Introduction
Japanese encephalitis virus (JEV) is a member of the
Flaviviridae family and infection is associated with nervous
damage and encephalitis (1–3).
Previous studies have demonstrated that JEV infection causes cell
death via the apoptotic pathway (4,5). In
addition, infection increases oxidative stress and activates
ERK/p38 MAPK signal transduction (6,7).
JEV-induced apoptosis via caspase activation has been previously
reported (8–10). However, the component of JEV which
induces cell death remains unclear.
Similar to JEV, dengue virus type 2 (DENV),
hepatitis C virus (HCV), West Nile virus (WNV) and langat
flavivirus are all members of the Flaviviridae family
(11–14). These viruses cause cell death in
infected cells, although their clinical presentation varies
(15–18). To date, a number of studies have
reported that induction of cell death by DENV, HCV, WNV and langat
flavivirus may be associated with non-structure protein 3 (NS3)
domains present in the viruses (19–22).
In addition, previous studies have demonstrated that JEV NS2B-NS3
fragments activate caspase-3 and induce apoptosis in human
medulloblastoma cells (23). Based
on these observations, we hypothesized that the JEV NS3 protein may
be important for JEV-induced apoptosis. Therefore, to examine
whether JEV NS3 proteins induce cell apoptosis, pEGFP-NS3 1–619
(expressing the NS3 intact protein) was transfected into cells.
Results indicate that the JEV NS3 protein induces apoptotic cell
death.
The JEV NS3 protein has important enzyme activities,
including helicase, protease and nucleoside triphosphatase
activities (24–26). Previous studies have cloned
C-terminal residues of JEV NS3 and identified RNA helicase activity
(27). In addition, a number of
studies have cloned N-terminal residues of NS3 using a PCR method,
identifying protease activity (28–30).
In order to examine which fragments of the JEV NS3 protein induce
cell apoptosis, pEGFP-NS3 163–619 or pEGFP-NS3 1–180 (expressing
the NS3 helicase and protease domains, respectively), were
transfected into cells and cell death percentage was calculated.
The current study demonstrates that the NS3 helicase and protease
domains induce cell death in a manner comparable with the JEV NS3
intact protein.
Cell death is divided into two pathways, necrosis
and apoptosis (31). In the
apoptosis pathway, chromatin condensation and DNA fragmentation are
observed (32,33) and the process is mediated by
caspase cascades (34,35). Two major caspase cascades have been
reported, including death receptor and mitochondria-mediated death
pathways (36). In the death
receptor pathway, initiator caspase-8 and executioner caspase-3 are
activated to induce cell apoptosis through death ligands, which
bind cell surface death receptors (37). In the mitochondrial death pathway,
initiator caspase-9 and executioner caspase-3 are activated to
mediate cell apoptosis via mitochondrial dysfunction (38). In this study, caspase-9 and -3 were
activated in cells transfected with pEGFP-NS3 1–619, pEGFP-NS3
163–619 and pEGFP-NS3 1–180. However, caspase-8 activity was
unchanged in groups compared with the control. Overall, results
demonstrate that JEV NS3 and the NS3 protease and helicase domains
induce cell death via the caspase-9/-3 cascade pathway.
Materials and methods
Plasmids and chemicals
pEGFP (expressing green fluorescent protein),
pEGFP-NS31–619 (expressing NS3 intact protein), pEGFP-NS3 1–180
(expressing NS3 protease domain) and pEGFP-NS3 163–619 ( expressing
NS3 helicase domain) were kindly donated by Dr Jaang-Jiun Wang
(Division of Pediatric Infectious Diseases, Emory University School
of Medicine, Atlanta, USA). Acetyl-Asp-Glu-Val-Asp-p-nitroanilide
(Ac-DEVD-pNA; caspase-3 substrate), Ac-Leu-Glu-His-Asp (LEHD)-pNA
(caspase-9 substrate) and Ac-Ile-Glu-Thr-Asp (IETD)-pNA (caspase-8
substrate) were obtained from Anaspec (Fremont, CA, USA). Gene
Jammer® transfection reagent was purchased from Agilent
Technologies (Santa Clara, CA, USA). Fetal bovine serum (FBS) was
obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
DMEM, non-essential amino acid, L-glutamine and
penicillin/streptomycin were obtained from Gibco-BRL (Carlsbad, CA,
USA).
Cell lines and cell culture
Vero (green monkey kidney epithelial) and HeLa cells
(human cervical cancer) were obtained from the Bioresources
Collection and Research Center (Hsin Chu, Taiwan). Cells were
cultured in DMEM supplemented with 10% heat-inactivated FBS, 2 mM
L-glutamine, 100 IU/ml penicillin/streptomycin and 0.1 mM
non-essential amino acids and maintained at 37°C in a humidified
atmosphere containing 5% CO2. The study was approved by
the ethics committee of Graduate Institute of Cancer Biology, China
Medical University, Taichung, Taiwan.
Transfection
Vero and Hela cells were incubated with 2 μg plasmid
(pEGFP, pEGFP-NS3 1–619, pEGFP-NS3 1–180 and pEGFP-NS3 163–619)
mixed with 6 μl Gene Jammer® transfection reagent and
added to 1 ml DMEM, in 6-well plates (2×105 cells/well)
for 3 hours. Following this, the medium was replaced with DMEM
supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, 100
IU/ml penicillin/streptomycin and 0.1 mM non-essential amino acids
and maintained at 37°C in a humidified atmosphere containing 5%
CO2. Following 16 h transfection, the plasmid expressed
the gene products.
Cell death percentage assay
Cells (1×105) were grown in a 24-well
plate. Following 24 h plasmid transfection, cell number was
calculated using the trypan blue exclusion method and a
hemocytometer. Percentage of dead cells was calculated using the
following formula: (100 - no. of cells in plasmid transfected
group/no. of cells in plasmid non-transfected group × 100.
Nuclear staining
Nuclear staining was performed by Hoechst 33342 as
described previously (39). In
brief, cells were treated with Hoechst 33342 (10 μg/ml) for 10 min.
Following this, DNA condensation and fragmentation were observed
under a fluorescence microscope (excitation, 352 nm; emission, 450
nm).
Caspase activity assay
Caspase activity was determined using a substrate
cleavage assay as described previously (39,40).
Briefly, cells were lysed with lysis buffer (50 mM Tris-HCl, 120 mM
NaCl, 1 mM EDTA and 1% NP-40; pH 7.5) in the presence of protease
inhibitors. Cell pellets were discarded following centrifugation at
15,000 × g for 30 min at 4°C. The substrate cleavage assay was
executed in a working reaction containing 40 μl cell lysate (80 μg
total protein), 158 μl reaction buffer (20% glycerol, 0.5 mM EDTA,
5 mM dithiothreitol and 100 mM HEPES; pH 7.5) and 2 μl fluorogenic
substrate (either Ac-LEHD-pNA, Ac-IETD-pNA or Ac-DEVD-pNA; 100 μM
final concentration). The reaction was incubated at 37°C for 6 h.
Cleavage of the fluorogenic substrate released p-NA, which was
measured at 405 nm in an ultra-microplate reader (Ceres UV 900,
BioTek Instruments, Inc., Winooski, VT, USA). Fold increase in
caspase activity was calculated using the following formula:
(A405sample -
A405control)/A405control.
Inhibition of caspase activity
Z-VAD-FMK is a general caspase inhibitor which
inhibits all caspase activity. Inhibition of caspase activity was
performed as described previously (39). Briefly, cells were pretreated with
10 mM Z-VAD-FMK prior to transfection. Following this, the
percentage of dead cells was determined as described for cell death
percentage assay.
Statistical analysis
Data were obtained from four independent triplicate
experiments and are presented as the mean values of the
representative triplicate experiment (mean ± SD). Statistical
differences between 2 groups were determined by the Student’s t
test.
Results
NS3 intact protein induces cell death
through the apoptosis pathway
Plasmid pEGFP expresses a green fluorescent protein
and plasmid pEGFP-NS3 1–619 expresses the JEV NS3 intact protein.
Following transfection of Vero cells with pEGFP or pEGFP-NS3 1–619
for 24 hours, cell counts were performed and the percentage of dead
cells was calculated. As demonstrated in Fig. 1, percentage of dead cells was ~8%
in the pEGFP-transfected group. However, the percentage of dead
cells was >20% in the pEGFP-NS3 1–619-transfected group,
indicating that JEV NS3 induces cell death. Next, nuclear
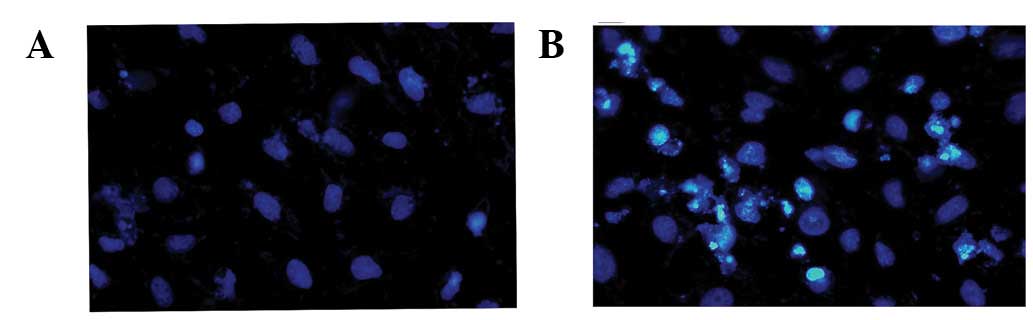
morphology was analyzed in transfected cells via the Hoechst 33342
staining method. Compared with pEGFP-transfected cells (Fig. 2A), chromatin condensation and DNA
fragmentation were found in pEGFP-NS3 1–619-transfected cells
(Fig. 2B). Results indicate that
the JEV NS3 intact protein induces cell death through the apoptosis
pathway.
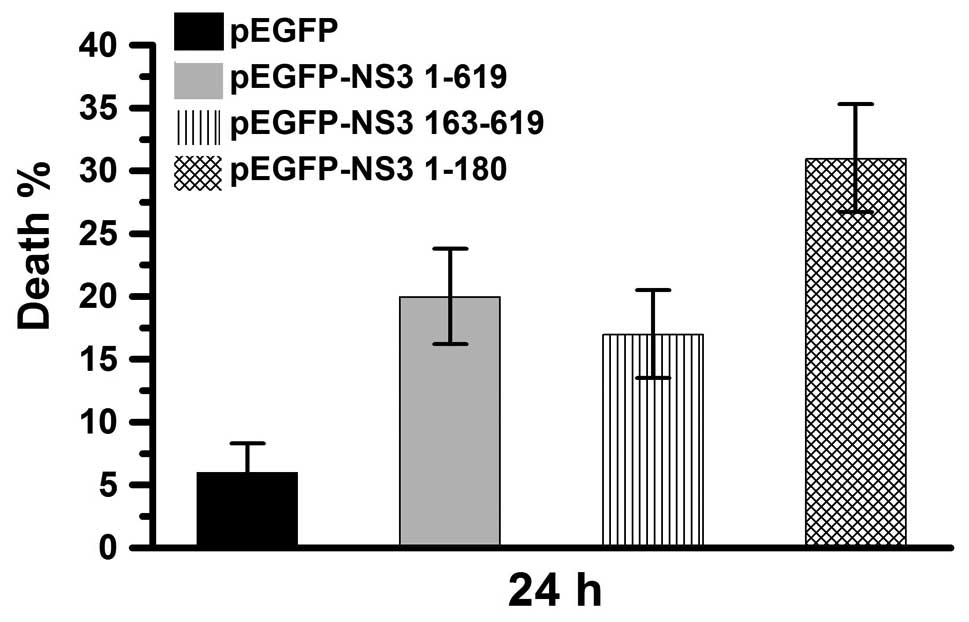
NS3 helicase and protease domains induce
cell death
JEV NS3 intact protein includes two major domains,
the protease and helicase domains. pEGFP-NS3 1–180 and pEGFP-NS3
163–619 (expressing the JEV NS3 protease and helicase domains,
respectively) were used in this study. The percentage of dead cells
was determined 24 h after transfection of Vero cells with pEGFP,
pEGFP-NS3 1–619, pEGFP-NS3 1–180 or pEGFP-NS3 163–619 (Fig. 3). In the pEGFP-transfected group,
cell death was ~6%. In the pEGFP-NS3 1–619 and pEGFP-NS3
163–619-transfected groups, cell death was ~20% and in the
pEGEP-NS3 1–180-transfected group, cell death was ~30%. These
results demonstrate that cell death is induced by the JEV NS3
intact protein as well as the the JEV NS3 helicase and protease
domains. In addition, the protease domain was observed to exert a
higher cytotoxic effect compared with the intact protein and
helicase domain.
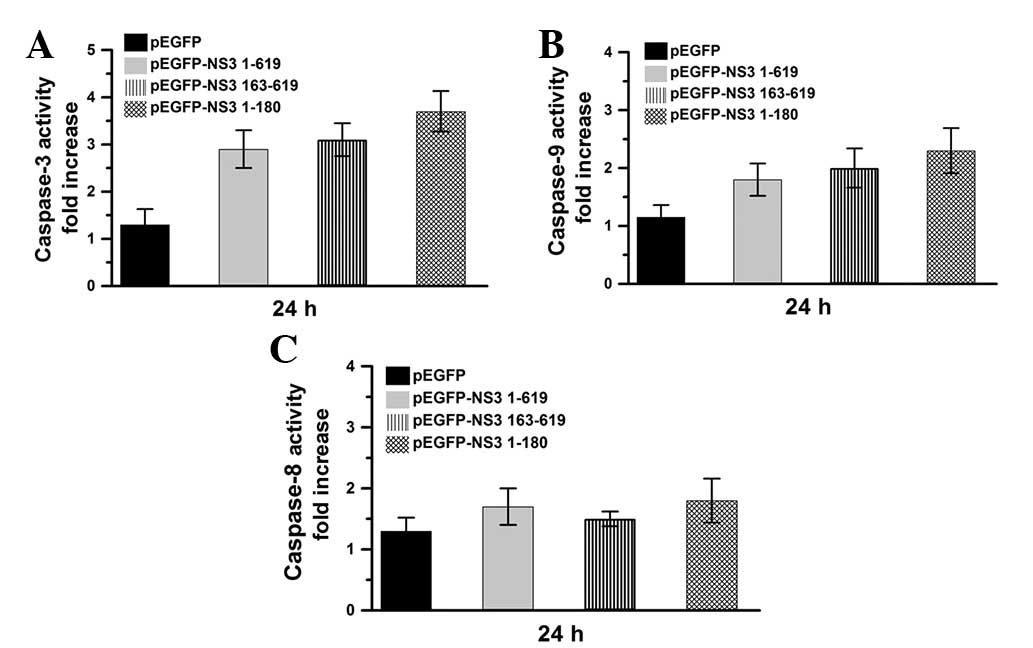
JEV NS3 intact protein and helicase and
protease domains induce the caspase-9/-3 cascade pathway
Caspase activities were also determined in the
current study, following 24 h transfection of Vero cells with
pEGFP, pEGFP-NS3 1–619, pEGFP-NS3 1–180 or pEGFP-NS3 163–619
(Fig. 4). Compared with the
pEGFP-transfected group, caspase-3 and -9 were markedly activated
in pEGFP-NS3 1–619, pEGFP NS31–180 and pEGFP-NS3
163–619-transfected groups (Fig. 4A
and B). However, compared with pEGFP, caspase-8 was not found
to be significantly activated in pEGFP-NS3 1–619, pEGFP-NS3 1–180
and pEGFP-NS3 163–619-transfected groups (Fig. 4C). Results indicate that the JEV
NS3 intact protein and protease and helicase domains activate the
caspase-9/-3 cascade pathway.
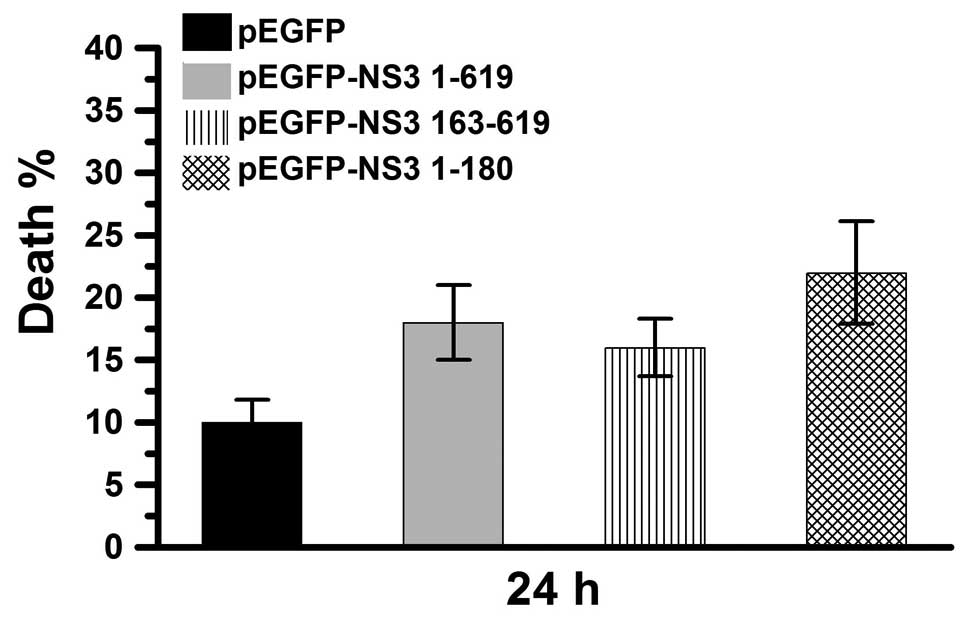
JEV NS3 protein and protease and helicase
domains induce cell death through caspase-independent pathways
To determine whether JEV NS3 intact protein- and
protease and helicase domain-induced cell death is dependent on
caspase activity, Vero cells were treated with Z-VA-FMK (a general
caspase inhibitor) prior to plasmid transfection and cell death
percentage was calculated. As demonstrated in Fig. 5, cell death was <10% in pEGFP-,
>15% in pEGFP-NS3 1–619- and pEGFP-NS3 163–619- and ~25% in
pEGFP-NS3 1–180-transfected groups. Therefore, the NS3 intact
protein and protease and helicase domains may also induce cell
death by caspase-independent pathways. In summary, this study
revealed that the JEV NS3 intact protein and protease and NS3
helicase domains induce cell death via caspase-9/-3 cascade and
caspase-independent pathways.
Discussion
A number of previous studies have demonstrated that
JEV infection induces cell death via a caspase-dependent cell death
pathway (4,8–10,41).
However, the involvement of various JEV proteins with cell death
remains unclear. A previous study indicated that JEV NS2-NS3
fragments induce cell death (23).
Consistent with these observations, the current study demonstrates
that the JEV NS3 protein alone is sufficient to trigger cell death
(Fig. 1). JEV NS3 protein has been
previously identified to exhibit protease and helicase enzyme
activities at the N- and C- terminals, respectively (25,42,43).
JEV NS3 protein is known to induce cell death; however, the enzyme
activities associated with this process are not known and were
analyzed in the present study. Results demonstrate that the
protease and helicase domains alone trigger cell death (Fig. 3), indicating that these activities
induce cell death following JEV infection.
Caspase-dependent death pathways include the
caspase-8/-3 and -9/-3 cascade pathways (36–38).
The caspase-8/-3 death pathway is induced by ligands acting on
death receptors located in the cell membrane (37,44).
However, the caspase-9/-3 death pathway is activated by
mitochondrial dysfunction (38,44).
Previous studies have reported that JEV infection triggers
activation of caspase-3, -8 and -9 (10,41),
indicating that JEV infection induces cell death through the death
receptor and mitochondrial pathways. However, the present results
indicate that the JEV NS3 protein and protease and helicase domains
activate caspase-9 and -3 activity only and do not activate
caspase-8 (Fig. 4). Therefore,
these fragments induce the caspase-9/-3 mitochondrial death pathway
only. To understand why the death receptor pathway is activated in
JEV-infected cells but not in JEV NS3-, protease- and
helicase-expressed cells, we hypothesized that the virus enters the
cell across the membrane and the viral capsid may interact with
death receptors leading to activation of the caspase-8/-3 death
pathway during JEV infection. The caspase-9/-3 pathway is not
activated until later in the infection process when NS3 protein is
expressed in cells. Therefore, results indicate that the JEV NS3
protein and protease and helicase domains markedly induce the
caspase-9/-3 but not the caspase-8/-3 cascades.
Numerous studies have demonstrated that cell death
is induced through the caspase-dependent and -independent pathways
(45–48). JEV infection is known to induce
cell death through a caspase-dependent pathway (10,23);
however, studies have not determined whether JEV-induced cell death
is also associated with caspase-independent pathways. The present
results demonstrate that inhibition of caspase activity, using the
general caspase inhibitor Z-VAD-FMK (39,49),
did not prevent cell death in pEGFP-NS3 1–619-, pEGFP-NS3 1–180-
and pEGFP-NS3 613–619-transfected cells (Fig. 5), indicating that these fragments
induce cell death through a caspase-independent pathway. Recent
studies have demonstrated that a number of compounds, including
isoegomaketone, transglutaminase 2 and blazeispirol A, induce cell
death through caspase-dependent and -independent pathways (50–52).
Consistent with these observations, the current study indicates
that the JEV NS3 protein, as well as the JEV NS3 protease and
helicase domains induce cell death through caspase-dependent
(caspase-9/-3 cascade) and -independent pathways (Figs. 4 and 5).
Acknowledgements
The present study was supported by grants from the
National Science Council of Taiwan (NSC99-2320-B-039-030-MY3,
NSC99-2632-B-039-001-MY3 and NSC100-2321-B-039-004) and The
University of Texas MD Anderson-China Medical University and
Hospital Sister Institution Fund (DMR-101-115).
References
|
1
|
Hall-Mendelin S, Jansen CC, Cheah WY, et
al: Culex annulirostris (Diptera: Culicidae) host feeding
patterns and Japanese encephalitis virus ecology in northern
Australia. J Med Entomol. 49:371–377. 2012. View Article : Google Scholar
|
|
2
|
Srivastava R, Kalita J, Khan MY and Misra
UK: Status of proinflammatory and anti-inflammatory cytokines in
different brain regions of a rat model of Japanese encephalitis.
Inflamm Res. 61:381–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xufang Y, Huanyu W, Shihong F, et al:
Etiological spectrum of clinically diagnosed Japanese encephalitis
cases reported in Guizhou Province, China, in 2006. J Clin
Microbiol. 48:1343–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liao CL, Lin YL, Wang JJ, et al: Effect of
enforced expression of human bcl-2 on Japanese encephalitis
virus-induced apoptosis in cultured cells. J Virol. 71:5963–5971.
1997.PubMed/NCBI
|
|
5
|
Liao CL, Lin YL, Shen SC, et al:
Antiapoptotic but not antiviral function of human bcl-2 assists
establishment of Japanese encephalitis virus persistence in
cultured cells. J Virol. 72:9844–9854. 1998.PubMed/NCBI
|
|
6
|
Tung WH, Tsai HW, Lee IT, et al: Japanese
encephalitis virus induces matrix metalloproteinase-9 in rat brain
astrocytes via NF-kappaB signalling dependent on MAPKs and reactive
oxygen species. Br J Pharmacol. 161:1566–1583. 2010. View Article : Google Scholar
|
|
7
|
Yang TC, Lai CC, Shiu SL, et al: Japanese
encephalitis virus down-regulates thioredoxin and induces
ROS-mediated ASK1-ERK/p38 MAPK activation in human promonocyte
cells. Microbes Infect. 12:643–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta N, Bhaskar AS and Lakshmana Rao PV:
Transcriptional regulation and activation of the mitogen-activated
protein kinase pathway after Japanese encephalitis virus infection
in neuroblastoma cells. FEMS Immunol Med Microbiol. 62:110–121.
2011. View Article : Google Scholar
|
|
9
|
Lee YH, Wei CW, Wang JJ and Chiou CT:
Rana catesbeiana ribonuclease inhibits Japanese encephalitis
virus (JEV) replication and enhances apoptosis of JEV-infected
BHK-21 cells. Antiviral Res. 89:193–198. 2011. View Article : Google Scholar
|
|
10
|
Tsao CH, Su HL, Lin YL, et al: Japanese
encephalitis virus infection activates caspase-8 and -9 in a
FADD-independent and mitochondrion-dependent manner. J Gen Virol.
89:1930–1941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Markoff LJ, Innis BL, Houghten R and
Henchal LS: Development of cross-reactive antibodies to plasminogen
during the immune response to dengue virus infection. J Infect Dis.
164:294–301. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fletcher NF and McKeating JA: Hepatitis C
virus and the brain. J Viral Hepat. 19:301–306. 2012. View Article : Google Scholar
|
|
13
|
Osorio HC, Ze-Ze L and Alves MJ:
Host-feeding patterns of Culex pipiens and other potential
mosquito vectors (Diptera: Culicidae) of West Nile virus
(Flaviviridae) collected in Portugal. J Med Entomol. 49:717–721.
2012.
|
|
14
|
Iacono-Connors LC and Schmaljohn CS:
Cloning and sequence analysis of the genes encoding the
nonstructural proteins of Langat virus and comparative analysis
with other flaviviruses. Virology. 188:875–880. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liao H, Xu J and Huang J: FasL/Fas pathway
is involved in dengue virus induced apoptosis of the vascular
endothelial cells. J Med Virol. 82:1392–1399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korner C, Tolksdorf F, Riesner K, et al:
Hepatitis C coinfection enhances sensitization of CD4(+) T-cells
towards Fas-induced apoptosis in viraemic and HAART-controlled
HIV-1-positive patients. Antivir Ther. 16:1047–1055.
2011.PubMed/NCBI
|
|
17
|
Kumar M, Verma S and Nerurkar VR:
Pro-inflammatory cytokines derived from West Nile virus
(WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and
neuronal death. J Neuroinflammation. 7:732010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prikhod’ko GG, Prikhod’ko EA, Cohen JI and
Pletnev AG: Infection with Langat Flavivirus or expression of the
envelope protein induces apoptotic cell death. Virology.
286:328–335. 2001.PubMed/NCBI
|
|
19
|
Shafee N and AbuBakar S: Dengue virus type
2 NS3 protease and NS2B-NS3 protease precursor induce apoptosis. J
Gen Virol. 84:2191–2195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gamlen T, Richards KH, Mankouri J, et al:
Expression of the NS3 protease of cytopathogenic bovine viral
diarrhea virus results in the induction of apoptosis but does not
block activation of the beta interferon promoter. J Gen Virol.
91:133–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramanathan MP, Chambers JA, Pankhong P, et
al: Host cell killing by the West Nile Virus NS2B-NS3 proteolytic
complex: NS3 alone is sufficient to recruit caspase-8-based
apoptotic pathway. Virology. 345:56–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Prikhod’ko GG, Prikhod’ko EA, Pletnev AG
and Cohen JI: Langat flavivirus protease NS3 binds caspase-8 and
induces apoptosis. J Virol. 76:5701–5710. 2002.PubMed/NCBI
|
|
23
|
Yang TC, Shiu SL, Chuang PH, et al:
Japanese encephalitis virus NS2B-NS3 protease induces caspase 3
activation and mitochondria-mediated apoptosis in human
medulloblastoma cells. Virus Res. 143:77–85. 2009. View Article : Google Scholar
|
|
24
|
Yamashita T, Unno H, Mori Y, et al:
Crystal structure of the catalytic domain of Japanese encephalitis
virus NS3 helicase/nucleoside triphosphatase at a resolution of 1.8
A. Virology. 373:426–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Borowski P, Heising MV, Miranda IB, Liao
CL, Choe J and Baier A: Viral NS3 helicase activity is inhibited by
peptides reproducing the Arg-rich conserved motif of the enzyme
(motif VI). Biochem Pharmacol. 76:28–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin CW, Huang HD, Shiu SY, et al:
Functional determinants of NS2B for activation of Japanese
encephalitis virus NS3 protease. Virus Res. 127:88–94. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Utama A, Shimizu H, Morikawa S, et al:
Identification and characterization of the RNA helicase activity of
Japanese encephalitis virus NS3 protein. FEBS Lett. 465:74–78.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bera AK, Kuhn RJ and Smith JL: Functional
characterization of cis and trans activity of the Flavivirus
NS2B-NS3 protease. J Biol Chem. 282:12883–12892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang YS, Liao CL, Tsao CH, et al:
Membrane permeabilization by small hydrophobic nonstructural
proteins of Japanese encephalitis virus. J Virol. 73:6257–6264.
1999.PubMed/NCBI
|
|
30
|
Lin CW, Lin KH, Lyu PC and Chen WJ:
Japanese encephalitis virus NS2B-NS3 protease binding to
phage-displayed human brain proteins with the domain of trypsin
inhibitor and basic region leucine zipper. Virus Res. 116:106–113.
2006. View Article : Google Scholar
|
|
31
|
Haeri M, Wöllert T, Langford GM and
Gilbert JL: Electrochemical control of cell death by
reduction-induced intrinsic apoptosis and oxidation-induced
necrosis on CoCrMo alloy in vitro. Biomaterials. 33:6295–6304.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
La Vignera S, Condorelli R, Vicari E,
D’Agata R and Calogero AE: Effects of varicocelectomy on sperm DNA
fragmentation, mitochondrial function, chromatin condensation and
apoptosis. J Androl. 33:389–396. 2012.PubMed/NCBI
|
|
33
|
Burattini S, Ferri P, Battistelli M, et
al: Apoptotic DNA fragmentation can be revealed in situ: an
ultrastructural approach. Microsc Res Tech. 72:913–923. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mu S, Tian X, Ruan Y, et al: Diosgenin
induces apoptosis in IGF-1-stimulated human thyrocytes through two
caspase-dependent pathways. Biochem Biophys Res Commun.
418:347–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan TL, Wang PW, Leu YL, Wu TH and Wu TS:
Inhibitory effects of Scutellaria baicalensis extract on
hepatic stellate cells through inducing G2/M cell cycle arrest and
activating ERK-dependent apoptosis via Bax and caspase pathway. J
Ethnopharmacol. 139:829–837. 2012.
|
|
36
|
Lee CS, Kwak SW, Kim YJ, et al: Guanylate
cyclase activator YC-1 potentiates apoptotic effect of licochalcone
A on human epithelial ovarian carcinoma cells via activation of
death receptor and mitochondrial pathways. Eur J Pharmacol.
683:54–62. 2012. View Article : Google Scholar
|
|
37
|
Yang C, Liu HZ and Fu ZX: PEG-liposomal
oxaliplatin induces apoptosis in human colorectal cancer cells via
Fas/FasL and caspase-8. Cell Biol Int. 36:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hua YY, Wang XS, Zhang Y, Yao CG, Zhang XM
and Xiong ZA: Intense picosecond pulsed electric fields induce
apoptosis through a mitochondrial-mediated pathway in HeLa cells.
Mol Med Rep. 5:981–987. 2012.PubMed/NCBI
|
|
39
|
Yiang GT, Yu YL, Chou PL, et al: The
cytotoxic protein can induce autophagocytosis in addition to
apoptosis in MCF-7 human breast cancer cells. In Vivo. 26:403–409.
2012.PubMed/NCBI
|
|
40
|
Tang CH, Hu CC, Wei CW and Wang JJ:
Synergism of Rana catesbeiana ribonuclease and IFN-gamma
triggers distinct death machineries in different human cancer
cells. FEBS Lett. 579:265–270. 2005.PubMed/NCBI
|
|
41
|
Lee CJ, Liao CL and Lin YL: Flavivirus
activates phosphatidylinositol 3-kinase signaling to block
caspase-dependent apoptotic cell death at the early stage of virus
infection. J Virol. 79:8388–8399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Deng X, Shi Z, Li S, et al:
Characterization of nonstructural protein 3 of a neurovirulent
Japanese encephalitis virus strain isolated from a pig. Virol J.
8:2092011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaczor A and Matosiuk D: Structure-based
virtual screening for novel inhibitors of Japanese encephalitis
virus NS3 helicase/nucleoside triphosphatase. FEMS Immunol Med
Microbiol. 58:91–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei CW, Hu CC, Tang CH, Lee MC and Wang
JJ: Induction of differentiation rescues HL-60 cells from Rana
catesbeiana ribonuclease-induced cell death. FEBS Lett.
531:421–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yu HY, Jin CY, Kim KS, et al:
Oleifolioside A mediates caspase-independent human cervical
carcinoma HeLa cell apoptosis involving nuclear relocation of
mitochondrial apoptogenic factors AIF and EndoG. J Agric Food Chem.
60:5400–5406. 2012. View Article : Google Scholar
|
|
46
|
Jang EH, Park CS and Kang JH: Bupropion,
an atypical antidepressant, induces endoplasmic reticulum stress
and caspase-dependent cytotoxicity in SH-SY5Y cells. Toxicology.
285:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhi B, Tang W and Zhang X: Enhancement of
shrimp antiviral immune response through caspase-dependent
apoptosis by small molecules. Mar Biotechnol (NY). 13:575–583.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Linsenbardt AJ, Breckenridge JM, Wilken GH
and Macarthur H: Dopaminochrome induces caspase-independent
apoptosis in the mesencephalic cell line, MN9D. J Neurochem.
122:175–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Furre IE, Møller MT, Shahzidi S, Nesland
JM and Peng Q: Involvement of both caspase-dependent and
-independent pathways in apoptotic induction by
hexaminolevulinate-mediated photodynamic therapy in human lymphoma
cells. Apoptosis. 11:2031–2042. 2006. View Article : Google Scholar
|
|
50
|
Cho BO, Jin CH, Park YD, et al:
Isoegomaketone induces apoptosis through caspase-dependent and
caspase-independent pathways in human DLD1 cells. Biosci Biotechnol
Biochem. 75:1306–1311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yoo JO, Lim YC, Kim YM and Ha KS:
Transglutaminase 2 promotes both caspase-dependent and
caspase-independent apoptotic cell death via the calpain/Bax
protein signaling pathway. J Biol Chem. 287:14377–14388. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Su ZY, Tung YC, Hwang LS and Sheen LY:
Blazeispirol A from Agaricus blazei fermentation product
induces cell death in human hepatoma Hep 3B cells through
caspase-dependent and caspase-independent pathways. J Agric Food
Chem. 59:5109–5116. 2011.PubMed/NCBI
|