Introduction
Paraquat dichloride (1,1′-dimethyl-4,4′-bipyridinium
dichloride, PQ), widely employed as an herbicide, is restricted in
a number of countries but is frequently used to control weeds. It
is fatal to humans when ingested, and is responsible for a high
number of fatalities in developing countries (1). In humans, the LD50 of PQ
is 3–5 mg/kg and its target organs are the kidney, liver, heart and
lung. The principal molecular mechanism of PQ toxicity involves
redox cycling and the generation of intracellular oxidative stress,
and the lung is the primary target of PQ where it exerts a
cytotoxic effect via the generation of reactive oxygen species
(ROS) (1,2). It has been demonstrated that the
acute phase may cause mortality within a few days from pulmonary
edema, cell infiltration and alveolar hemorrhage (1,2). In
the late phase of PQ poisoning, patients develop lung fibrosis
followed by infiltrations of profibroblast cells with oxidative
damage to lung tissues which contributes to extremely high
morbidity and mortality. Despite intensive research into the
pathobiological mechanisms of PQ, no effective treatments for PQ
poisoning have thus far been developed.
Phytochemicals have been highlighted as a possible
therapeutic modality for a variety of diseases, including cancer
and diabetes, due to their putative efficacies and safety. In
particular, dietary phytochemicals, including resveratrol,
(−)-epigallocatechin gallate (EGCG), [6]-gingerol, myricetin
curcumin, quercetin and luteolin, have been recognized as
antioxidants and directly regulate a variety of signal pathways in
human diseases (3). The
antioxidant function of the acai berry in a variety of human
diseases has become the subject of some attention (4–8). The
acai berry, the fruit of Euterpe oleraceae Martius, is a
small round berry that grows in the Amazon region and is used
medicinally as an antidiarrheal agent (8). Additionally, acai berry extract and
juice have been used for foods and dietary supplements as they
contain a variety of nutrients, including proteins, lipids,
vitamins A, C and E, thiamine and flavan-3-ols, hydroxybenzoic
acids, polyphenols and anthocyanins, predominantly cyanidin
3-O-rutinoside and cyanidin 3-O-glucuronide (9–11).
Additionally, the acai berry has already been recognized as a
possible pharmacological candidate in a variety of human diseases,
including cancer, due to its strong ROS scavenging effect and
anti-inflammatory effects induced via the regulation of
cyclooxygenase (COX)-1 and -2 inhibitors (4,11,12).
In animal models, the acai berry has been shown to have a
protective effect on cigarette-induced emphysema in mice (6). However, the acai berry has numerous
potential effects in a variety of human diseases and its function
in PQ poisoning has yet to be clearly elucidated.
Proteomics is a technique that has been intensively
employed in the past 10 years in a variety of life sciences in
efforts to evaluate the mechanisms of the expression of genes
and/or proteins that operate as regulators or modifiers during
transcription, translation and post-translation (13,14).
The powerful and comprehensive information provided by proteomics
research has been frequently applied to the discovery of new drugs,
toxins and even treatments for human diseases such as cancer. This
technique has emerged and expanded to other -omics, including
epigenomics, metabolomics and genomics. In the toxicological
sciences, proteomics is an appropriate technique for the discovery
of target genes, biomarkers and therapeutic reagents. In the
current study, among 40 spots, five proteins were identified the
levels of which were altered >2-fold in the PQ-exposed rats
treated with acai berry extract relative to the control group.
Calcium binding protein 1 (CaBP1), FK506 binding protein 4 (FKBP4),
secreted protein acidic and rich in cysteine (Sparc, also known as
osteonectin) and S100A6 (also known as calcyclin) proteins were
downregulated by acai berry, and Protein Kinase Substrate 80H-K was
upregulated by acai berry under PQ poisoning conditions. The data
indicated that these proteins play pivotal roles in PQ-induced
acute lung damage and may function as early biomarkers of
PQ-induced injury. Furthermore, these data allowed us to speculate
that the acai berry may contribute to the diminution of PQ-induced
early lung cell damage via the regulation of the expression of
these proteins and their functions. In order to gain insight into
the mechanism of action of these proteins on PQ-poisoned acute lung
damage and the clinical relevance of the acai berry in the context
of PQ intoxication, further research will be required.
Materials and methods
Chemicals and reagents
MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and
PQ were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Male CD(SD)IGS rats, 6–7 weeks old, were purchased
from Orientbio (Seongnam, Korea) and allowed to acclimate to a
standard rodent diet with free access to water for 7 days prior to
the initiation of the experiment. The rats in the control group
(n=5) were treated with phosphate-buffered saline and sacrificed on
the 7th day. The acai berry (n=5) and PQ treatment (n=5) groups
were treated via intraperitoneal administration of 200 ng/ml
PBS-diluted acai berry and 25 mg/kg PQ solution, respectively, and
sacrificed on the 7th day. The PQ with acai berry group (n=5) rats
were first treated via intraperitoneal injection of 25 mg/kg PQ
solution followed by 200 ng/ml acai berry treatment 2 h and 6 h
afterward. During the experiment, the rats were carefully monitored
for signs of PQ poisoning. On the 7th day, the rats were sacrificed
and the lungs were collected for proteomic analysis. Animal studies
followed the guidelines of the Institutional Animal Care and Use
Committee of Soonchunhyang University.
Cell culture
Human alveolar type II-like epithelial A549 cells
(ATCC# CCL-185) were purchased from the American Type Culture
Collection (Manassas, VA, USA) and the cells were maintained in
standard DMEM/F-12 Ham supplemented with 10% (v/v) heat-inactivated
FBS and 1% (v/v) antibiotics (100 U/ml penicillin, 100 μg/ml
streptomycin and 0.25 μg/ml amphotericin B; Invitrogen, Carlsbad,
CA, USA) at 37°C in an incubator with a humidified atmosphere of 5%
CO2.
MTT assay
The MTT assay was carried out at various
time-points, including 12, 24, 48 and 72 h after PQ treatment
coupled with acai berry treatment. In brief, 80% confluent A549
lung adenocarcinoma cells were seeded on 96-well plates prior to
the beginning of the experiment. The cells were treated with PQ and
acai berry time- and dose-dependently and then were stained for 2 h
with MTT (2 mg/ml). The medium was removed and the formazan
crystals produced were dissolved via the addition of 200 μl
dimethyl sulfoxide. The absorbance at 540 nm was then measured
using a SpectraMax 250 microplate reader (Molecular Probes, Eugene,
OR, USA).
Proteomics
Sample preparation
Tissues were homogenized in sample lysis buffer and
the proteins were extracted. Insoluble material was discarded via
centrifugation at 15,000 × g for 1 h and the concentration of
proteins was measured via Bradford assay (Bio-Rad, Hercules, CA,
USA).
2D PAGE
Isoelectric focusing (IEF) using 200 μg samples was
conducted at 20°C using a Multiphor II electrophoresis unit in
accordance with the manufacturer’s instructions (Amersham
Biosciences, Pittsburgh, PA, USA). Prior to running the second
dimension, the strips were incubated for 10 min with equilibration
buffer (50 mM Tris-Cl, pH 6.8, containing 6 M urea, 2% SDS and 30%
glycerol), first with 1% DTT and second with 2.5% iodoacetamide.
Equilibrated strips were inserted onto SDS-PAGE gels (20×24 cm,
10–16%) and 2D gels were run. When the runs were completed, the 2D
gels were subjected to silver staining via a modification of the
method devised by Oakley et al(15).
Image analysis
Digitalized images were quantitatively analyzed
using the PDQuest (version 7.0, Bio-Rad) software in accordance
with the manufacturer’s instructions. Each spot was normalized by
total valid spot intensity and the spots whose expression levels
had altered >2-fold were selected.
In-gel digestion
Protein spots were enzymatically digested in-gel in
a manner similar to that described previously (16) and were modified by porcine trypsin
(Promega, Madison, WI, USA). The gel pieces were washed with 50%
acetonitrile and subsequently dehydrated. The spots were then dried
to remove the solvent, rehydrated with trypsin (8–10 ng/μl)
solution in 50 mM ammonium bicarbonate (pH 8.7) and then incubated
for 8–10 h at 37°C.
Identification of proteins by
MALDI-TOF/TOF
Samples were analyzed using an Applied Biosystems
4700 proteomics analyzer with TOF/TOFTM ion optics. MS and MS/MS
data were acquired with a Nd:YAG laser with a repetition rate of
200 Hz and up to 4000 shots were accumulated for each spectrum. MS
and MS/MS data were acquired using the instrument default
calibration, without applying internal or external calibration.
Sequence tag searches were conducted using the MASCOT program run
by the National Resource for the Mass Spectrometric Analysis of
Biological Macromolecules and Matrixscience Company (http://www.matrixscience.com/).
Quantitative RT-PCR
Total RNAs were extracted from lung tissues using
RNA isolation kits (Qiagen, Hilden, Germany). cDNAs were generated
using the first-strand cDNA synthesis kit (Intron, Seongnam, Korea)
and qRT-PCR assays were conducted using a Bio-Rad real-Time PCR kit
with a rat Sparc-specific primer set comprising: forward primer,
5′-GTGTGCAGCAATGACAACAAG-3′; and reverse primer;
5′-TCAGCTCAGAATCCAGGCAG-3′).
Statistical analyses
The results were shown as the means ± standard
deviation (SD) from triplicate samples of three independent
experiments. Statistical significance was analyzed using a paired
Student’s t-test with P<0.05 considered as significant.
Results
Acai berry has a protective effect
against PQ-induced A549 cytotoxicity
In order to investigate the protective effects of
the acai berry, we initially conducted an MTT cytotoxicity assay of
PQ-treated lung adenocarcinoma A549 cells (Fig. 1). Prior to performing these
experiments, we tested the toxicity of the acai berry to the A549
cells by MTT assay. Concentrations of the acai berry ≤50 μg/ml
evidenced no cytotoxicity (data not shown). Acai berry and PQ
co-treatment was also evaluated. Fig.
1 shows that the viability of the cells was reduced markedly in
the PQ-treated group. The cell viability began to reduce at 12 h
and reached almost 40% at 72 h, whereas the cell viability of the
control cells did not change. The viability of the A549 cells
treated with PQ and 30 μg/ml acai berry extract was unchanged at 12
h compared with that of the control cells, but subsequently
decreased gradually until 72 h. However, the cytotoxicity of PQ was
markedly diminished by co-treatment with acai berry relative to
that of the group treated with PQ alone at 72 h. The data indicated
that acai berry exerts a protective effect against PQ-induced cell
cytotoxicity.
In order to assess the clinical relevance of the
results, we designed an animal model system. We used CD(SD)IGS rats
assigned randomly to the following groups prior to administration:
acai berry (group A, n=5), PQ (group PQ, n=5), PQ with acai berry
(group PA, n=5) and control (group C, n=5). After acclimation, the
rats of group PA were treated via the intraperitoneal
administration of 200 ng/ml PBS-diluted acai berry at 2 and 6 h
later, 25 mg/kg PQ was administered. Following PQ administration,
the rats were carefully monitored for any signs of PQ poisoning. On
the 7th day, the rats were euthanized and their lungs were
collected. In order to confirm PQ poisoning, we conducted micro-CT
analysis of the rat lungs. Notably, we observed no significant
differences in the lung tissues among the groups (data not shown).
The data indicated that the low dose of PQ treatment may not induce
severe or acute lung phenotypes. However, we subsequently conducted
proteomic analysis of the rat lung tissues, since global gene
expression may be altered during chemical treatments before the
tissues demonstrate any severe pathological features.
Proteomic analysis
In order to assess whether gene expression was
altered during the PQ-induced acute lung injury, we conducted
duplicate conventional proteomic analyses using 200 μg protein
samples from the four groups. In Fig.
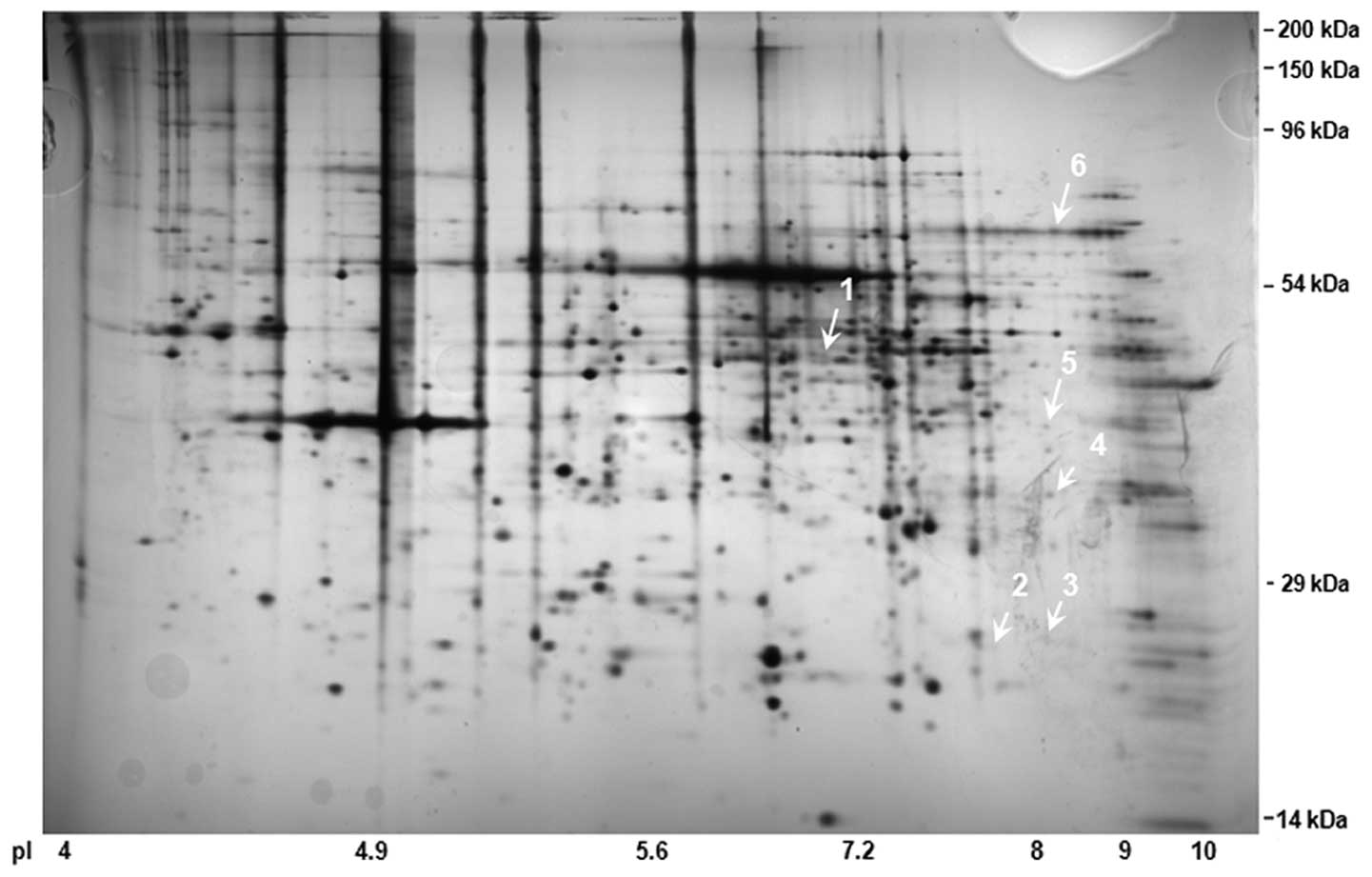
2, a combined 2D-SDS PAGE image is presented with >800
spots. By comparing the duplicated 2D-SDS-PAGE gels, we analyzed
and identified 6 differentially expressed protein spots among the
40 spots, the expression levels of which were substantially altered
>2-fold relative to basal expression levels. The substantially
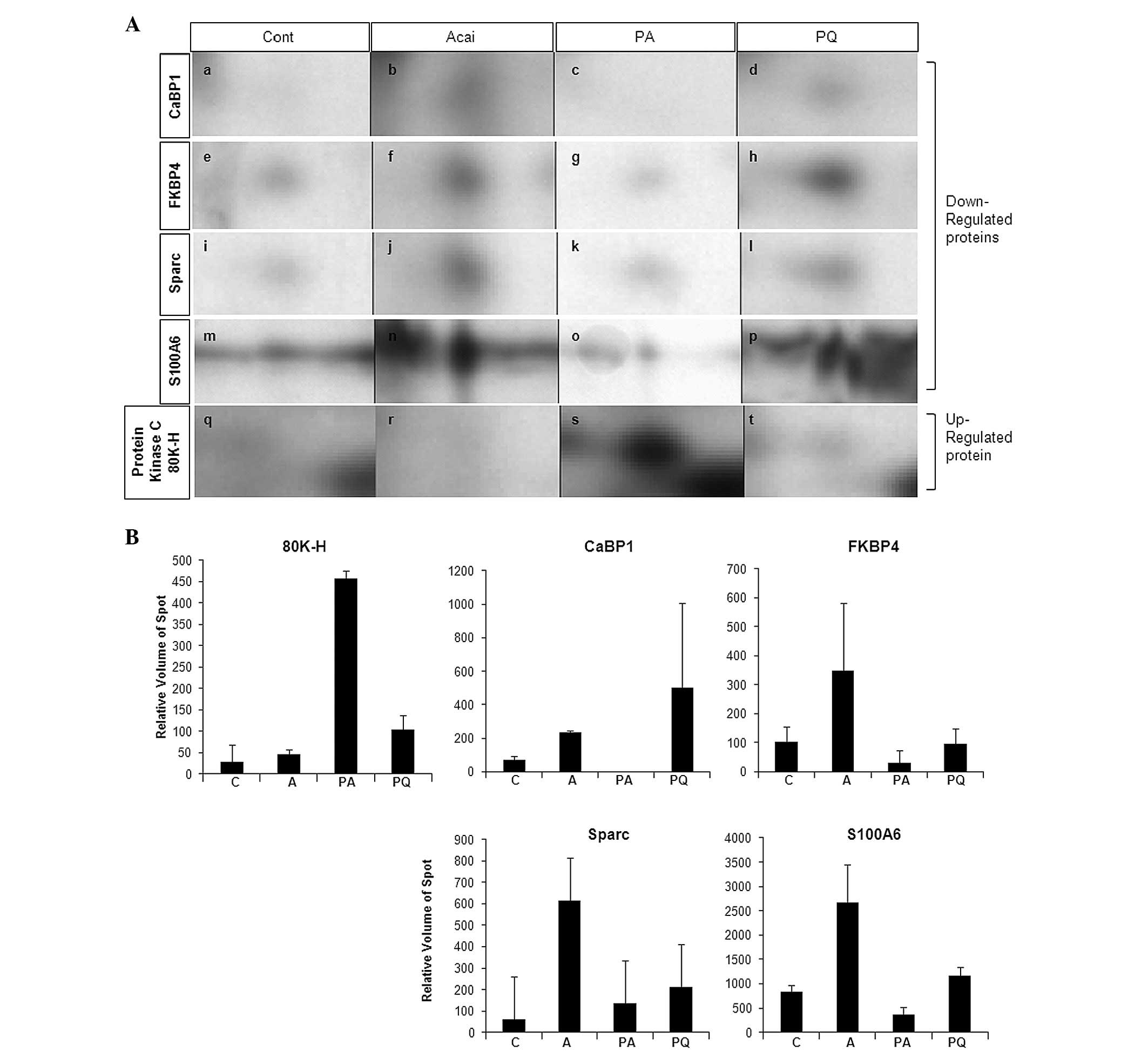
altered protein expression spots are shown in Fig. 3A and the relative volumes of the
spots are shown in Fig. 3B. These
spots consisted of one upregulated and five downregulated protein
spots for the PA group. To characterize the spots, we utilized
MS/MS-TOF and analyzed the spots via the MASCOT analysis program.
The results of Mascot analysis revealed that the upregulated spot
for the PA group was protein kinase C substrate 80K-H protein with
a Mowse score of 53 (P<0.05; Table
I). Spot 2 was matched with CaBP1 with a Mowse score of 44
(P<0.05) and the peptides covered 3% of the entire CaBP1
protein. Spot 4 was identified as FKBP4 with a score of 34
(P<0.05) and a peptide coverage of 3%. Notably, spot 5 matched
highly with Sparc with a Mowse score of 116 (P<0.05) and a
peptide coverage of 14% from the 301 amino acid polypeptide. S100A6
(spot 6) was characterized by a score of 47 (P<0.05) and 30%
peptide coverage. However, spot 3 was not characterized by Mascot
analysis, owing to a non-significant match to the proteins in the
protein database.
 | Table IA list of characterized and altered
proteins from PQ-induced lung injury in rats following acai berry
treatment |
Table I
A list of characterized and altered
proteins from PQ-induced lung injury in rats following acai berry
treatment
| SPOT | Annotation | Identified
protein | Mowse score | Coverage (%) | Change in group
PA |
|---|
| 1 | gi|149020437 | Protein kinase C
substrate 80K-H | 53 | 2 | Up |
| 2 | gi|488838 | CaBP1 | 44 | 3 | Down |
| 3 | | No significant
hits | - | - | Down |
| 4 | gi|22324680 | FK506 binding protein
4 | 34 | 3 | Down |
| 5 | gi|600381 | Sparc
(osteonectin) | 116 | 14 | Down |
| 6 | gi|16758986 | S100-A6 | 47 | 30 | Down |
In order to confirm the alteration of protein
expression in vivo, we conducted quantitative RT-PCR using a
Sparc-specific primer set (Fig.
4). This revealed that Sparc mRNA was induced in the PQ
group but not in the acai berry-treated group. However,
Sparc gene expression was altered slightly in the PA rat
group.
Discussion
PQ is broadly employed as an herbicide for weed
control all over the world, particularly in third world countries,
and remains one of the principal causes of fetal mortality in
humans as the result of accidental or intentional ingestion
(8). Acute PQ poisoning in animal
and human models leads to mortality within a few days due to severe
pulmonary fibrosis, but the early molecular mechanisms of
PQ-induced lung fibrosis remain incompletely understood.
Furthermore, studies of the molecular mechanisms relevant to
PQ-induced lung fibrosis using in vitro and in vivo
animal models have yet to generate clear results. Thus far,
intensive research has been conducted to develop treatments for PQ
poisoning, but no effective treatments have been developed.
Proteomics is a powerful tool for the analysis of
global gene expression and the display of differential protein
expression governed by functional genes in comparison among groups,
as well as heterogeneous diseases such as diabetes and cancer
(14). Proteomics has been
highlighted and expanded into a variety of research areas for
clinical application. In this regard, proteomics is an extremely
useful tool for the discovery of biomarkers, pharmacological
targets and drugs. However, few studies have been conducted in
which the conventional proteomics analysis technique has been
employed in the context of PQ-induced poisoning. Hence, we utilized
proteomics technology to investigate proteins that are
differentially expressed during treatment with PQ and/or acai berry
extract.
In this study, combined 2DE gels revealed ~800 spots
that were be identified via MALDI-TOF/TOF analysis. We selected 40
spots that were found have >2-fold altered expression and 6
spots were ultimately selected for further investigation. The most
highly matched spot was Sparc; the spot covered 14% of the peptide
portion on the entire Sparc protein with a Mowse score of 116.
Sparc is a secreted glycoprotein with multiple functions in tissue
remodeling, wound healing, cell proliferation and a variety of
diseases, including cancer (17).
Although the biological functions of Sparc remain controversial,
its functions have been demonstrated in the context of idiopathic
pulmonary fibrosis via the suppression of apoptosis through the
activation of β-catenin (18). The
results of in vivo and in vitro studies using siRNA
and null mice have demonstrated that Sparc gene silencing
may significantly reduce collagen expression (19,20).
A growing body of data has accumulated to suggest that Sparc is a
biomarker for fibrosis. Our findings suggest that Sparc expression
was induced by PQ treatment but reduced by acai berry treatment and
indicate that Sparc may be a useful biomarker of PQ-induced
poisoning.
S100A6 consists of 90 amino acids and is a member of
the S100 domain family within the EF-hand calcium binding protein
superfamily, which is associated with a variety of cellular and
extracellular processes (21).
Researchers have demonstrated that S100A6 performs crucial
functions in pulmonary fibroblast proliferation and responds to
stress conditions, including mechanical tension in pulmonary IMR-90
cells and oxidation by exobiotics (22–24).
It has been demonstrated that the activation of S100A6 promotes
apoptosis in cells under oxidative stress and also induces the
ROS-dependent activation of JNK and caspases -3 and -7 in
neuroblastoma cells (25).
Proteomics and MS/MS analysis results indicated that
S100A6 was downregulated in the PQ plus acai berry treatment group.
In Fig. 3B, the S100A6 expression
induced by PQ treatment is revealed to be repressed ~5-fold by acai
berry treatment. The data indicate that acai berry may exploit a
mechanism that represses PQ-induced apoptosis and ultimately exerts
a protective effect against PQ poisoning. However, in order to
define the mechanism of S100A6 regulation utilized by the acai
berry, further study may be necessary.
CaBP1 is a calcium -binding protein and carries out
a modulatory function in the gating of voltage-gated calcium
channels (26,27). However, the function of CaBP1 in
other physiological contexts and its implication with regard to
human diseases has yet to be thoroughly evaluated. Our proteomics
results showed that PQ-induced CaBP1 expression was downregulated
by acai berry treatment. These data lead us to speculate that the
acai berry may deliver and trigger signaling via a
calcium-dependent antioxidant pathway. However, further studies of
the functions of CaBP1 in the PQ-induced ROS signal pathway are
required.
FKBP4 (FKBP52) is a member of the immunophilin
proteins and functions as a co-chaperone with heat-shock protein 90
(HSP90) (28). The results of a
recent study has demonstrated that FKBP52 regulates oxidative
stress via the unique antioxidant, peroxiredoxin-6 (PRDX6), which
functions independently of other peroxiredoxins and antioxidant
proteins (29). However, FKBP4
expression was shown to be downregulated by acai berry treatment in
this study, thus suggesting the existence of an alternative
antioxidant pathway exploited by the acai berry under PQ exposure
conditions.
The protein kinase C substrate 80K-H (80K-H) was the
only upregulated protein spot identified in the PA group in this
study. The protein kinase C substrate 80K-H (80K-H) forms a complex
with the epithelial Ca2+ channel transient receptor
potential cation channel V5 (TRPV5) and is able to regulate the
activity of the channel (30).
Barnard et al demonstrated that the mechanism
underlying the protective effect of the acai berry against PQ
involves a calcium-dependent reaction (31). Notably, in this study, the calcium
regulation-related genes, S100A6, CaBP1 and protein kinase C
substrate 80K-H, were substantially altered by either PQ or the
acai berry. The data indicate that the acai berry may employ
calcium signaling for its protective effects against PQ-induced ROS
signaling.
In summary, the proteomic data for the herbicide PQ
and phytochemical acai berry extract have allowed us to propose
several target biomarkers for acute PQ poisoning. Furthermore, our
data indicate the possibility that the acai berry contains a
therapeutic phytochemical reagent that may help to alleviate or
protect against PQ intoxication.
Acknowledgements
The authors would like to express their gratitude to
Dr Gil, Hyo-Wook (SoonChunHyang University Hospital, Cheonan,
Korea) for his valuable comments.
References
|
1
|
Dinis-Oliveira RJ, Duarte JA,
Sánchez-Navarro A, Remião F, Bastos ML and Carvalho F: Paraquat
poisonings: mechanisms of lung toxicity, clinical features, and
treatment. Crit Rev Toxicol. 38:13–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith LL: Mechanism of paraquat toxicity
in lung and its relevance to treatment. Hum Toxicol. 6:31–36. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phytochemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie C, Kang J, Burris R, et al: Acai juice
attenuates atherosclerosis in ApoE deficient mice through
antioxidant and anti-inflammatory activities. Atherosclerosis.
216:327–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Udani JK, Singh BB, Singh VJ and Barrett
ML: Effects of Acai (Euterpe oleracea Mart.) berry
preparation on metabolic parameters in a healthy overweight
population: A pilot study. Nutr J. 10:452011.
|
|
6
|
de Moura RS, Pires KM, Santos Ferreira T,
et al: Addition of açaí (Euterpe oleracea) to cigarettes has
a protective effect against emphysema in mice. Food Chem Toxicol.
49:855–863. 2011.
|
|
7
|
Schauss AG, Clewell A, Balogh L, et al:
Safety evaluation of an açai-fortified fruit and berry functional
juice beverage (MonaVie Active(®)). Toxicology.
278:46–54. 2010.
|
|
8
|
Schauss AG, Wu X, Prior RL, et al:
Phytochemical and nutrient composition of the freeze-dried
amazonian palm berry, Euterpe oleraceae mart. (acai). J
Agric Food Chem. 54:8598–8603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Del Pozo-Insfran D, Brenes CH and Talcott
ST: Phytochemical composition and pigment stability of Açai
(Euterpe oleracea Mart.). J Agric Food Chem. 52:1539–1545.
2004.PubMed/NCBI
|
|
10
|
Lichtenthäler R, Rodrigues RB, Maia JG,
Papagiannopoulos M, Fabricius H and Marx F: Total oxidant
scavenging capacities of Euterpe oleracea Mart. (Açaí)
fruits. Int J Food Sci Nutr. 56:53–64. 2005.
|
|
11
|
Rodrigues RB, Lichtenthäler R, Zimmermann
BF, et al: Total oxidant scavenging capacity of Euterpe
oleracea Mart. (açaí) seeds and identification of their
polyphenolic compounds. J Agric Food Chem. 54:4162–4167.
2006.PubMed/NCBI
|
|
12
|
Sun X, Seeberger J, Alberico T, et al:
Açai palm fruit (Euterpe oleracea Mart.) pulp improves
survival of flies on a high fat diet. Exp Gerontol. 45:243–251.
2010.
|
|
13
|
Banks RE, Dunn MJ, Hochstrasser DF, et al:
Proteomics: new perspectives, new biomedical opportunities. Lancet.
356:1749–1756. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Plymoth A and Hainaut P: Proteomics beyond
proteomics: toward clinical applications. Curr Opin Oncol.
23:77–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oakley BR, Kirsch DR and Morris NR: A
simplified ultrasensitive silver stain for detecting proteins in
polyacrylamide gels. Anal Biochem. 105:361–363. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shevchenko A, Wilm M, Vorm O and Mann M:
Mass spectrometric sequencing of proteins silver-stained
polyacrylamide gels. Anal Chem. 68:850–858. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clark CJ and Sage EH: A prototypic
matricellular protein in the tumor microenvironment - where there’s
SPARC, there’s fire. J Cell Biochem. 104:721–732. 2008.PubMed/NCBI
|
|
18
|
Chang W, Wei K, Jacobs SS, Upadhyay D,
Weill D and Rosen GD: SPARC suppresses apoptosis of idiopathic
pulmonary fibrosis fibroblasts through constitutive activation of
beta-catenin. J Biol Chem. 285:8196–8206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JC, Lai S, Guo X, et al: Attenuation
of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res
Ther. 12:R602010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Savani RC, Zhou Z, Arguiri E, et al:
Bleomycin-induced pulmonary injury in mice deficient in SPARC. Am J
Physiol Lung Cell Mol Physiol. 279:L743–L750. 2000.PubMed/NCBI
|
|
21
|
Leśniak W, Słomnicki ŁP and Filipek A:
S100A6 - new facts and features. Biochem Biophys Res Commun.
390:1087–1092. 2009.
|
|
22
|
Zhang SP, Wu YW, Wu ZZ, Liu HY, Nie JH and
Tong J: Up-regulation of RAGE and S100A6 in rats exposed to
cigarette smoke. Environ Toxicol Pharmacol. 28:259–264. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leśniak W, Szczepańska A and KuŸnicki J:
Calcyclin (S100A6) expression is stimulated by agents evoking
oxidative stress via the antioxidant response element. Biochim
Biophys Acta. 1744:29–37. 2005.PubMed/NCBI
|
|
24
|
Breen EC and Tang K: Calcyclin (S100A6)
regulates pulmonary fibroblast proliferation, morphology, and
cytoskeletal organization in vitro. J Cell Biochem. 88:848–854.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Słomnicki ŁP, Nawrot B and Leśniak W:
S100A6 binds p53 and affects its activity. Int J Biochem Cell Biol.
41:784–790. 2009.PubMed/NCBI
|
|
26
|
Haeseleer F, Sokal I, Verlinde CL, et al:
Five members of a novel Ca(2+)-binding protein (CABP)
subfamily with similarity to calmodulin. J Biol Chem.
275:1247–1260. 2000.PubMed/NCBI
|
|
27
|
Oz S, Tsemakhovich V, Christel CJ, Lee A
and Dascal N: CaBP1 regulates voltage-dependent inactivation and
activation of Ca(V)1.2 (L-type) calcium channels. J Biol Chem.
286:13945–13953. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davies TH and Sanchez ER: Fkbp52. Int J
Biochem Cell Biol. 37:42–47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirota Y, Acar N, Tranguch S, et al:
Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6)
signaling protects pregnancy from overt oxidative stress. Proc Natl
Acad Sci USA. 107:15577–15582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gkika D, Mahieu F, Nilius B, Hoenderop JG
and Bindels RJ: 80K-H as a new Ca2+ sensor regulating
the activity of the epithelial Ca2+ channel transient
receptor potential cation channel V5 (TRPV5). J Biol Chem.
279:26351–26357. 2004.PubMed/NCBI
|
|
31
|
Barnard JW, Womack WA, Smith SM, Engerson
TD and Taylor AE: Lung protection against paraquat is calcium
dependent. J Appl Physiol. 72:498–504. 1992.PubMed/NCBI
|