Introduction
Acute cardiovascular events, including myocardial
infarction, stroke and sudden death, which are induced by
atherosclerosis, remain the principal causes of morbidity and
mortality globally (1). Increasing
epidemiological evidence implies that inflammation contributes to
the development and progression of atherosclerotic disease.
However, the underlying mechanisms of atherosclerosis are not
clear. Recent data suggest that peroxisome proliferator-activated
receptor α (PPAR-α) has multiple effects, which may be beneficial
to alleviate atherosclerotic lesions.
The evolving view of atherosclerosis as a metabolic
complication has directed attention toward PPARs as nuclear
receptors which have three subtypes, PPAR-α, β/δ and γ (2). PPAR-α has been detected in the liver,
kidney, heart and skeletal muscle, since hepatocytes and smooth
muscle cells, as well as macrophages and endothelial cells, are
represented in these organs (3).
PPAR-α regulates fatty acid catabolism and glucose homeostasis,
while it inhibits cytokine-induced expression of vascular
cell-adhesion molecule-1 (VCAM-1), intercellular adhesion
molecule-1 (ICAM-1), C-reactive protein (CRP) and interleukin
(IL)-6 as a result of potentially modulating inflammation (4–9).
Among pro- and anti-inflammatory cytokines, tumor necrosis factor-α
(TNF-α) and IL-10 play predominant roles in the formation of
atherosclerosis (10). Another
problem we focus on in this study is P-selectin. A previous study
has shown that PPAR-α decreases platelet-derived growth factor-BB
(PDGF-BB) via suppression of the PDGF-B gene in bone marrow
megakaryocytes (11). PPAR-α
expression and its modulatory role in inflammation have been
studied under in vitro conditions. However, there are few
data on the expression and role of PPAR-α in vivo.
In order to further explore the expression and
mechanisms of PPAR-α, our objectives were to investigate: i) PPAR-α
expression in an atherosclerotic rabbit model of plaque rupture
induced by high-fat diet and balloon angioplasty; ii) the
correlation between PPAR-α and TNF-α, IL-10 and P-selectin.
Materials and methods
Animal model
Adult male New Zealand White rabbits weighing
2.0–2.5 kg (total n=35), were purchased from Shanghai Experimental
Animal Breeding Co., China. After adaptation to the environment for
1 week, New Zealand White rabbits were randomly divided into 7
groups: control (n=5), high-fat diet 6-week (n=5), high-fat diet
8-week (n=5), high-fat diet 10-week (n=5), high-fat diet +
balloon-injury 6-week (n=5), high-fat diet + balloon-injury 8-week
(n=5) and high-fat diet + balloon-injury 10-week (n=5). Rabbits
were housed in a temperature-, humidity- and light-controlled room
with free access to water. The control group was provided with a
normal diet, the high-fat group received only the high-fat diet,
and the high-fat diet + balloon-injury group received the high-fat
diet and balloon-injury. The high-fat diet was composed of
cholesterol (2%), lard (10%) and normal diet (88%). All experiments
were performed according to the Experimental Animal Center of
Shanghai First People’s Hospital (SYXK, Shanghai, 2009-0086) and
Use Committee guidelines.
Preparation of balloon injury
Rabbits (n=15) were allowed to adapt to the
environment for one week, and then femoral artery balloon-injury
was carried out after rabbits were fed a high-fat diet for 4 weeks.
Animals were anesthetized with an intravenous infusion of ketamine
(0.5ml/kg Ketalar; Parke-Davis, Detroit, MI, USA) and xylazine
(0.25ml/kg Rompun; Bayer, Leverkusen, Germany). Femoral artery
deendothelialization was induced by a 3-F Fogarty balloon catheter
(Baxter, Shanghai, China). Using ophthalmic scissors to cut a
V-shaped small hole in the artery wall, a balloon catheter (1:15
diluted heparin saline infiltration) was inserted retrograde into
the iliac artery to ~15 cm, with a connection to a 20-ml injector,
and ~10 ml air was injected (~2 atm) to fill the balloon. The
balloon was slowly pulled back to the femoral artery, and then the
stretch was repeated twice for 30 sec each time at an interval of 1
min, to ensure intimal injury. The catheter was removed, the
proximal end was ligated and the distal end was sutured with
subcutaneous tissue and skin. To prevent infection, penicillin
sodium liquid was used to clean wounds, and 400,000 units of
penicillin sodium was injected intramuscularly for 3 days after the
surgery.
Two weeks following the initial injury, 5 rabbits
were sacrificed from the high-fat diet + balloon-injury 6-week
group. The high-fat diet + balloon-injury 8-week group and high-fat
diet + balloon-injury 10-week group were created using the same
method.
Histological assessment of artery
damage
The artery was sliced transversely, and a
midventricular slice was fixed in 10% formalin for 24 h, embedded
in paraffin and cut into 3-mm sections for histological assessment.
Paraffin sections were stained with hematoxylin and eosin. The
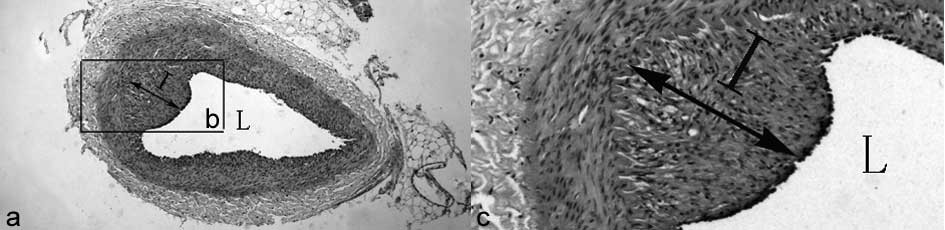
percentage of vessel wall lumen occlusion was calculated as 1 - [L
area/(I+L area) × 100] (L, lumen; I, intima), as described by a
previous study (12). The
calculation method is shown in Figure
1. All measurements were made by an investigator blinded to the
treatment and injury conditions.
Immunohistochemistry
Paraffin sections of the artery were deparaffinized,
and endogenous peroxidase activity was inactivated with 3%
H2O2 for 10 min. The primary antibody (mouse
anti-rabbit PPAR-α, no. NB300–537; Novus, Cambridge, UK) or normal
blocking serum was added and incubated overnight. Biotin-conjugated
goat anti-mouse immunoglobulin G (IgG) was used as the secondary
antibody and incubated for 30 min. An avidin-biotin enzyme reagent
was sequentially added and incubated for 20 min. A peroxidase
substrate was added and incubated until the desired stain intensity
developed. Finally, sections were covered with a glass coverslip
and observed using a light microscope. The intensity of positive
staining in tissue was analyzed by integrated optical density (IOD)
using Image-Pro Plus software (IPP; Media Cybernetics, Rockville,
MD, USA). Briefly, 4 ×20 TIF-format images from five individual
rabbits in each group were analyzed in a blinded manner. At the end
of the analysis, the IOD and area were obtained, as well as the
lumen area and internal elastic lamina area. The PPAR-α expression
determined by immunohistochemistry (IHC) was expressed as
(IOD/area) × 100 in accordance with a previous study (13).
Western blot analysis
Specimens were washed with ice-cold PBS and lysed
for 20 min on ice with lysis buffer. Following lysis (no. SC-003;
Invent, Lund, Sweden), the lysates were centrifuged for 4 min at
12,000 rpm, and the supernatants were collected in a fresh tube
kept on ice. Protein concentrations in each sample were determined
using a BCA assay. Total proteins (100 μg) were mixed with loading
buffer containing the anionic denaturing detergent sodium dodecyl
sulfate (SDS), boiled for 5 min, and then resolved by 10% SDS
polyacrylamide gel electrophoresis. The proteins were transferred
onto a PVDF membrane. After blocking the membrane in TBST
containing non-fat milk for 1 h at 4°C under agitation, the
membrane was washed three times in TBST and incubated for 2 h with
anti-rabbit PPAR-α antibody (1:200 dilution, no. NB300–537; Novus)
or GAPDH monoclonal antibody (1:200 dilution, no. 20028, Abmart
Company, Shanghai, China). After washing three times in TBST, the
membrane was incubated with HRP-conjugated goat anti-mouse IgG
(1:1,000) at room temperature for 1 h and then washed three times
with TBST. Immunobands were detected using a streptavidin
amplification reagent (no. WBKL SOO 50; Millipore, Billerica, MA,
USA) according to the manufacturer’s instructions.
RNA extraction and real-time PCR
Total RNA was extracted from the specimen arteries
using TRIzol® reagent according to the manufacturer’s
instructions (Invitrogen, Carlsbad, CA, USA). Total RNA (1 μg) was
used as a template to produce cDNA using a reverse transcription
kit (BioDev, Beijing, China). Real-time quantitative PCR was
performed by monitoring the increase in fluorescence of the
SYBR-Green dye using GreenMaster mix (Genaxxon BioScience, Ulm,
Germany) according to the manufacturer’s instructions. The primer
sequences used to amplify PPAR-α were 5′-gttccggtggcgttgat-3′
(sense) and 5′-gcggtcgcatttgtc-3′ (antisense). The primer sequences
used to amplify GAPDH were 5′-ccactttgtgaagctcatttcct-3′ (sense)
and 5′-tcgtcctcctctggtgctct-3′ (antisense). PCR amplification was
performed with Taq polymerase for 32 cycles at 95°C for 45
sec, 62°C for 30 sec, and 72°C for 1 min (for PPAR-α and GAPDH).
The 2−ΔΔCT method was used to determine the relative
change in PPAR-α and GAPDH gene expression. Values are expressed as
relative quantities (RQ) compared with mRNA expression in the
control group.
Enzyme-linked immunosorbent assay (ELISA)
for TNF-α, IL-10 and P-selectin
Fresh blood (3 ml) was taken from all animals via
the femoral vein and samples were centrifuged at 3,000 rpm for 10
min at 4°C. The supernatant was stored in a clean centrifuge tube
and frozen at −20°C. Concentrations of plasma TNF-α, IL-10 and
P-selectin were assayed with an ELISA kit (R&D, Minneapolis,
MN, USA) according to the manufacturer’s instructions. The minimum
detectable concentration of the kit was <1.0 pg/ml. The kit did
not cross-react with other soluble structural analogs.
Statistical analysis
The data were analyzed using the program SPSS 11.5
for Windows. Quantitative data are presented as the means ± SD. For
comparison between multiple groups, data were analyzed by ANOVA and
with the Student-Newman-Keuls post hoc analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Histological changes
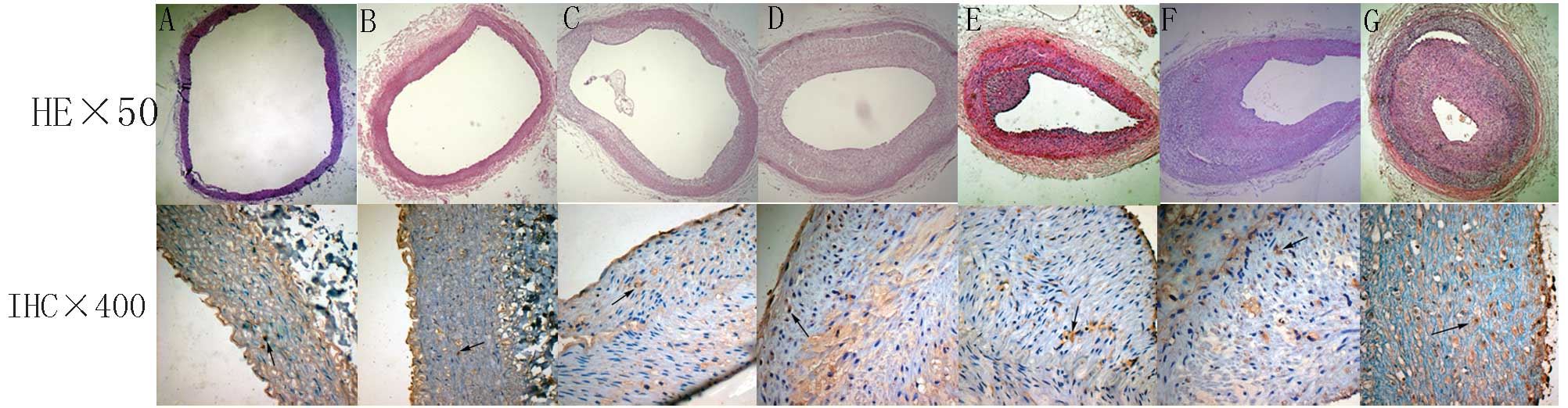
Hematoxylin and eosin-stained sections of aorta were
examined for signs of atheroma (Fig.
2). The femoral artery of the control group had the following
features: complete intimal endothelial cells arranged in neat rows;
clear and intact internal elastic membrane; medial smooth muscle
cell volume, size and arrangement were normal; and there was no
inflammatory cell infiltration and lipid accumulation. Arterial
lesions of the high-fat diet group show that the intimal membrane
is composed mainly of foam cells, a small amount of inflammatory
cell aggregation and no marked cap. In the high-fat diet +
balloon-injury 6-week group, there was a significant fibrous cap.
The fibrous cap had a few lipids, foam cells and other cell debris.
In the high-fat diet + balloon-injury 10-week group it was shown
that the fibrous cap contained smooth muscle cells, foam cells and
a lipid core in the intima, in addition to atherosclerotic lesions.
With the development of lesions, the fibrous cap of the
atherosclerotic material was gradually increased.
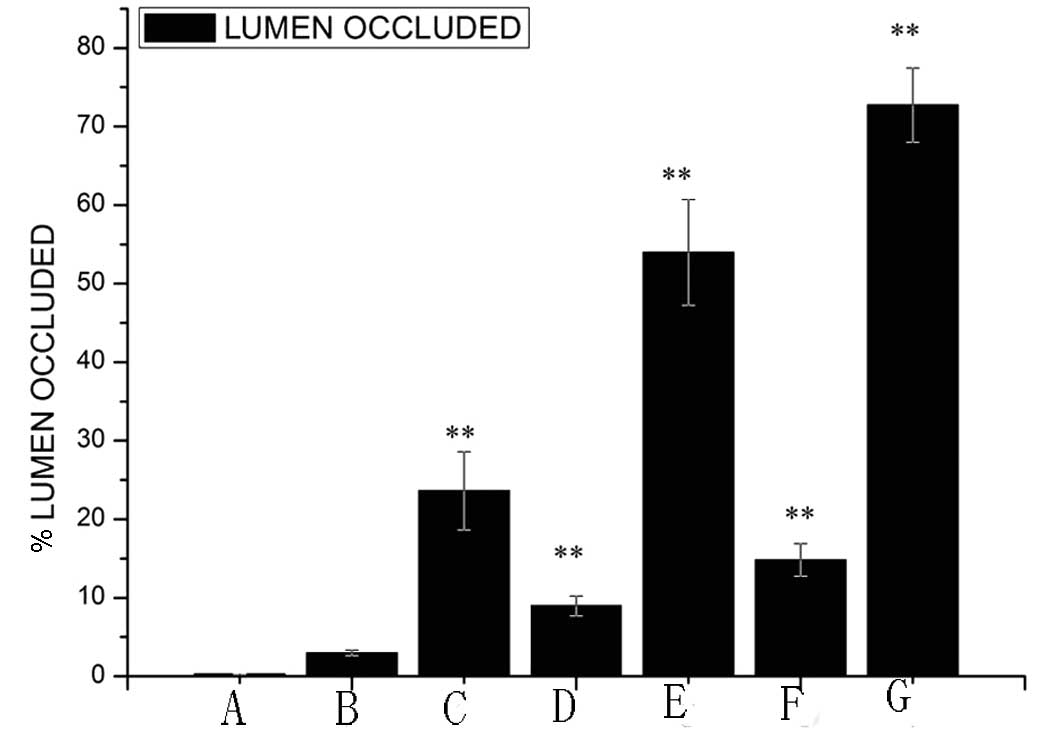
The percentage of vessel wall lumen occlusion was
calculated (Fig. 3). The results
showed that there was no marked occlusion of the lumen (0.30±0.03%,
mean ± SD) in the control group. There was a significant difference
between the control group and the high-fat diet 6-week group
(1.98±0.34%, P<0.05), high-fat diet 8-week group (8.97±1.2%,
P<0.01) and high-fat diet 10-week group (14.82±2.09%,
P<0.01). In the high-fat diet 10-week group, the percentage of
vessel wall lumen occlusion was higher than in the 6-week group.
Compared with the control group, the percentage of vessel wall
lumen occlusion in the high-fat diet + balloon-injury group was
markedly increased (P<0.01). The high-fat diet + balloon-injury
6-group had >23.62±4.97% occlusion of the lumen, the high-fat
diet + balloon-injury 8-week group had >53.97±6.7% occlusion of
the lumen and the high-fat diet + balloon-injury 10-week group had
>72.73±4.7% occlusion of the lumen. There was a significant
difference between the various timepoint groups (P<0.01).
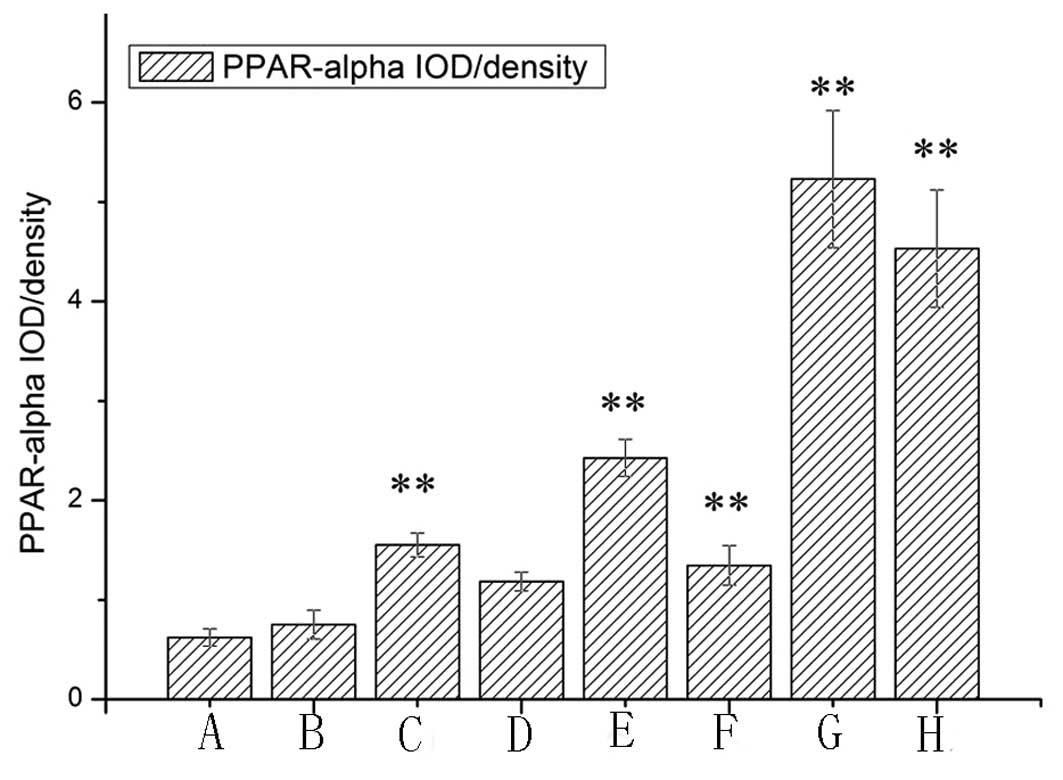
Location and expression of PPAR-α
protein
IHC showed that PPAR-α with high staining intensity
was seldom detected in the cytoplasm of the control group (Figs. 2 and 4). PPAR-α was detected at high staining
intensities in the majority of lesions. Positive staining of PPAR-α
was located in the cytoplasm of macrophages near the intima. In the
high-fat diet and high-fat diet + balloon-injury groups, PPAR-α was
higher than in the control group (P<0.01), with the exception of
the high-fat diet 6-week group (P>0.05). In the high-fat diet
group, PPAR-α increased significantly from weeks 6 to 8
(P<0.01). In the high-fat diet group, from weeks 8 to 10, PPAR-α
increased markedly (P<0.05). In the high-fat diet +
balloon-injury group, from weeks 6 to 10, PPAR-α increased
significantly (P<0.01).
According to the degree of occlusion, we divided the
high-fat diet + balloon-injury 10-week group into <60 and ≥60%
groups. In the <60% group, PPAR-α expression was higher than in
the ≥60% group (P<0.05).
Expression of PPAR-α protein
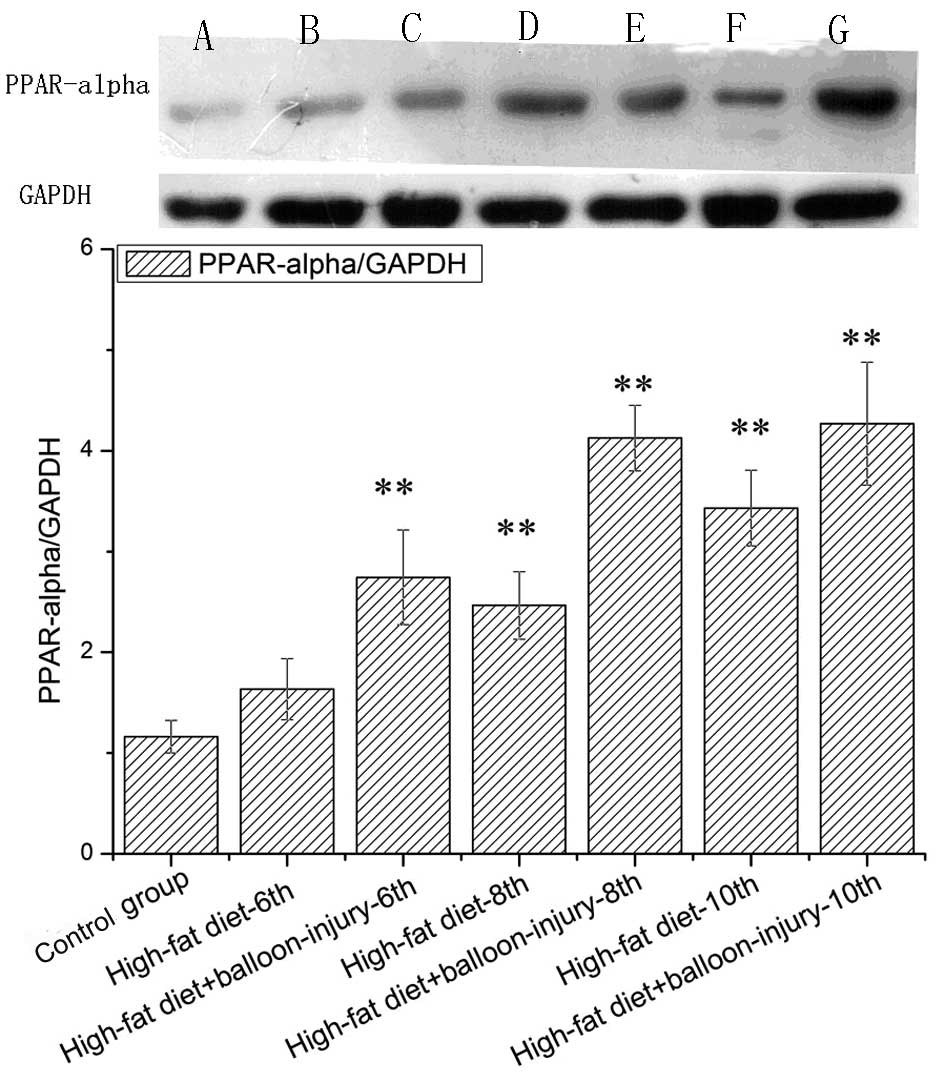
Western blotting was used to determine whether the
PPAR-α protein level correlated with changes in its mRNA levels
(Fig. 5). There was no difference
between the control group and the 6-week high-fat diet group
(P>0.05). In the high-fat diet group, PPAR-α expression was
higher than the control group at the 8- and 10-week points
(P<0.01). Furthermore, in the high-fat diet + balloon injury
group, PPAR-α protein was expressed at significantly higher levels
than in the control group. At the 10-week point, PPAR-α was 3.6
times more highly expressed compared with control group. From the
6-week point to the 8-week point, PPAR-α expression increased by
50.39%. No such change was observed from weeks 8 to 10
(P>0.05).
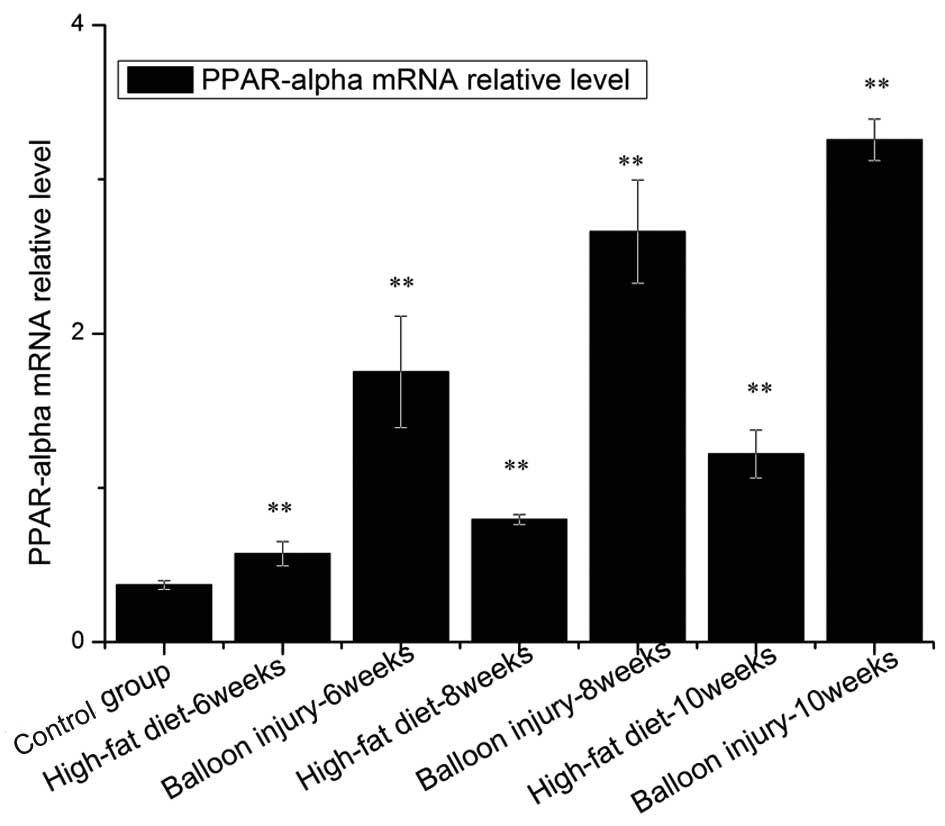
Expression of PPAR-α gene
The expression level of PPAR-α mRNA and protein
tended to increase in proportion to the severity of the lesion type
(Fig. 6). Compared with the
control group, PPAR-α mRNA was significantly increased in the
high-fat diet or balloon injury groups (P<0.01). In the high-fat
diet group, the increased level of PPAR-α was 7.18-fold higher
after rabbits were administered a 2% cholesterol diet for 6 weeks.
As shown in Fig. 6, in the
high-fat diet + balloon-injury group, the expression of PPAR-α was
increased by 8.7-fold at week 10. Moreover, in the high-fat diet
group, PPAR-α mRNA was more highly elevated at week 8 than at week
6 (P<0.01). PPAR-α mRNA at week 10 was higher than at week 8
(P<0.01). In the high-fat diet + balloon-injury group, we also
observed that PPAR-α was increased significantly from weeks 6 to 10
(P<0.01), while this change was larger than in the high-fat diet
group (P<0.01).
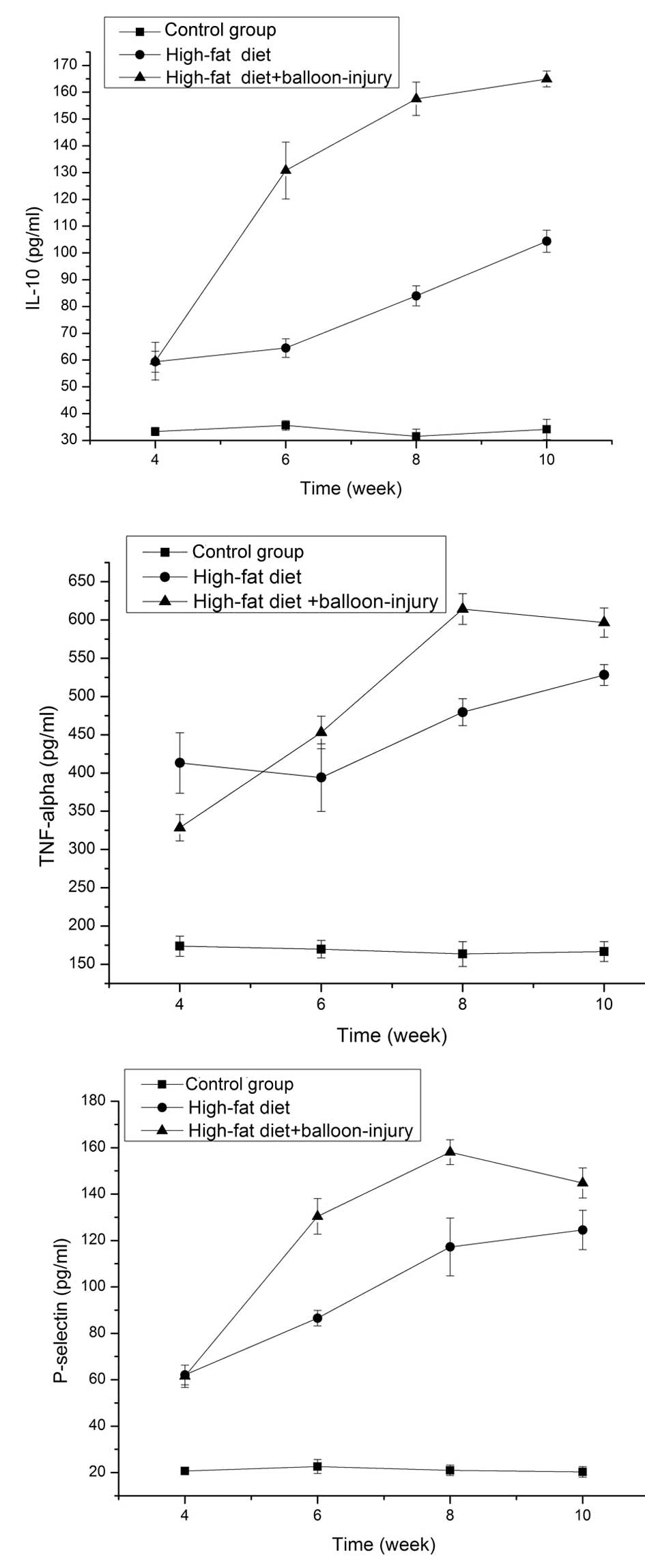
IL-10 in the serum
In the high-fat diet and high-fat diet +
balloon-injury groups, IL-10 levels were higher than in the control
group (P<0.01). In the high-fat diet group, IL-10 was higher at
week 4 than at week 6 (P<0.01). In addition, there was marked
growth from weeks 6 to 8 (P<0.01), as well as from weeks 8 to 10
(P<0.01). IL-10 increased to 3.31 times the control group level
at week 10. In the balloon-injury group, the expression level of
IL-10 was also significantly higher than in the control group
(P<0.01). From weeks 4 to 8, there was a significant difference
(P<0.01). However, the growth rate became slower between the
high-fat diet + balloon-injury 8-week group and the high-fat diet +
balloon-injury 10-week group (P<0.05). At 10 weeks, IL-10 was
5.25 times higher compared to the control group (Fig. 7).
TNF-α in the serum
In the high-fat diet and high-fat diet +
balloon-injury groups, TNF-α levels were higher than in the control
group (P<0.01). The expression of TNF-α showed no marked
difference between weeks 4 and 6 in the high-fat diet group
(P>0.05). From weeks 6 to 8, the level of TNF-α showed a
significant difference (P<0.01). In the high-fat diet 10-week
group, TNF-α was increased by 3.23-fold compared with the control
group. From weeks 4 to 8, the expression of TNF-α was increased in
the high-fat diet + balloon-injury group (P<0.01). From weeks 8
to 10, the level of TNF-α was decreased (P>0.05). At the 8-week
time point, TNF-α was increased by 3.75 times compared with the
control group (Fig. 7).
P-selectin in the serum
In the high-fat diet and high-fat diet +
balloon-injury groups, P-selectin levels were higher than in the
control group (P<0.01). In the high-fat diet group, the level of
P-selectin was significantly different between weeks 4 and 8
(P<0.01). However, the rate of growth decreased between the
high-fat diet 8-week and high-fat diet 10-week groups (P<0.05).
In the high-fat diet 10-week group, P-selectin was 6.13-fold
higher than in the control group. In the high-fat diet +
balloon-injury group, there was a significant difference between
weeks 4 and 8 (P<0.01). However, P-selectin was lower in the
high-fat diet + balloon-injury 10-week group than in the high-fat
diet + balloon-injury 8-week group (P<0.05). P-selectin was
7.78-fold higher than the control group in the high-fat diet +
balloon-injury 8-week group (Fig.
7).
Discussion
Due to atherosclerosis, cardiovascular disease is
currently the major cause of disability and mortality. Therefore,
investigating the etiopathogenesis and pathogenicity of
atherosclerosis and taking effective measures to delay and reverse
the progression of atherosclerosis have become important topics of
study. However, atherosclerosis has a complex multifactorial
pathophysiology and a number of risk factors work together to shape
the formation of plaques. Activated PPAR-α is important in the
regulation of cellular development and differentiation and the
metabolism of body fuel including carbohydrates, lipids and
proteins (13,14). Although PPAR-α has been
demonstrated to be involved in atherosclerosis, the potential
mechanisms of PPAR-α remain to be determined. The present study was
undertaken in order to test our hypothesis that PPAR-α activity may
have vascular protective effects in atherosclerosis. The major
finding of our present study was that PPAR-α activation played a
role in atherosclerosis, leading to partial inhibition of the
development of lesions. We further found that PPAR-α influenced
atherosclerosis via decreasing inflammation and P-selectin.
Collectively, our present observations highlight PPAR-α as a
promising therapeutic agent for the treatment of vascular injury in
a high-fat diet and balloon-injury model.
A previous study showed that lipolytic products are
capable of activating PPAR-α in brown adipocytes, thereby expanding
the oxidative capacity in order to match enhanced fatty acid supply
(15). Furthermore, activation of
PPAR-α has a critical role in controlling the cholesterol cycle in
macrophages. Scavenger receptor class B type I (SR-B I), which
plays a role in cholesterol efflux from macrophages, is upregulated
by PPAR-α ligands in macrophages, leading to increased lipolysis
(16). These collective effects of
PPAR-α should enhance transport of cholesterol from peripheral
tissues to the liver and promote proatherogenic responses. In this
study, we demonstrated that PPAR-α mRNA and protein were
significantly increased in high-fat diet or high-fat diet +
balloon-injury groups (P<0.01), as compared with the control
group. Furthermore, the expression of PPAR-α was significantly
higher in the high-fat diet + balloon-injury group than in the
high-fat diet group at the same time point. In the
immunohistochemical experiment, our results revealed that a high
expression of PPAR-α occurred in macrophages of the high-fat diet
and high-fat diet + balloon-injury groups, particularly in the
plaque shoulder, but the expression of PPAR-α was low in the
control group. Notably, in the high-fat diet + balloon-injury
10-week group we found that in the <60% group, PPAR-α was higher
than in the ≥60% group (P<0.01). In addition, Sueyoshi et
al(17) showed that PPAR-α
mRNA increased significantly in atherosclerosis and tended to
increase in proportion to the severity of the lesion, which is in
line with the findings of the present study. In our model, high
triglyceride load may cause PPAR-α activation. There is abundant
evidence that PPAR-α inhibited extracellular matrix metalloprotease
activity (18) and foam cell
formation (19). Our results
suggest that PPAR-α is involved in the chain of events leading to
atherosclerosis in a protective manner, and that combined therapy
including PPAR-α agonists may have a synergistic effect on
inhibiting atherosclerosis.
A complex network of inflammatory cytokines and
chemokines plays a major role in mediating, amplifying and
perpetuating the atherosclerosis process (20). Among pro- and anti-inflammatory
cytokines, TNF-α and IL-10 play critical roles in the formation of
atherosclerosis (21). As an
anti-inflammatory cytokine, IL-10 in atherosclerotic plaques in
mice and in the peripheral circulation of patients was higher
(22). Previous studies on certain
other inflammatory diseases, such as Alzheimer’s disease (AD) and
Crohn’s disease, found that PPAR-α and IL-10 have significant
interactions (23,24). On the contrary, TNF-α is an
important proinflammatory cytokine that may stimulate production of
a host of other cytokines (25).
Decreased TNF-α significantly improves endothelial and adipose
tissue dysfunction in pre-diabetic patients with coronary artery
disease (CAD) (26). The levels of
these cytokines were assessed in our study. The analyses
demonstrated that the concentration of IL-10 and TNF-α
significantly increased in high-fat diet and high-fat diet +
balloon-injury groups (P<0.01). Notably, the level of IL-10
increased between the 8th and 10th week in the high-fat diet +
balloon-injury group, while the level of TNF-α decreased. Based on
our results and other known characteristics of PPAR-α, we
hypothesize that IL-10 activation may occur with atherosclerosis,
which may explain some of the beneficial effects of PPAR-α on
vascular cells. PPAR-α may cause this anti-inflammatory effect
through TLR, MAPK and NF-κB pathways (27–30).
The absence of PPAR-α is capable of upregulating
P-selectin in acute pancreatitis induced by cerulean (31). However, the role of PPAR-α on
P-selectin in atherosclerosis remains to be elucidated. The
inflammatory response is the main process by which inflammatory
factors are involved in atherosclerotic lesions. During the
occurrence and development of atherosclerotic lesions, lipid
accumulation plays an important role which results from
inflammatory cells ingratiating. P-selectin is involved in these
two stages (32). There are few
data available on P-selectin and artery atherosclerosis. Our
experiments found that P-selectin levels were lower in the high-fat
diet + balloon-injury 10-week group than in the high-fat diet +
balloon-injury 8-week group (P<0.05). Anti-P-selectin therapy
may be a powerful tool in inhibiting atherosclerotic lesion
progression.
In conclusion, we demonstrated that PPAR-α was
associated with atheromatous plaque formation in the
hypercholesterolemic and balloon-injury model. PPAR-α expression
was an adaptive response that results in a slow progression of
atherosclerosis, although vascular occlusions were serious in the
high-fat diet + balloon-injury group. If the experimental time or
treatment with PPAR-α agonists is extended, the lesions may become
smaller. We further observed that PPAR-α protected against
atherosclerosis by increasing IL-10, suppressing TNF-α and
regulating P-selectin, although a detailed analysis of the
molecular mechanisms in atherosclerosis is required in order to
increase understanding. Previous studies have reported beneficial
effects of PPAR-α agonists such as fibrates, bezafibrate and
WY14643 (33,34). These results suggest that PPAR-α
agonists can be expected to protect against the progression of
atherosclerosis in hyperlipidemic patients.
Acknowledgements
The study was supported by the Shanghai Rising-Star
Program (08QA1404100 grant) and the National Natural Science
Foundation of China (30971265).
References
|
1
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medh JD: Peroxisome proliferator-activated
receptors in atherosclerosis. Curr Opin Lipidol. 10:69–71. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oyekan A: PPARs and their effects on the
cardiovascular system. Clin Exp Hypertens. 33:287–293. 2011.
View Article : Google Scholar
|
|
4
|
Shah A, Rader DJ and Millar JS: The effect
of PPAR-alpha agonism on apolipoprotein metabolism in humans.
Atherosclerosis. 210:35–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kraja AT, Province MA, Straka RJ, Ordovas
JM, Borecki IB and Arnett DK: Fenofibrate and metabolic syndrome.
Endocr Metab Immune Disord Drug Targets. 10:138–148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashizume S, Akaike M, Azuma H, et al:
Activation of peroxisome proliferator-activated receptor α in
megakaryocytes reduces platelet-derived growth factor-BB in
platelets. J Atheroscler Thromb. 18:138–147. 2011.
|
|
7
|
Wagner JD, Shadoan MK, Zhang L, et al: A
selective peroxisome proliferator-activated receptor alpha agonist,
CP-900691, improves plasma lipids, lipoproteins, and glycemic
control in diabetic monkeys. J Pharmacol Exp Ther. 333:844–853.
2010. View Article : Google Scholar
|
|
8
|
Marx N, Sukhova GK, Collins T, Libby P and
Plutzky J: PPARalpha activators inhibit cytokine-induced vascular
cell adhesion molecule-1 expression in human endothelial cells.
Circulation. 99:3125–3131. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mueller M, Hobiger S and Jungbauer A: Red
clover extract: a source for substances that activate peroxisome
proliferator-activated receptor alpha and ameliorate the cytokine
secretion profile of lipopolysaccharide-stimulated macrophages.
Menopause. 17:379–387. 2010.
|
|
10
|
Wofford JL, Kahl FR, Howard GR, McKinney
WM, Toole J and Crouse JR III: Relation of extent of extracranial
carotid artery atherosclerosis as measured by B-mode ultrasound to
the extent of coronary atherosclerosis. Arterioscler Thromb.
11:1786–1794. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buchanan MR and Brister SJ: Inhibition of
chronic vessel wall intimal hyperplasia following acute
anticoagulant treatment: relative effects of heparin and dermatan
sulphate. Thromb Res. 91:157–167. 1998. View Article : Google Scholar
|
|
12
|
Jia XL, Li SY, Dang SS, et al: Increased
expression of chondroitin sulphate proteoglycans in rat
hepatocellular carcinoma tissues. World J Gastroenterol.
18:3962–3976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Campioli E, Batarseh A, Li J and
Papadopoulos V: The endocrine disruptor mono-(2-ethylhexyl)
phthalate affects the differentiation of human liposarcoma cells
(SW 872). PLoS One. 6:e287502011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soymorphin-5, a soy-derived μ-opioid
peptide, decreases glucose and triglyceride levels through
activating adiponectin and PPARα systems in diabetic KKAy mice. Am
J Physiol Endocrinol Metab. 302:E433–E440. 2012.PubMed/NCBI
|
|
15
|
Mottillo EP, Bloch AE, Leff T and
Granneman JG: Lipolytic products activate peroxisome
proliferator-activated receptor (PPAR) α and δ in brown adipocytes
to match fatty acid oxidation with supply. J Biol Chem.
287:25038–25048. 2012.
|
|
16
|
Chinetti G, Gbaguidi FG, Griglio S, et al:
CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and
regulated by activators of peroxisome proliferator-activated
receptors. Circulation. 101:2411–2417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sueyoshi S, Mitsumata M, Kusumi Y, et al:
Increased expression of peroxisome proliferator-activated receptor
(PPAR)-alpha and PPAR-gamma in human atherosclerosis. Pathol Res
Pract. 206:429–438. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duhaney TAS, Cui L, Rude MK, et al:
Peroxisome proliferator-activated receptor alpha-independent
actions of fenofibrate exacerbates left ventricular dilation and
fibrosis in chronic pressure overload. Hypertension. 49:1084–1094.
2007. View Article : Google Scholar
|
|
19
|
Dushkin M, Khoshchenko O, Posokhova E and
Schvarts YS: Agonists of PPAR-alpha, PPAR-gamma, and RXR inhibit
the formation of foam cells from macrophages in mice with
inflammation. Bull Exp Biol Med. 144:713–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stoll G and Bendszus M: Inflammation and
atherosclerosis: novel insights into plaque formation and
destabilization. Stroke. 37:1923–1932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kabłak A, Ziembicka TP, Stępień E, et al:
Relationship between carotid intima-media thickness, cytokines,
atherosclerosis extent and a two-year cardiovascular risk in
patients with arteriosclerosis. Kardiol Pol. 69:1024–1031.
2011.PubMed/NCBI
|
|
22
|
Meng X, Zhang K, Li J, et al: Statins
induce the accumulation of regulatory T cells in atherosclerotic
plaque. Mol Med. 18:598–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heun R, Kölsch H, Ibrahim-Verbaas CA, et
al: Interactions between PPAR-α and inflammation-related cytokine
genes on the development of Alzheimer’s disease, observed by the
Epistasis Project. Int J Mol Epidemiol Genet. 3:39–47. 2012.
|
|
24
|
Lee JW, Bajwa PJ, Carson MJ, et al:
Fenofibrate represses interleukin-17 and interferon-gamma
expression and improves colitis in interleukin-10-deficient mice.
Gastroenterology. 133:108–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Donnelly SC, Strieter RM, Reid PT, et al:
The association between mortality rates and decreased
concentrations of interleukin-10 and interleukin-1 receptor
antagonist in the lung fluids of patients with the adult
respiratory distress syndrome. Ann Intern Med. 125:191–196. 1996.
View Article : Google Scholar
|
|
26
|
Rizza S, Cardellini M, Porzio O, et al:
Pioglitazone improves endothelial and adipose tissue dysfunction in
pre-diabetic CAD subjects. Atherosclerosis. 215:180–183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parameswaran N and Patial S: Tumor
necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene
Expr. 20:87–103. 2010.
|
|
28
|
Hou X, Shen YH, Li C, et al: PPARalpha
agonist fenofibrate protects the kidney from hypertensive injury in
spontaneously hypertensive rats via inhibition of oxidative stress
and MAPK activity. Biochem Biophys Res Commun. 394:653–659. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rinaldi B, Donniacuo M, Esposito E, et al:
PPARα mediates the anti-inflammatory effect of simvastatin in an
experimental model of zymosan-induced multiple organ failure. Br J
Pharmacol. 163:609–623. 2011.
|
|
30
|
Matta R, Barnard JA, Wancket LM, et al:
Knockout of Mkp-1 exacerbates colitis in Il-10-deficient mice. Am J
Physiol Gastrointest Liver Physiol. 302:G1322–G1335. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Genovese T, Mazzon E, Di Paola R, et al:
Role of peroxisome proliferator-activated receptor-alpha in acute
pancreatitis induced by cerulein. Immunology. 118:559–570.
2006.PubMed/NCBI
|
|
32
|
Johnson RC, Chapman SM, Dong ZM, et al:
Absence of P-selectin delays fatty streak formation in mice. J Clin
Invest. 99:1037–1043. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koh KK, Han SH, Quon MJ, Yeal Ahn J and
Shin EK: Beneficial effects of fenofibrate to improve endothelial
dysfunction and raise adiponectin levels in patients with primary
hypertriglyceridemia. Diabetes Care. 28:1419–1424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakamachi T, Nomiyama T, Gizard F, et al:
PPARalpha agonists suppress osteopontin expression in macrophages
and decrease plasma levels in patients with type 2 diabetes.
Diabetes. 56:1662–1670. 2007. View Article : Google Scholar : PubMed/NCBI
|