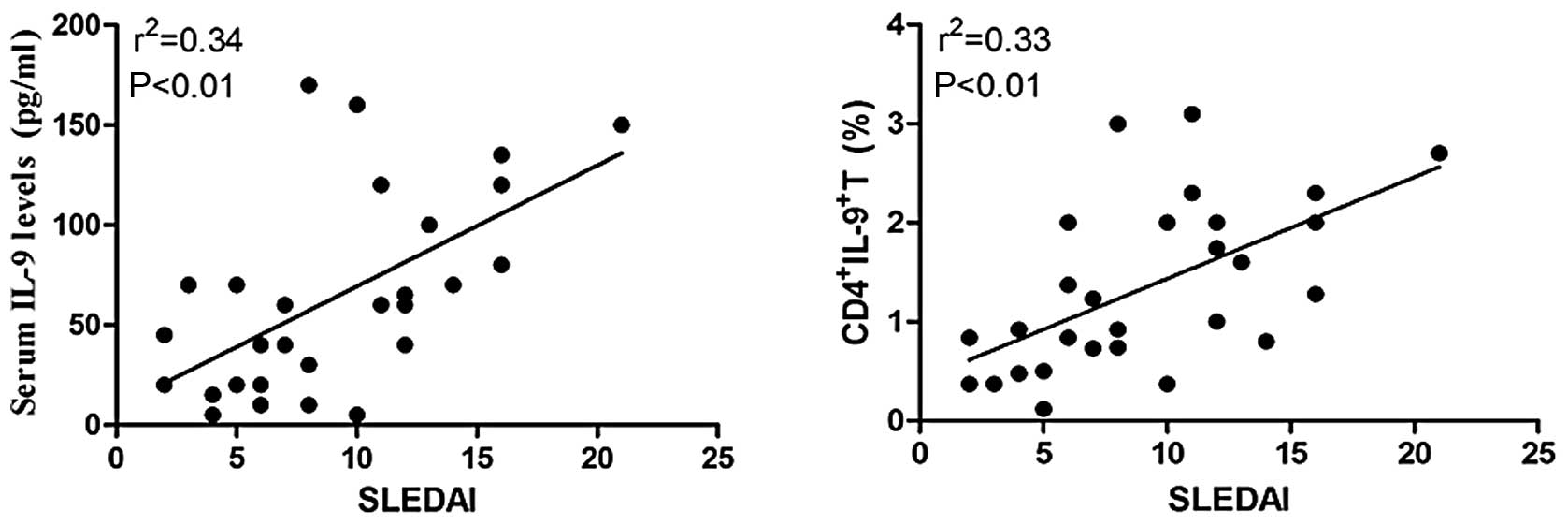
Association of serum IL-9 levels and
percentages of CD4+IL-9+ T cells with the
clinical features of SLE patients
To determine whether IL-9 expression and the
percentages of CD4+IL-9+ T cells were
associated with disease activity, it was observed that the
percentages of CD4+IL-9+ T cells and the
serum IL-9 levels were correlated with SLEDAI. The results showed
that both the percentages of CD4+IL-9+ T
cells and the serum levels of IL-9 were positively associated with
SLEDAI (Fig. 4). Additionally,
potential association of serum IL-9 levels and the percentages of
CD4+IL-9+ T cells with major clinical
features of SLE patients was analyzed, but no correlation was found
(Fig. 5).
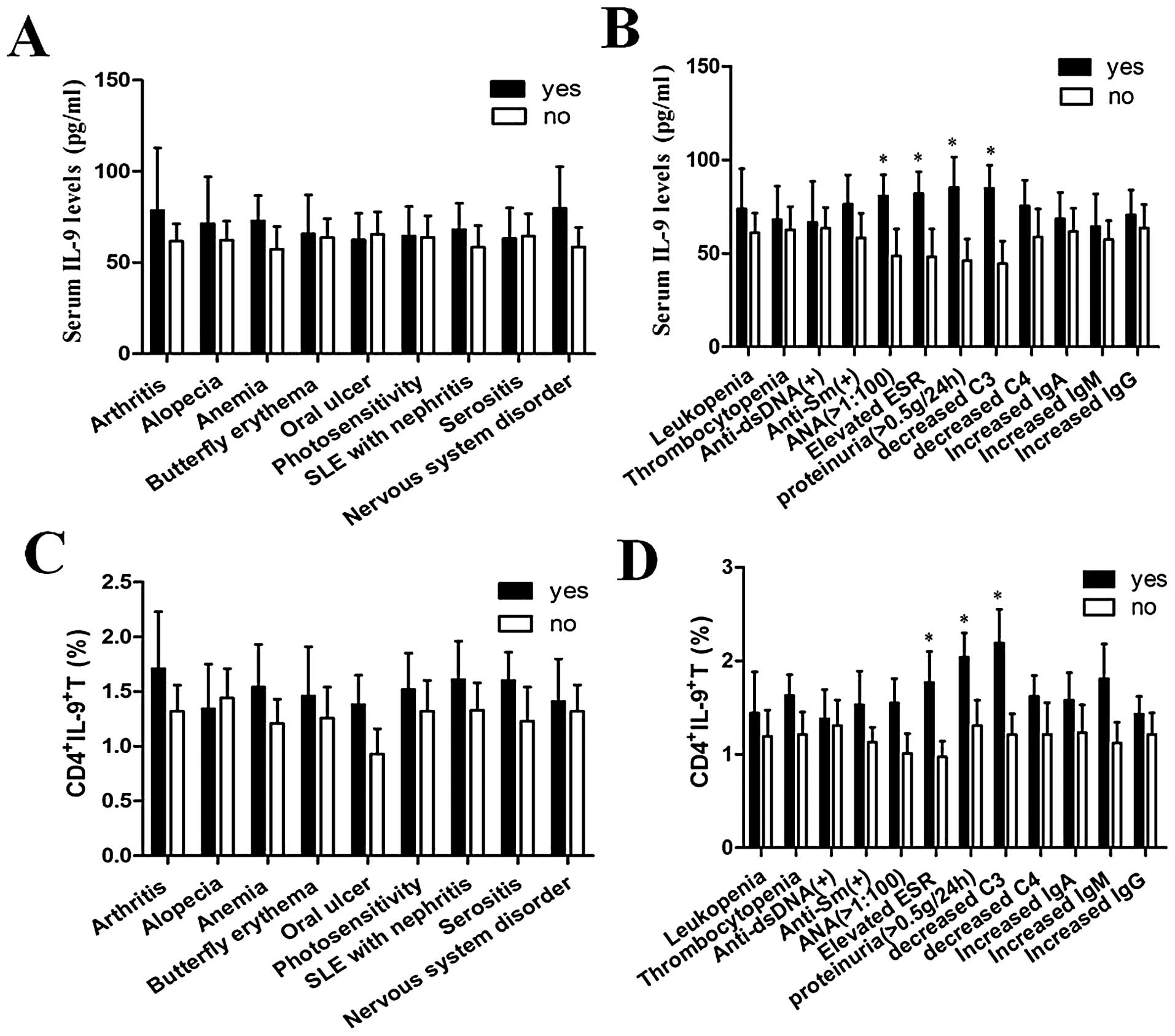
Association of serum IL-9 levels and the
percentages of CD4+IL-9+ T cells with the
laboratory parameters of SLE patients
In order to analyze the relation between the
expression of IL-9 and the laboratory parameters of SLE patients,
we correlated serum IL-9 levels and the percentages of
CD4+IL-9+ T cells with certain laboratory
parameters. The results showed that serum IL-9 levels were
significantly increased in SLE patients with decreased C3,
increased ANA, elevated ESR and high proteinuria (P<0.05 for
each). The percentages of CD4+IL-9+ T cells
were also significantly increased in SLE patients with decreased
C3, elevated ESR and high proteinuria (P<0.05 for each).
However, there was no significant difference between the expression
of IL-9 and other laboratory parameters (Fig. 5).
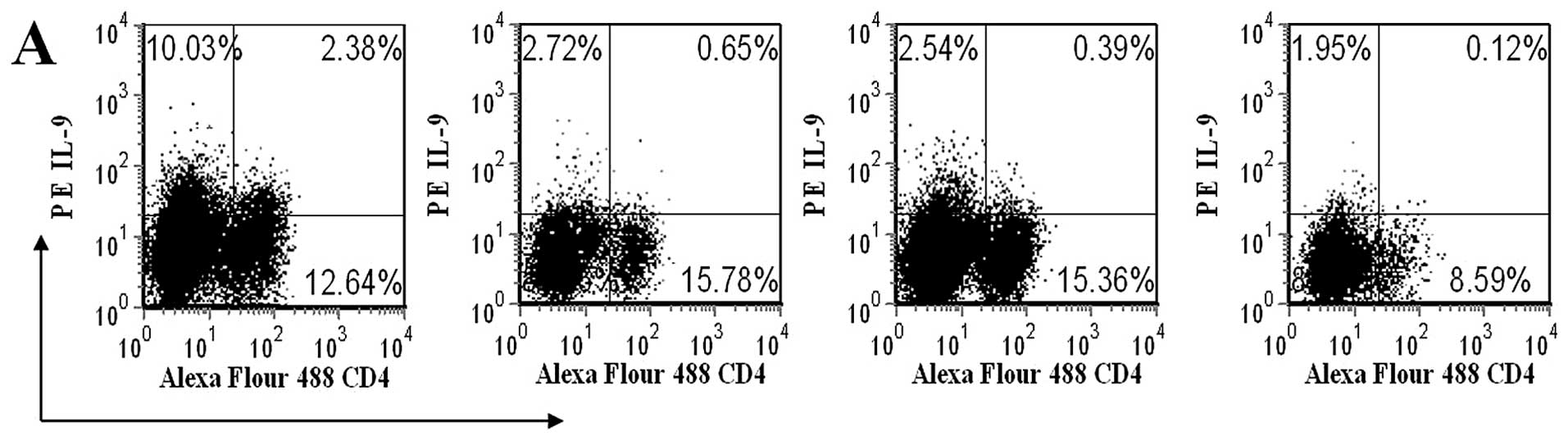
Glucocorticoid influence on serum IL-9
levels and the percentages of CD4+IL-9+ T
cells
Finally, in order to explore the effect of
glucocorticoids on serum IL-9 levels and the percentages of
CD4+IL-9+ T cells, 8 active SLE patients who
were not administered any immuno-modulating medication were
assessed. Notably, the percentages of
CD4+IL-9+ T cells and the protein levels of
IL-9 in the 8 untreated active SLE patients gradually decreased l,
2 and 3 weeks following treatment with methylprednisolone (0.8
mg/kg/day) when compared with those prior to treatment (Fig. 6).
Discussion
According to Nowak et al(13), IL-9 receptor deficiency attenuates
EAE disease, indicating that IL-9 is correlated with EAE. Poulin
et al(14) found that IL-9
mRNA and protein were highly expressed in a mouse model of fully
mismatched heart transplantation. A previous study also
demonstrated that serum levels of IL-9 and additional pathologic
cytokines were elevated after the second immunization of rheumatoid
arthritis (RA) (15), while Khan
et al(16) further
confirmed that IL-9 and some another inflammatory cytokines were
descended following treatment with rituximab, indicating that IL-9
plays a pro-inflammatory role in RA. Additional evidence indicates
that IL-9 cytokine may contribute to the development of autoimmune
diseases.
To elucidate whether IL-9 expression is abnormal in
the peripheral blood of SLE patients, IL-9 mRNA expression level
and serum IL-9 levels were analyzed in SLE patients and healthy
controls. The results of the present study indicate that IL-9
production was significantly increased in SLE patients compared
with healthy controls. IL-9 production was also significanlty
increased in active SLE patients, indicating that the elevation of
IL-9 levels may trigger the inflammatory process in SLE.
Previous studies have shown that CD4+ T
cell subsets, including Tregs, Th1, Th2, Th17 and Th9, produce IL-9
under specific conditions. In the present study, the percentages of
IL-9 expression in CD4+ T cells were analyzed using flow
cytometry. It was found that the percentages of
CD4+IL-9+ T cells from the peripheral blood
of active and inactive SLE patients were significantly increased
compared with the healthy controls, suggesting that
CD4+IL-9+ T cells may play an important role
in the pathogenesis of SLE. In the present study, it was shown that
the percentages of CD4+IL-9+ T cells were
significantly increased; however, the question of which subset of
the CD4+ T cells was the major contributor still remains
to be elucidated. There is controversial evidence concerning the
subtype of T cells producing this cytokine, which include but are
not limited to Th1, Th2, Th9, Th17 and Treg cells.
In the present study, a strong positive correlation
between the percentages of CD4+IL-9+ T cells
and SLEDAI in SLE patients was found, while the similar correlation
was found between serum IL-9 levels and SLEDAI. Additionally, serum
IL-9 levels were significantly increased in SLE patients with
decreased C3, increased ANA, elevated ESR and high proteinuria.
Similarly, the percentages of CD4+IL-9+ T
cells were significantly increased in SLE patients with decreased
C3, elevated ESR and high proteinuria, indicating that the
percentages of CD4+IL-9+ T cells and serum
IL-9 levels are correlated with disease activity and severity. The
percentages of CD4+IL-9+ T cells and serum
IL-9 levels tended to be higher in patients with leukopenia,
thrombocytopenia, increased immunoglobulin, as well as patients
positive for anti-dsDNA and anti-Sm. Nevertheless, a significant
difference was not reached. Moreover, the percentages of
CD4+IL-9+ T cells and serum IL-9 were not
significantly increased in SLE patients with the typical clinical
features of SLE compared with patients without the clinical
features (Fig. 5). This was
probably due to the low number of the patients included, or due to
the heterogeneity of SLE patients. Further studies are needed to
confirm these preliminary results. In the present study, an
influence of treatment on the percentages of
CD4+IL-9+ T cells and serum IL-9 levels was
not found between untreated active SLE and those active SLE who
were administered treatment with immunodepressant prior to
relapse.
Glucocorticoids affect cytokine synthesis in T cells
by binding to and activating cytoplasmic glucocorticoid receptors
which translocate to the nucleus and regulate the transcription of
target genes. Brink et al(17) reported that even low doses of
steroids inhibit cytokine synthesis in SLE patients. Similarly, in
the present study, the effects of glucocorticoids on serum IL-9
levels and CD4+IL-9+ T cells in SLE patients
were investigated. Notably, the percentages of
CD4+IL-9+ T cells and the protein levels of
IL-9 in 8 untreated active SLE patients were gradually decreased l,
2 and 3 weeks after treatment with methylprednisolone, indicating
that glucocorticoids downregulate the expression of IL-9 and
IL-9-producing CD4+ T cells in SLE patients. These
results suggest that the abnormality of IL-9 and
CD4+IL-9+ T cells may play a role in the
inflammatory process of SLE.
The pro-inflammatory role of IL-9 in SLE patients
may be related to Th17 cells. It has been recently shown that IL-9,
as a dominant cytokine, is produced by Th17 cells (18). Th17 cells produce large quantities
of IL-9 which, in turn, further amplify Th17 cells which have been
shown to be an important subset in human autoimmune diseases,
including RA (16), multiple
sclerosis (19) and SLE (3). Additionally, there is evidence
suggesting that IL-9 production in Th17 cells is pathogenic during
autoimmunity. According to Beriou et al(20), it has been suggested that the Th17
population in patients with type 1 diabetes have an increased
propensity to secrete IL-9. IL-9R deficiency has been shown to
attenuate EAE disease and to have a decreased accumulation of Th17
cells in a mouse model, indicating that IL-9 is pathogenic in
autoimmunity (10). However, there
has been a number of studies showing an anti-inflammatory activity
of IL-9 in human autoimmune diseases (18,21),
potentially due to the fact that IL-9 enhances the suppressive
function of regulatory T cells (18). Thus, the functions and mechanisms
of IL-9 in SLE as well as additional autoimmune diseases remains to
be elucidated.
In conclusion, the present study demonstrated
increased mRNA levels of IL-9, serum IL-9 levels and percentages of
CD4+IL-9+ T cells in SLE patients,
particularly inactive SLE, which were correlated with disease
activity and severity. Thus, IL-9 and
CD4+IL-9+ T cells might play an impotrant
role in SLE. Finally, treatment with a high-dose of
methylprednisolone was demonstrated to reduce serum IL-9 levels and
the percentages of CD4+IL-9+ T cells,
indicating that IL-9 and CD4+IL-9+ T cells
are involved in the inflammatory process of SLE. The use of
IL-9-specific antibodies in the treatment of several conditions,
such as EAE (13),
collagen-induced arthritis (14),
transplantation (22), chronic
active EBV infection (23) and
human anaplastic large-cell lymphoma cells (24), has been previously implemented.
Targeting IL-9 in human SLE could be a promising approach in the
future. Therefore, further studies are needed confirm and extend
the current results.
Acknowledgements
This study was partly supported by the National
Natural Science Foundation of China (grant no. 81200507) and the
Natural Science Foundation of Jiangsu Province (grant no.
SBK201121707).
References
|
1
|
Rahman A and Isenberg DA: Systemic lupus
erythematosus. N Engl J Med. 358:929–939. 2008. View Article : Google Scholar
|
|
2
|
Herrmann M, Voll RE and Kalden JR:
Etiopathogenesis of systemic lupus erythematosus. Immunol Today.
9:424–426. 2000. View Article : Google Scholar
|
|
3
|
Pan HF, Ye DQ and Li XP: Type 17 T-helper
cells might be a promising therapeutic target for systemic lupus
erythematosus. Nat Clin Pract Rheumatol. 4:352–353. 2008.PubMed/NCBI
|
|
4
|
Knoops L and Renauld JC: IL-9 and its
receptor: from signal transduction to tumorigenesis. Growth
Factors. 22:207–215. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Teige I, Birnir B, et al:
Neuron-mediated generation of regulatory T cells from
encephalitogenic T cells suppresses EAE. Nat Med. 12:518–525. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugimoto T, Ishikawa Y, Yoshimoto T, et
al: Interleukin 18 acts on memory T helper cells type 1 to induce
airway inflammation and hyperresponsiveness in a naive host mouse.
J Exp Med. 199:535–545. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elyaman W, Bradshaw EM, Uyttenhove C, et
al: IL-9 induces differentiation of TH17 cells and enhances
function of FoxP3+ natural regulatory T cells. Proc Natl
Acad Sci USA. 106:885–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jager A, Dardalhon V, Sobel RA, et al:
Th1, Th17 and Th9 effector cells induce experimental autoimmune
encephalomyelitis with different pathological phenotypes. J
Immunol. 183:7169–7177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soroosh P and Doherty TA: Th9 and allergic
disease. Immunology. 127:450–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nowak EC and Noelle RJ: Interleukin-9 as a
T helper type 17 cytokine. Immunology. 131:169–173. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan EM, Cohen AS, Fries JF, et al: The
1982 revised criteria for the classification of systemic
lupuserythematosus. Arthritis Rheum. 25:1271–1277. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bombardier C, Gladman DD, Urowitz MB, et
al: Derivation of the SLEDAI. A disease activity index for lupus
patients The Committee on Prognosis Studies in SLE. Arthritis
Rheum. 35:630–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nowak EC, Weaver CT, Turner H, et al: IL-9
as a mediator of Th17-driven inflammatory disease. J Exp Med.
206:1653–1660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poulin LF, Richard M, Le Moine A, et al:
Interleukin-9 promotes eosinophilic rejection of mouse heart
allografts. Transplantation. 76:572–577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuji F, Yoshimi M, Katsuta O, et al:
Point mutation of tyrosine 759 of the IL-6 family cytokine
receptor, gp130, augments collagen-induced arthritis in DBA/1J
mice. BMC Musculoskelet Disord. 10:232009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan IH, Krishnan VV, Ziman M, et al: A
comparison of multiplex suspension array large-panel kits for
profiling cytokines and chemokines in rheumatoid arthritis
patients. Cytometry B Clin Cytom. 76:159–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brink I, Thiele B, Burmester GR, et al:
Effects of anti-CD4 antibodies on the release of IL-6 and TNF-alpha
in whole blood samples from patients with systemic lupus
erythematosus. Lupus. 8:723–730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elyaman W, Bradshaw EM, Uyttenhove C, et
al: IL-9 induces differentiation of TH17 cells and enhances
function of FoxP3+ natural regulatory T cells. Proc Natl
Acad Sci USA. 106:12885–12890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tzartos JS, Manuel AF, Matthew JC, et al:
Interleukin-17 production in central nervous system-infiltrating T
cells and glial cells is associated with active disease in multiple
sclerosis. Am J Pathol. 172:146–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beriou G, Bradshaw EM, Lozano E, et al:
TGF-b induces IL-9 production from human Th17 cells. J Immunol.
185:46–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koichi Y, Ayumi Y, Yoshihide A, et al:
Serum interleukin 9 levels are increased in patients with systemic
sclerosis: association with lower frequency and severity of
pulmonary fibrosis. J Rheumatol. 38:2193–2197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu LF, Lind EF, Gondek DC, et al: Mast
cells are essential intermediaries in regulatory T-cell tolerance.
Nature. 442:997–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L, Aozasa K, Oshimi K, et al:
Epstein-Barr virus (EBV) encoded RNA promotes growth of
EBV-infected T cells through interleukin-9 induction. Cancer Res.
64:5332–5337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu L, Lai R, Lin Q, et al: Autocrine
release of interleukin-9 promotes Jak3-dependent survival of
ALK+ anaplastic large-cell lymphoma cells. Blood.
108:2407–2415. 2006. View Article : Google Scholar : PubMed/NCBI
|