Introduction
The p63 gene is a member of the p53 gene family and
has two different promoter usage-generating proteins that contain
(TA) or lack (ΔN) an NH2-terminus. The p53 and p63 molecules differ
in function and expression profiles. p63 is critical for the
development of various epithelial organs or tissues and is also
essential for the proliferative potential of stem cells in the
epidermis (1–4). ΔNp63 functions as a dominant negative
regulator of the TA isoforms of p63 and p53, which have been
revealed to inhibit apoptosis and promote stem cell proliferation
in vitro(5,6). In contrast to p53, the role of ΔNp63
in tumors remains unclear and complex (7,8).
Previous studies have demonstrated that ΔNp63 is overexpressed in
carcinomas of squamous epithelial origin (9–11)
and may play a role in promoting tumorigenesis (12,13).
Our previous study demonstrated that ΔNp63 is overexpressed in
human bladder carcinoma.
Cell-cell and cell-extracellular matrix interaction
is crucial for tumor transformation and tumor invasion (14,15),
in which the tight junction is an important constituent. Absence of
tight junctions or defects in these complexes has been associated
with the development of the neoplastic phenotype in epithelial
cells (16–18). The disruption of tight junctions
leads to cohesion loss, invasiveness and the lack of
differentiation, thereby promoting tumorigenesis (19). Claudin-1 is a tight junction
protein expressed in epithelial and endothelial cells (20).
The mechanism by which ΔNp63 promotes tumor cell
development, including adhesion, proliferation and polarity, is
unknown. Previous studies have reported that ΔNp63 induces cancer
cell invasion (21,22). Specific downstream genes of ΔNp63
have been described (21–25) in association with cell junctions.
Claudin-1 is an important p63 target gene required for normal skin
development (26). As a connexin,
it acts in a similar manner to these proteins to affect events
important for cancer cell development.
In the present study, ΔNp63 expression in human
bladder carcinoma UM-UC-3 cells was reduced in vitro. These
results indicate that ΔNp63 is located in the nucleus. In addition,
ΔNp63 silencing decreases invasion and metastasis of UM-UC-3 cells
and reduces claudin-1 expression. These results indicate that
claudin-1, as a ΔNp63 target gene, is associated with cell invasion
and migration in UM-UC-3 bladder cancer cells.
Materials and methods
Cell culture and transfection
The human bladder carcinoma cell line, UM-UC-3, was
purchased from the Institute of Cell Research (Chinese Academy of
Sciences, Shanghai, China). The study was approved by the ethics
committee of North Sichuan Medical College, Nan Chong, P.R. China.
Cells were cultured in RPMI-1640 medium (Gibco, Shanghai, China)
supplemented with 10% fetal bovine serum (FBS; Sijixin Inc.,
Beijing, China) and 1% penicillin-streptomycin (Invitrogen,
Shanghai, China). All cells were cultured at 37°C with 5%
CO2. The expression plasmid that encodes ΔNp63 was
kindly provided by Dr He Yunfeng (The First Affiliated Hospital,
Chongqing Medical University, Chongqing, China)and has a structure
consisting of two 19 bp stem-targeting ΔNp63 mRNA, a 9 bp loop and
a short poly(A)6 sequence. The sequences of two
oligonucleotides were as follows: forward,
5′-GATCCGTGCCCAGACTCAATTTAGTTTCAAGACGA
CTAAATTGAGTCTGGGCATTTTTTGTCTTCAAGACG
ACTAAATTGAGTCTGGGCATTTTTTGTCGACA-3′ and reverse,
5′-AGCTTGTCGACAAAAAATGCCCAGACT CAATTTAGTCGTCTTGAAACTAAATTGAGTCTGGGC
ACG-3′. The sequences of the negative control shRNA were as
follows: forward, 5′-GATCCGACTTCATAAGGCGCA
TGCTTCAAGACGGCATGCGCCTTATGAAGTCTTTTTT GTCGACA-3′ and reverse,
5′-AGCTTGTCGACAAAAAAG ACTTCATAAGGCGCATGCCGTCTTGAAGCATGCGCC
TTATAAGTCG-3′. Transfection was performed using Lipofectamine 2000
(Invitrogen) according to the manufacturer’s instructions.
Cell invasion and migration assay
Cell invasiveness was determined using a Transwell
chamber (6.5 mm in diameter with polyvinylpyrrolidone-free
polycarbonate filter of 8-μm pore size; Corning Inc., Corning, NY,
USA) precoated with 30 μg Matrigel (BD Biosciences, San Jose, CA,
USA). Approximately 100 μl of cells (105) transfected
with siRNA or control plasmid were added to the upper compartment
of the Transwell chamber. Then, 600 μl of 10% FBS medium was added
to the lower chamber. Following 24 h incubation at 37°C, the
non-invading cells in the upper surface of the filter were removed
using a cotton swab. The cells that penetrated into the lower
surface of the filter were stained with trypan blue. Finally, the
invading cells were counted under a microscope using a 10×
objective in four random fields. Cells were plated in six-well
plates for the migration assay. A wound was created on the
monolayer cells when the cells reached full confluence by scraping
a gap using a micropipette tip. The plate was then washed with
serum-free RPMI-1640 medium to clean the dissociated cells. Cells
were then incubated with serum-free RPMI-1640 medium at 37°C in 5%
CO2. Cells that migrated into the unit length area were
counted five times for each group at 0, 12 and 24 h following
scraping.
Cell heterogeneity adhesion assay
Cells (~1×105/ml) were added into a
96-well plate covered with collagen IV and incubated at 37°C in 5%
CO2 for 90 min. The plate was washed with
phosphate-buffered saline (PBS) to clean the dissociated cells.
Approximately 20 μl of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich, St. Louis, MO, USA) was then added to the culture
medium. Following incubation for 10 min at room temperature, the
culture medium was removed and then 200 μl dimethylsulfoxide was
added into each well. Absorbance (A value) was measured at 570 nm.
Each sample was assayed four times. The cell adhesion rate was
compared with the ratio of adherent cells and the total A value of
the cells.
Confocal microscopy
Cells were seeded on polylysine (10 μg/ml)-coated
glass chamber slides at a density of 2,000 cells/chamber and
washed, fixed in ice-cold 4% paraformaldehyde for 15 min and
permeabilized in 100 mM phosphate buffer containing 0.2% Triton
X-100 (Sigma-Aldrich) for 4 min. Cells were then incubated with 5%
bovine serum albumin (BSA) and immunolabeled with anti-ΔNp63
(1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
anti-claudin-1 antibodies (1:500; Santa Cruz Biotechnology, Inc.)
at room temperature for 1 h. Normal goat IgG was used instead of
anti-p63 in specific experiments to serve as the negative control.
Following incubation with the primary antibodies, the cells were
washed and incubated for 1 h with fluorescein
isothiocyanate-conjugated anti-ΔNp63 antibodies (1:500; Santa Cruz
Biotechnology, Inc.) and Cy3-conjugated anti-claudin-1 antibodies
(1:500; Santa Cruz Biotechnology, Inc.) for 1 h. Additional washes
were performed and the cells were mounted using fluorescent
mounting medium (Applygen Technologies, Inc., Beijing, China).
Cells were viewed under a Leica SP2 upright microscope and the
images were captured in LCS Light (Leica).
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was isolated using an RNeasy mini kit
(Qiagen, Hilden, Germany) and treated with DNase I (Qiagen).
Real-time PCR was conducted using an iCycler (Bio-Rad) with an iQ
SYBR-Green Supermix (Bio-Rad), according to the manufacturer’s
instructions. The primer sequences designated from the coding
region of the human gene cDNA were as follows: ΔNp63,
5′-CAGCCCATTGACTTGAACTTTG-3′ (sense) and
5′-TGTTATAGGGACTGGTGGACGA-3′ (antisense); claudin-1,
5′-GAGGATGGCTGTCATTGGG-3′ (sense) and 5′-CTTGGTGTTGGGTAAGAGGTTG-3′
(antisense). The internal controls were as follows: 5′-TGACGTGGA
CATCCGCAAAG-3′ (sense) and 5′-CTGGAAGGTGGACAG CGAGG-3′ (antisense).
The PCR conditions were as follows: 94°C for 4 min, followed by 35
cycles at 94°C for 20 sec, 60°C for 30 sec and 72°C for 30 sec,
with data acquisition during each cycle. Melting curve analysis was
conducted following PCR cycling to verify the purity and quality of
the PCR product.
Western blot analysis
Cells were seeded into 100-cm2 flasks.
Confluent cell layers were washed with ice-cold PBS and lysed for
30 min at 4°C, with 1% NP-40, 0.1% Triton X-100, 30 mM sodium
phosphate (pH 7.4) containing 1 mM sodium orthovanadate, 2.5 mM
Tris-HCl (pH 7.5), 100 mM NaCl and 10 μg/ml leupeptin and aprotinin
24 h after plating. The homogenate was then centrifuged at 12,000 ×
g for 20 min at 4°C. The supernatant liquid was collected and the
protein was quantified with the Bio-Rad protein colorimetric assay.
Protein was separated using 8% sodium dodecyl sulfate
polyacrylamide gel electrophoresis following addition of the sample
buffer to the cellular extract and boiling the samples at 95°C for
5 min. The protein was transferred onto a polyvinylidene difluoride
membrane (Millipore, Bedford, MA, USA) and the membrane was then
blocked for 1 h at room temperature with 5% BSA in Tris-buffered
saline containing 0.05% Tween-20 (TBST). Then, the blots were
washed and incubated overnight at 4°C in TBST containing 1% BSA
with primary antibodies against ΔNp63 (1:200), claudin-1 (1:200)
and GAPDH (1:3,000). The membranes were washed three times with
TBST, incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (1:2,500 dilution in
TBST containing 1% BSA) for 120 min at room temperature and then
washed three times with TBST. Following the chemiluminescence
reaction, bands were detected by exposing the blots to X-ray films
for the appropriate time. For quantitative analysis, bands were
detected and evaluated densitometrically with UVP Gelatin image
processing system Labworks 4.6 software and normalized against
GAPDH density.
Statistical analysis
Results are expressed as mean ± SD. One-way ANOVA
was used to determine the levels of difference between all groups.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were conducted using the SPSS
statistical software program (SPSS Inc., Chicago, IL, USA).
Results
ΔNp63 protein expression and
localization
Our results showed that ΔNp63 mRNA expression is
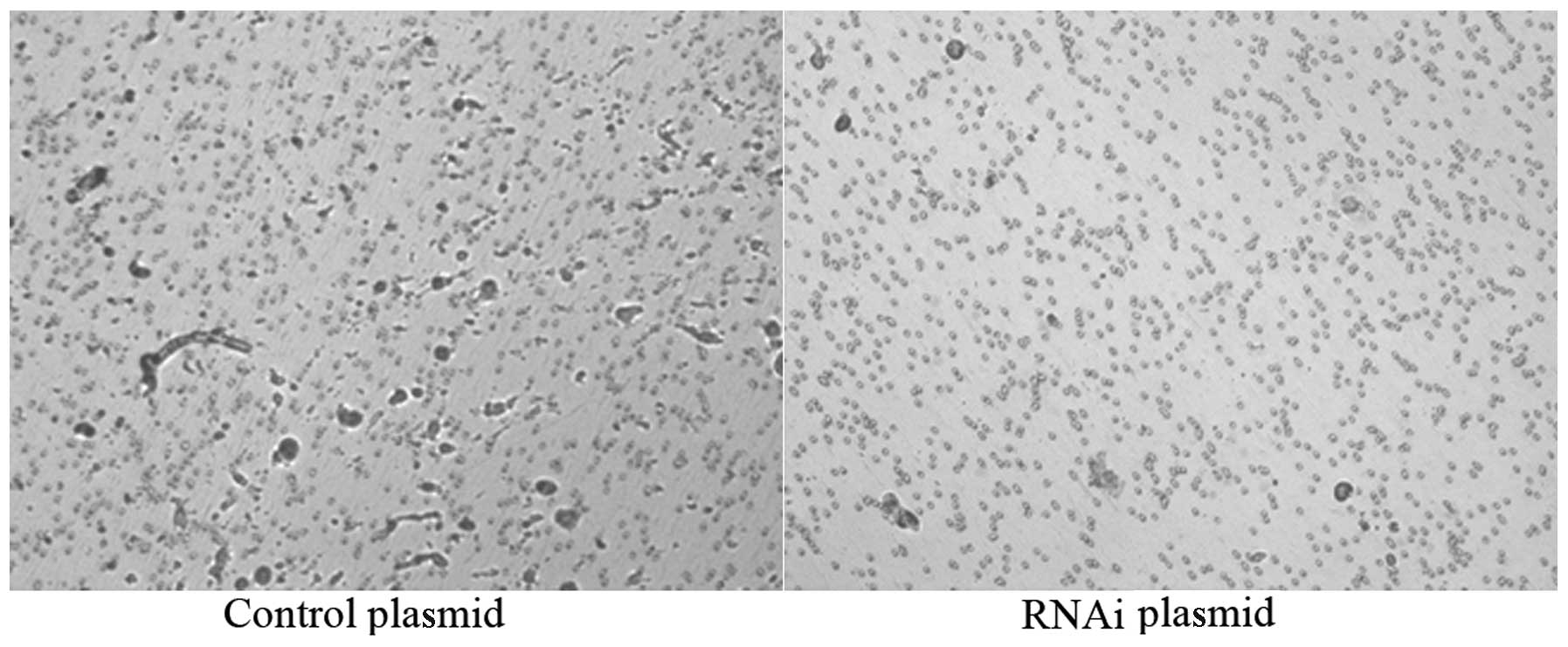
inhibited by ΔNp63 siRNA in vitro. In the current study,
green fluorescent protein (in the UM-UC-3 cells transfected with
the ΔNp63 control and ΔNp63-interfering plasmid) was observed under
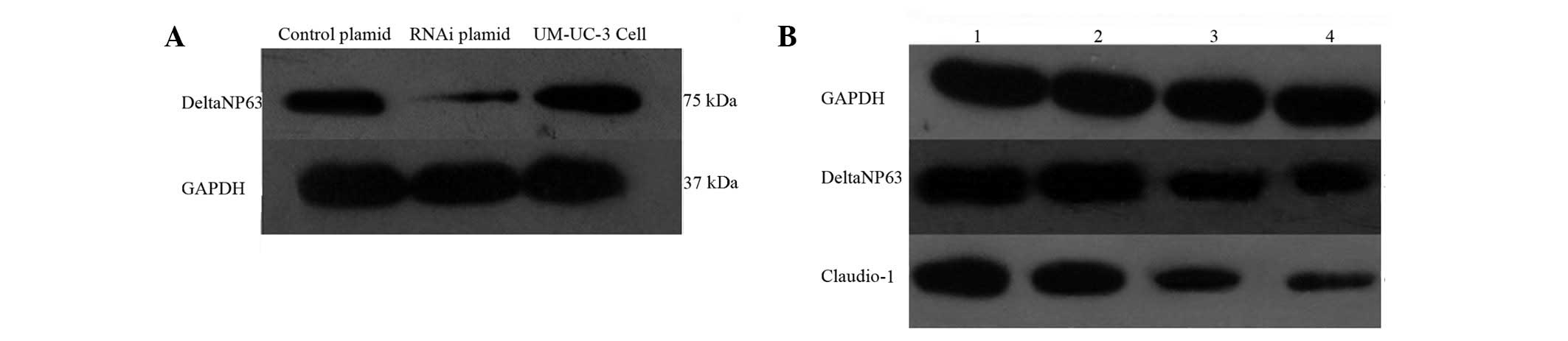
an inverted fluorescence microscope. ΔNp63 protein expression
levels were also determined. The control and ΔNp63 siRNA-treated
UM-UC-3 cells were characterized through western blot analysis,
using GAPDH as the internal control. The relative ratio of the
ΔNp63 protein expression with that of GAPDH in the UM-UC-3 cells
was determined following stable transfection with the control or
siRNA plasmid. Results indicate that ΔNp63 protein expression was
inhibited by the ΔNp63-interfering plasmid in vitro
(Fig. 1A). UM-UC-3 cells were
detected under laser confocal microscopy to determine the
functional position of the ΔNp63-interfering plasmid in the cells.
The result indicates that ΔNp63 is largely localized to the nuclei
of the UM-UC-3 cells. In addition, a sporadic distribution of ΔNp63
was revealed in the cell membrane. However, ΔNp63 protein
expression was reduced and localized to the cell nucleus around the
cell membrane in the ΔNp63-transfected UM-UC-3 cells. By contrast,
ΔNp63 protein expression was inhibited by the ΔNp63-interfering
plasmid and localized on the cell membrane of the UM-UC-3
cells.
Downregulation of invasion and
metastasis
ΔNp63 knockdown in the UM-UC-3 cell line was used to
examine the effect of ΔNp63-interfering plasmid on bladder cancer
invasion in vitro. The Transwell chamber precoated with 30
μg Matrigel was used for the invasion assay. Results reveal that
control exhibited 11.25±1.2 cells, whereas the interfering plasmid
group had 5.5±0.7 cells following stable transfection. The
invasiveness of UM-UC-3 cells transfected with ΔNp63-interfering
plasmid was found to have decreased significantly (P<0.05;
Fig. 2). Collagen IV-covered
96-well plates were used for cell heterogeneity adhesion and MTT
assays. The result indicates that the cell adhesion capacity
following stable transfection with ΔNp63 was lower compared with
the control and negative plasmid groups (P<0.05).
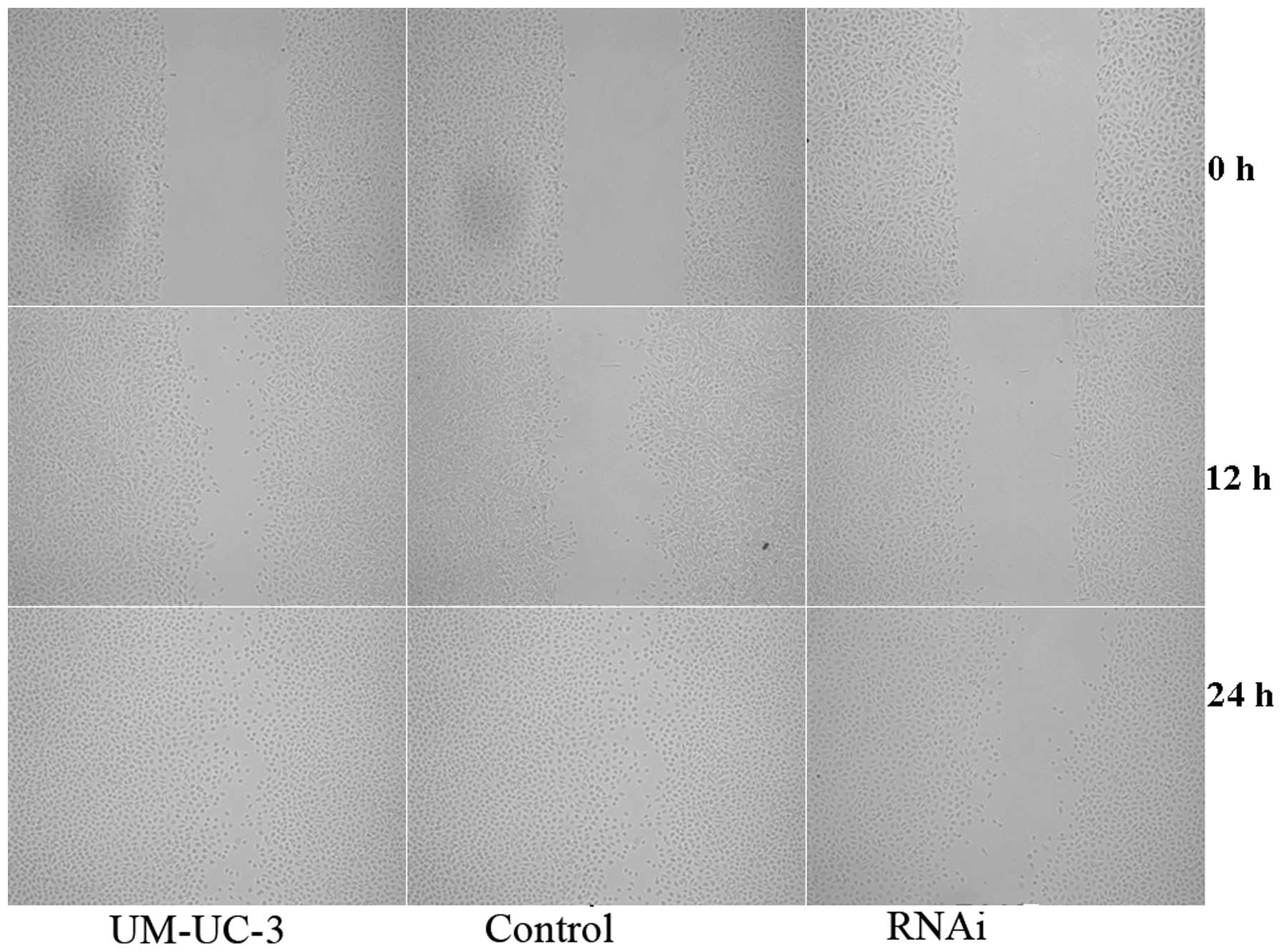
A cell scratch experiment was performed to examine
the effect of ΔNp63-interfering plasmid on cell migration in
vitro. Cells were photographed and the number of cells that
migrated per unit area was counted at 0, 12 and 24 h following
scraping. At 12 h, the negative group had 14.2±3.7
cells/mm2; control had 13.9±3.3 cells/mm2 and
interfering plasmid had 6.2±2.3 cells/mm2. At 24 h, the
negative group had 22.0±1.2 cells/mm2, control had
18.2±2.1 cells/mm2 and interfering plasmid had 12.6±1.4
cells/mm2. The migration ability of the cells in the
negative plasmid and control groups was higher than that of the
interfering plasmid (Fig. 3)
A series of experiments demonstrated that the
invasion and metastasis of bladder cancer is suppressed in
vitro through stable transfection with ΔNp63.
Claudin-1 expression
ΔNp63 protein expression was reduced and localized
to the cell nucleus. The cell membrane did not have the same ΔNp63
protein distribution as that associated with the promotion of
bladder cancer cell invasion and metastasis. However, we focused on
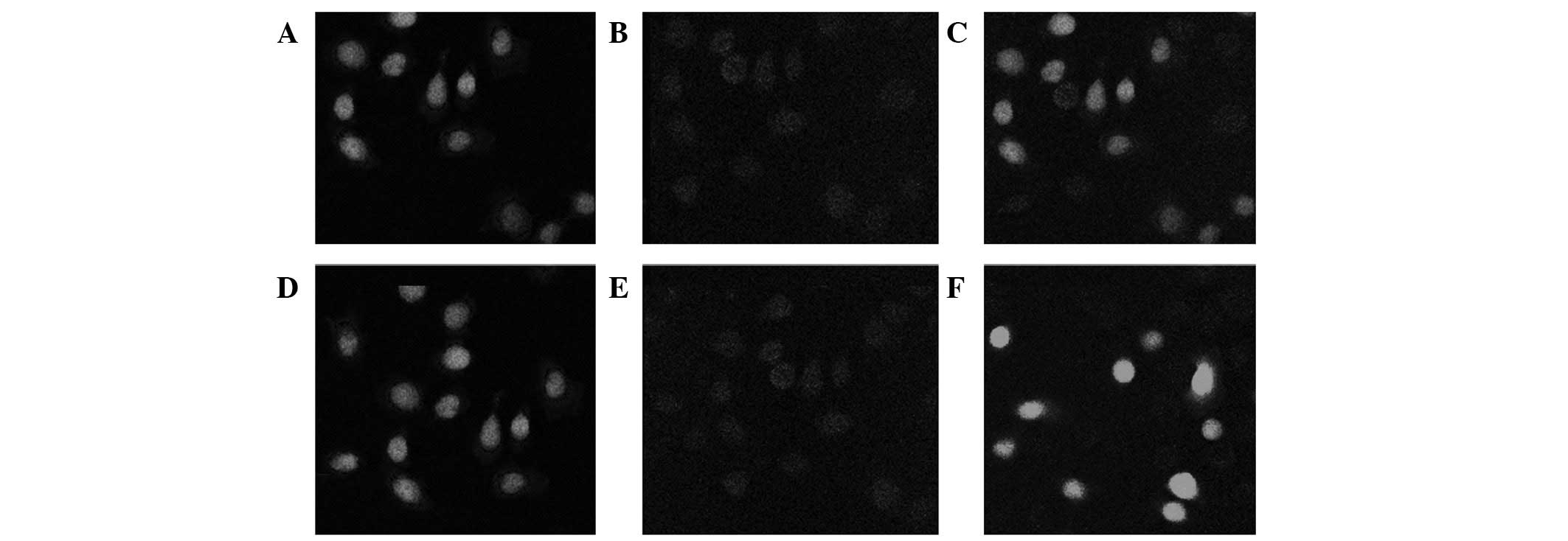
its regulation by ΔNp63. Double mark confocal microscopy was
performed to verify the binding of ΔNp63 with the claudin-1
promoter and subsequently determine its potential role as a
transcriptional regulator of claudin-1. Results indicate that
claudin-1 expression was reduced in the UM-UC-3 cells transfected
with ΔNp63 (Fig. 4).
The ability of ΔNp63 to induce claudin-1 expression
was investigated to verify whether the binding of ΔNp63 to the
claudin-1 gene is associated with changes in claudin-1 gene
expression. UM-UC-3 cells were transfected with si-ΔNp63 plasmid,
negative plasmid or empty vector and the relative
claudin-1-transcript endogenous levels were examined using
real-time PCR and western blot analysis. The cells transfected with
the si-ΔNp63 plasmid revealed significantly decreased claudin-1
expression at mRNA and protein levels (Fig. 1B).
In the current study, ΔNp63 was demonstrated to
downregulate claudin-1 expression and promote the invasion and
migration of claudin-1 in UM-UC-3 cells using a series of
assays.
Discussion
Cancer development is a multi-step process through
which cells accumulate genetic mutations. During the development of
human cancer, tumor cells detach and invade adjacent tissues. The
tumor cells may then succeed in forming new colonies. Therefore,
tumor invasion and migration are crucial steps in tumor
development. The molecular mechanism of tumor invasion involves
altered interactions between tumor cells and their environment, as
well as intracellular and intercellular events, including cell
proliferation, loss of cell-cell adhesion, acquisition of cell
motility and loss of cell polarity. p63 is a member of the p53
family and a number of studies have analyzed p63 functions.
However, the role of p63 in tumors is not well understood.
Previously, p63 downregulation was demonstrated to increase cell
migration and invasiveness of cancer cell lines (20,21).
Carroll et al(27) reported
that decreased p63 causes the downregulation of cell
adhesion-associated genes. The loss of p63 expression in bladder
cancer is associated with progression to more invasive and
metastatic tumors (28).
The mechanism by which ΔNp63 downregulation
increases the invasiveness of cancer cells still needs to be
elucidated. Kommagani et al(24) found that the vitamin D receptor is
a direct target of ΔNp63-α inhibited cell invasion in A431 human
epidermoid carcinoma cell line. Fukushima et al(23) demonstrated that exogenous ΔNp63-α
expression attenuates invasiveness by downregulating N-cadherin
expression and ERK activity in bladder cancer. Decreased ΔNp63
expression accompanied by N-cadherin upregulation during
muscle-invasive recurrence of bladder cancer among patients with
ΔNp63 promotes the activity of bladder cancer cells. Therefore,
ΔNp63 regulates cancer cell connexins. Lopardo et
al(26) reported that
claudin-1 is an important p63 target protein in epithelial cell
development. Since claudin-1 is known to play a role in the
formation of tight junctions, its regulation by ΔNp63 was the focus
of the present study. Therefore, the expression of claudin-1 was
adjusted using ΔNp63 in bladder cancer cells.
In the current study, decreased ΔNp63 expression
resulted in decreased tumor invasiveness, consistent with previous
studies (21–24). In addition, claudin-1 expression
was downregulated by loss of ΔNp63 in bladder cancer cells. These
results may provide a new mechanism of action for ΔNp63 in the
invasiveness of bladder cancer. ΔNp63 is likely to promote a
crucial step in invasion by affecting claudin-1 expression. Further
studies must be conducted to determine the mechanism of ΔNp63
downregulation, which enables bladder cancer cells to become
invasive through claudin-1.
References
|
1
|
Signoretti S, Waltregny D, Dilks J, et al:
p63 is a prostate basal cell marker and is required for prostate
development. Am J Pathol. 157:1769–1775. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Signoretti S, Pires MM, Lindauer M, et al:
p63 regulates commitment to the prostate cell lineage. Proc Natl
Acad Sci USA. 102:11355–11360. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mills AA, Zheng B, Wang XJ, Vogel H, Roop
DR and Bradley A: p63 is a p53 homologue required for limb and
epidermal morphogenesis. Nature. 398:708–713. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Senoo M, Pinto F, Crum CP and McKeon F:
p63 is essential for the proliferative potential of stem cells in
stratified epithelia. Cell. 129:523–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacobs WB, Govoni G, Ho D, et al: p63 is
an essential proapoptotic protein during neural development.
Neuron. 48:743–756. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moll UM and Slade N: p63 and p73: roles in
development and tumor formation. Mol Cancer Res. 2:371–386.
2004.PubMed/NCBI
|
|
7
|
Mills AA: p63: oncogene or tumor
suppressor? Curr Opin Genet Dev. 16:38–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flores ER, Sengupta S, Miller JB, et al:
Tumor predisposition in mice mutant for p63 and p73: evidence for
broader tumor suppressor functions for the p53 family. Cancer Cell.
7:363–373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi K, Wu L, Caballero OL, et al:
Frequent gain of the p40/p51/p63 gene locus in primary head and
neck squamous cell carcinoma. Int J Cancer. 86:684–689. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park BJ, Lee SJ, Kim JI, et al: Frequent
alteration of p63 expression in human primary bladder carcinomas.
Cancer Res. 60:3370–3374. 2000.PubMed/NCBI
|
|
11
|
Choi HR, Batsakis JG, Zhan F, Sturgis E,
Luna MA and El-Naggar AK: Differential expression of p53 gene
family members p63 and p73 in head and neck squamous tumorigenesis.
Hum Pathol. 33:158–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dohn M, Zhang S and Chen X: p63alpha and
deltaNp63alpha can induce cell cycle arrest and apoptosis and
differentially regulate p53 target genes. Oncogene. 20:3193–3205.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ihrie RA, Marques MR, Nguyen BT, et al:
Perp is a p63-regulated gene essential for epithelial integrity.
Cell. 120:843–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Numa F, Hirabayashi K, Kawasaki K, et al:
Syndecan-1 expression in cancer of the uterine cervix: association
with lymph node metastasis. Int J Oncol. 20:39–43. 2002.PubMed/NCBI
|
|
15
|
Sawada N, Murata M, Kikuchi K, et al:
Tight junctions and human diseases. Med Electron Microsc.
36:147–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tobioka H, Isomura H, Kokai Y, Tokunaga Y,
Yamaguchi J and Sawada N: Occludin expression decreases with the
progression of human endometrial carcinoma. Hum Pathol. 35:159–164.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morin PJ: Claudin proteins in human
cancer: promising new targets for diagnosis and therapy. Cancer
Res. 65:9603–9606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaihara T, Kawamata H, Imura J, et al:
Redifferentiation and ZO-1 reexpression in liver-metastasized
colorectal cancer: possible association with epidermal growth
factor receptor-induced tyrosine phosphorylation of ZO-1. Cancer
Sci. 94:166–172. 2003. View Article : Google Scholar
|
|
19
|
Myal Y, Leygue E and Blanchard AA: Claudin
1 in breast tumorigenesis: revelation of a possible novel ‘claudin
high’ subset of breast cancers. J Biomed Biotechnol. 2010 May
13;(Epub ahead of print).
|
|
20
|
Tsukita S and Furuse M: Claudin-based
barrier in simple and stratified cellular sheets. Curr Opin Cell
Biol. 14:531–536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higashikawa K, Yoneda S, Tobiume K, et al:
Snail-induced down-regulation of DeltaNp63alpha acquires invasive
phenotype of human squamous cell carcinoma. Cancer Res.
67:9207–9213. 2007. View Article : Google Scholar
|
|
22
|
Barbieri CE, Tang LJ, Brown KA and
Pietenpol JA: Loss of p63 leads to increased cell migration and
up-regulation of genes involved in invasion and metastasis. Cancer
Res. 66:7589–7597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukushima H, Koga F, Kawakami S, et al:
Loss of DeltaNp63alpha promotes invasion of urothelial carcinomas
via N-cadherin/Src homology and collagen/extracellular
signal-regulated kinase pathway. Cancer Res. 69:9263–9270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kommagani R, Leonard MK, Lewis S, Romano
RA, Sinha S and Kadakia MP: Regulation of VDR by deltaNp63alpha is
associated with inhibition of cell invasion. J Cell Sci.
122:2828–2835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimomura Y, Wajid M, Shapiro L and
Christiano AM: P-cadherin is a p63 target gene with a crucial role
in the developing human limb bud and hair follicle. Development.
135:743–753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lopardo T, Lo Iacono N, Marinari B, et al:
Claudin-1 is a p63 target gene with a crucial role in epithelial
development. PLoS One. 3:e27152008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carroll DK, Carroll JS, Leong CO, et al:
p63 regulates an adhesion programme and cell survival in epithelial
cells. Nat Cell Biol. 8:551–561. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koga F, Kawakami S, Fujii Y, et al:
Impaired p63 expression associates with poor prognosis and
uroplakin III expression in invasive urothelial carcinoma of the
bladder. Clin Cancer Res. 9:5501–5507. 2003.
|