Introduction
Isoflavones are a flavonoid subclass that includes
daidzein (7,4-dihydroxyisoflavone) and genistein
(4′,5,7-trihydroxyisoflavone), which are the predominant
isoflavones in legumes, such as soybeans, and are commonly found in
a variety of human foods (1,2).
Several epidemiological studies have revealed an inverse
association between isoflavone consumption and the risk of breast
cancer in Asians, indicating that dietary intervention may reduce
the risk of breast cancer (3–5).
Based on this hypothesis, a number of studies have
investigated the pharmacological activities of isoflavones as
candidate anticancer agents. Many have reported that isoflavones
significantly inhibit breast cancer cell growth in
vitro(6–8), and that this growth inhibition
stimulates the signal transduction pathway leading to apoptosis.
Moreover, our previous studies support the theory that isoflavones,
including genistein and daidzein, exhibit anticancer activity
against breast cancer both in vivo(9,10)
and in vitro(11,12).
Breast cancer is one of the cancers most frequently
diagnosed in women. According to a US National Cancer Institute
(NCI) report, one in eight women in the US (~13.3%) will develop
breast cancer during their lifetime (13,14).
Breast cancer is a group of heterogeneous diseases that manifest in
several clinical, molecular and histopathological forms; this makes
effective chemotherapy difficult. The response to a cancer drug may
differ according to biological factors including estrogen receptor
(ER) status, progesterone receptor status and c-erbB-2 expression.
The present study compared the anticancer activity of daidzein and
genistein via their effect on ERα and c-erbB-2 expression in breast
cancer cell lines.
Materials and methods
Cell culture
Human breast cancer MCF-7, SK-BR-3 and ZR-75-1 cell
lines were purchased from the KCLB (Korean Cell Line Bank, Seoul,
Korea) and were used in the present study. Each cell line was
routinely maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS) and antibiotics
(50 U/ml penicillin and 50 μg/ml streptomycin; Gibco, Carlsbad, CA,
USA) at 37°C in a humidified atmosphere containing 5%
CO2. Each cell type was plated at a density of
0.8–1.2×106 cells/well and 2.5–5.0×103
cells/well on 6- and 96-well plates for
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and immunoblotting assays, respectively. Daidzein and genistein
were purchased from LC Laboratories® (Woburn, MA, USA)
and dissolved in dimethyl sulfoxide (DMSO; final concentration 0.1%
in medium).
MTT assay
Each cell type was exposed to daidzein or genistein
at 1, 10, 100 and 200 μM for 72 h. Following treatment, the plated
cells were incubated with MTT (0.5 mg/ml final concentration) for 4
h at 37°C. After discarding the medium from the plates, 100 μl DMSO
was added to each well. The plates were placed for 5 min at room
temperature with agitation, so that complete dissolution of
formazan was achieved. The absorbance of the MTT formazan was
determined at 540 nm by a UV/VIS spectrophotometric plate reader
(Emax; Molecular Devices, Silicon Valley, CA, USA).
Immunoblotting assay
Each cell type was exposed to daidzein or genistein
at 1, 10, 100 and 200 μM for 72 h. Cells were lysed in RIPA buffer
(1% NP-40, 150 mM NaCl, 0.05% DOC, 1% SDS, 50 mM Tris, pH 7.5)
containing protease inhibitor for 1 h at 4°C. The supernatant was
separated by centrifugation and the protein concentration was
determined by Bradford protein assay kit II (Bio-Rad Laboratories,
Hercules, CA, USA). Proteins were transferred onto nitrocellulose
membranes (0.45 μm). The membranes were blocked with a 1% bovine
serum albumin (BSA) solution for 1.5 h and washed twice with
phosphate-buffered saline (PBS) containing 0.2% Tween-20 and
incubated with the respective primary antibodies (ERα, C-erbB-2,
β-actin; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C. The next day, the immunoreaction was continued
with the secondary rabbit anti-rabbit horseradish
peroxidase-conjugated antibody after washing for 2 h at room
temperature. The specific protein bands were detected using an
Opti-4CN Substrate kit (Bio-Rad Laboratories).
Apoptosis detection
Apoptotic morphological changes were determined by
DAPI (4′,6-diamidino-2-phenyl-indole) staining on ZR-75-1 cells
exposed to daidzein or genistein at a concentration of 100 μM for
72 h. After harvesting the cells exposed to daidzein or genistein,
the cells were seeded onto poly-l-lysine-coated slides and fixed
with 4% methanol-free formaldehyde solution for 30 min. Mounting
medium with DAPI (Molecular Probes, Invitrogen) was then dispersed
over the entire section of slides. Mounted slides were stored at
4°C without light. Each slide was observed under an Axio vision 4.0
fluorescence microscope (Carl Zeiss Inc., New York, NY, USA).
Statistical analysis
The values are expressed as the means ± standard
deviation (SD). Data were analyzed by unpaired Student’s t-test or
one-way analysis of variance followed by Dunnett’s multiple
comparison test (SigmaStat, San Jose, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
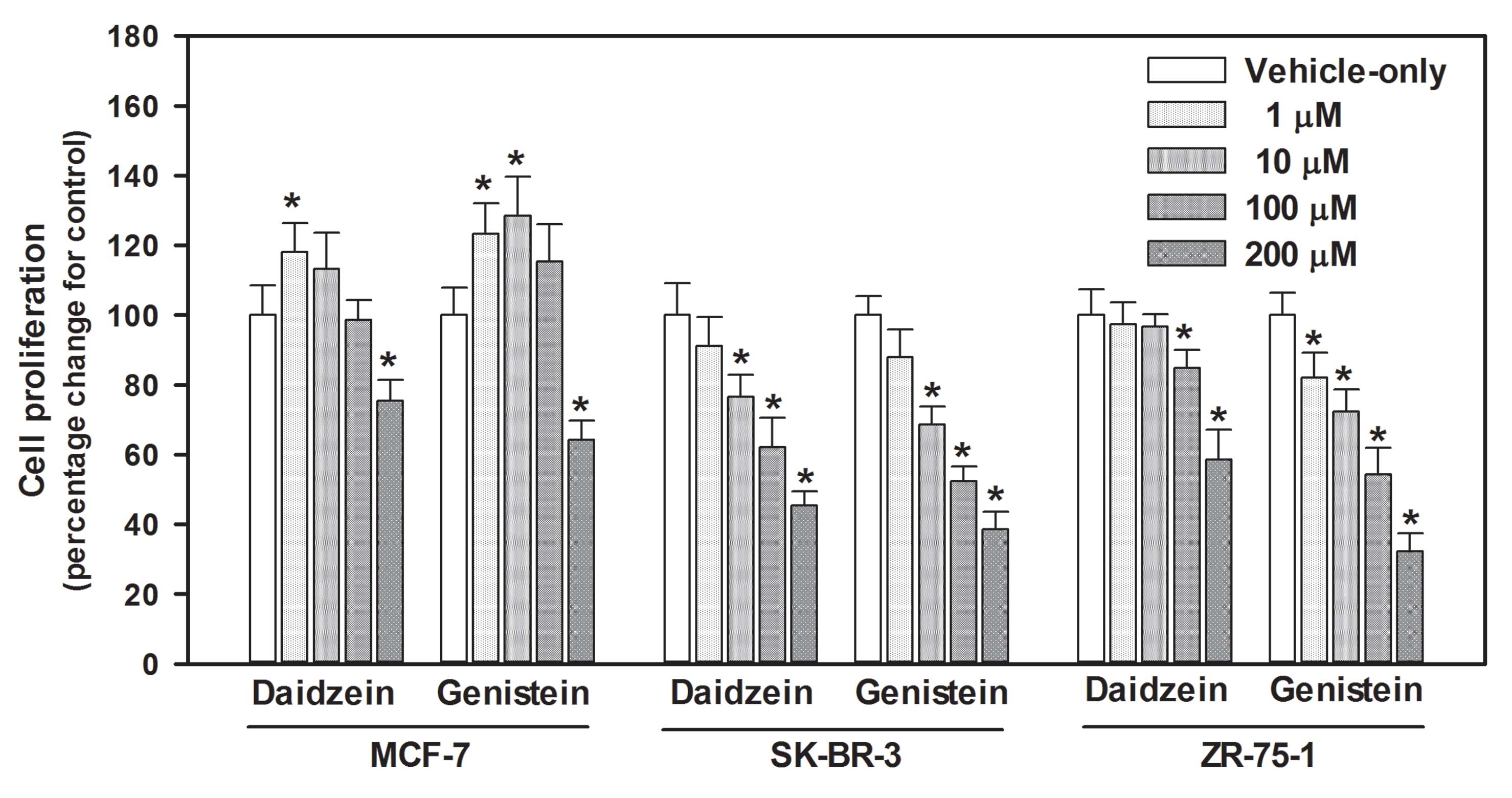
Antiproliferative activities
We compared the antiproliferative effects of
daidzein and genistein on MCF-7, SK-BR-3 and ZR-75-1 cells
(Fig. 1). Exposure of MCF-7 cells
to various concentrations (1, 10, 100 and 200 μM) of daidzein and
genistein for 72 h resulted in similar biphasic antiproliferative
effects. Although daidzein and genistein at higher concentrations
(200 μM) inhibited proliferation by 24.64 and 35.79%, respectively,
compared with the control, both compounds stimulated cell growth at
lower concentrations (<10 μM). Daidzein and genistein (1 and 10
μM, respectively) induced cell growth by ~18 and 28%,
respectively.
The two compounds greatly inhibited growth of
SK-BR-3 cells in a dose-dependent manner. Statistically significant
inhibition of cell proliferation was first observed in cells
treated with 10 μM daidzein and genistein (23.46 and 35.79%
inhibition, respectively). The IC50 values for daidzein
and genistein were 211.70 and 138.13 μM, respectively.
In ZR-75-1 cells, the antiproliferative effects of
genistein were stronger than those of daidzein under the same
conditions. Genistein significantly inhibited proliferation of
ZR-75-1 cells in a dose-dependent manner; the effect was similar to
that of genistein on SK-BR-3 cells. Daidzein significantly
inhibited cell growth at 100 and 200 μM, but not at concentrations
<10 μM. Inhibition of proliferation by higher daidzein
concentrations was decreased by 15–41% compared with control
levels.
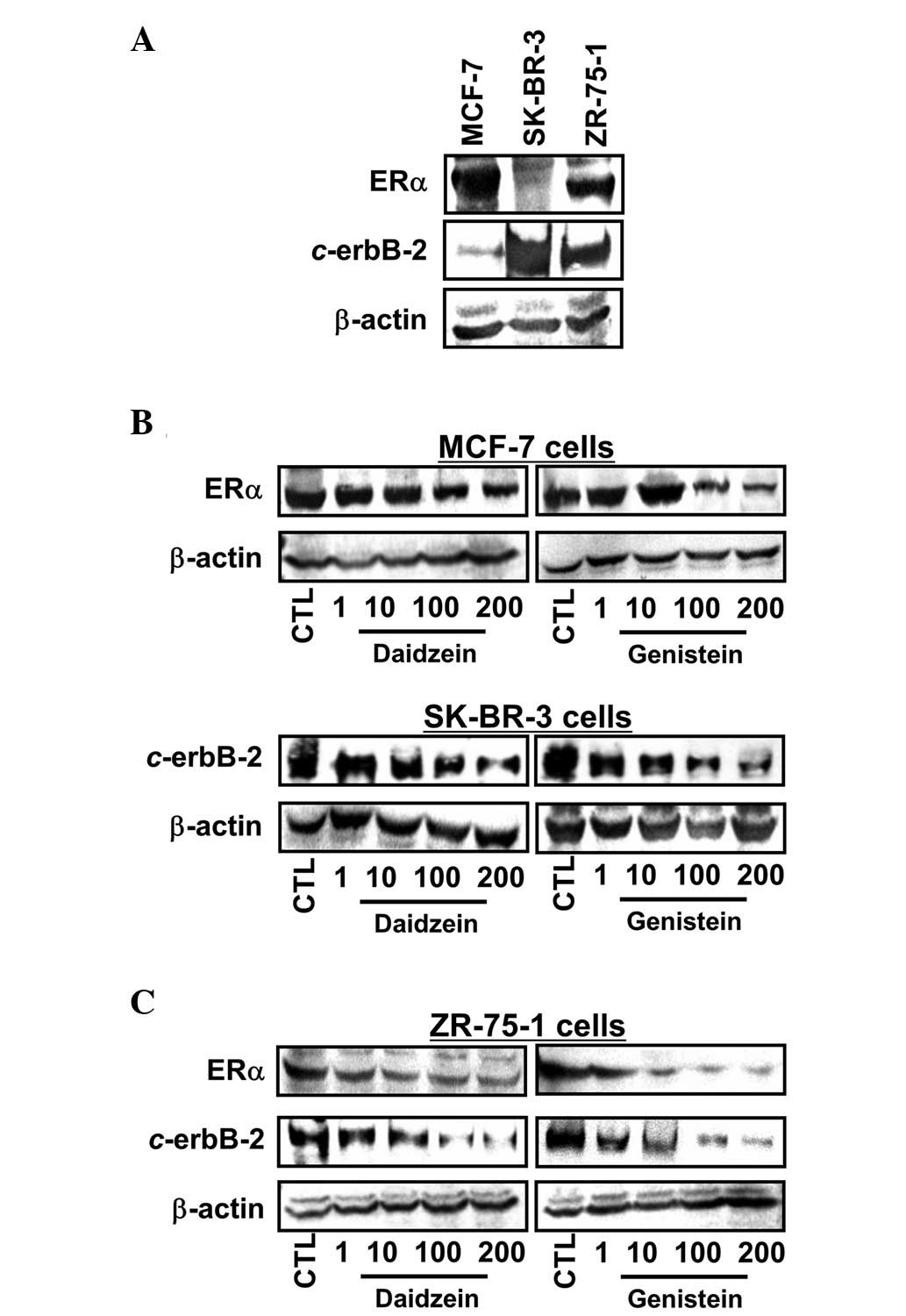
ERα and c-erbB-2 expression
ERα and c-erbB-2 expression was determined in the
three cell lines (Fig. 2A).
ERα-positive MCF-7 cells exhibited a basal level of c-erbB-2, while
SK-BR-3 cells overexpressed c-erbB-2. In ZR-75-1 cells, expression
of ERα and c-erbB-2 was observed. As presented in Fig. 2B and C, when cells were exposed to
1, 10, 100 and 200 μM daidzein and genistein for 72 h, ERα
expression by MCF-7 cells exposed to genistein showed a pattern
similar to the antiproliferative effects and no stimulation by
lower daidzein concentrations was detected. However, both daidzein
and genistein reduced ERα expression at higher concentrations (100
and 200 μM). Notably, ERα expression in ZR-75-1 cells was
downregulated by daidzein and genistein at the concentrations used;
again, the effect of genistein was greater. c-erbB-2 expression was
decreased by daidzein and genistein in a dose-dependent manner in
SK-BR-3 and ZR-75-1 cells, regardless of their basal c-erbB-2
levels.
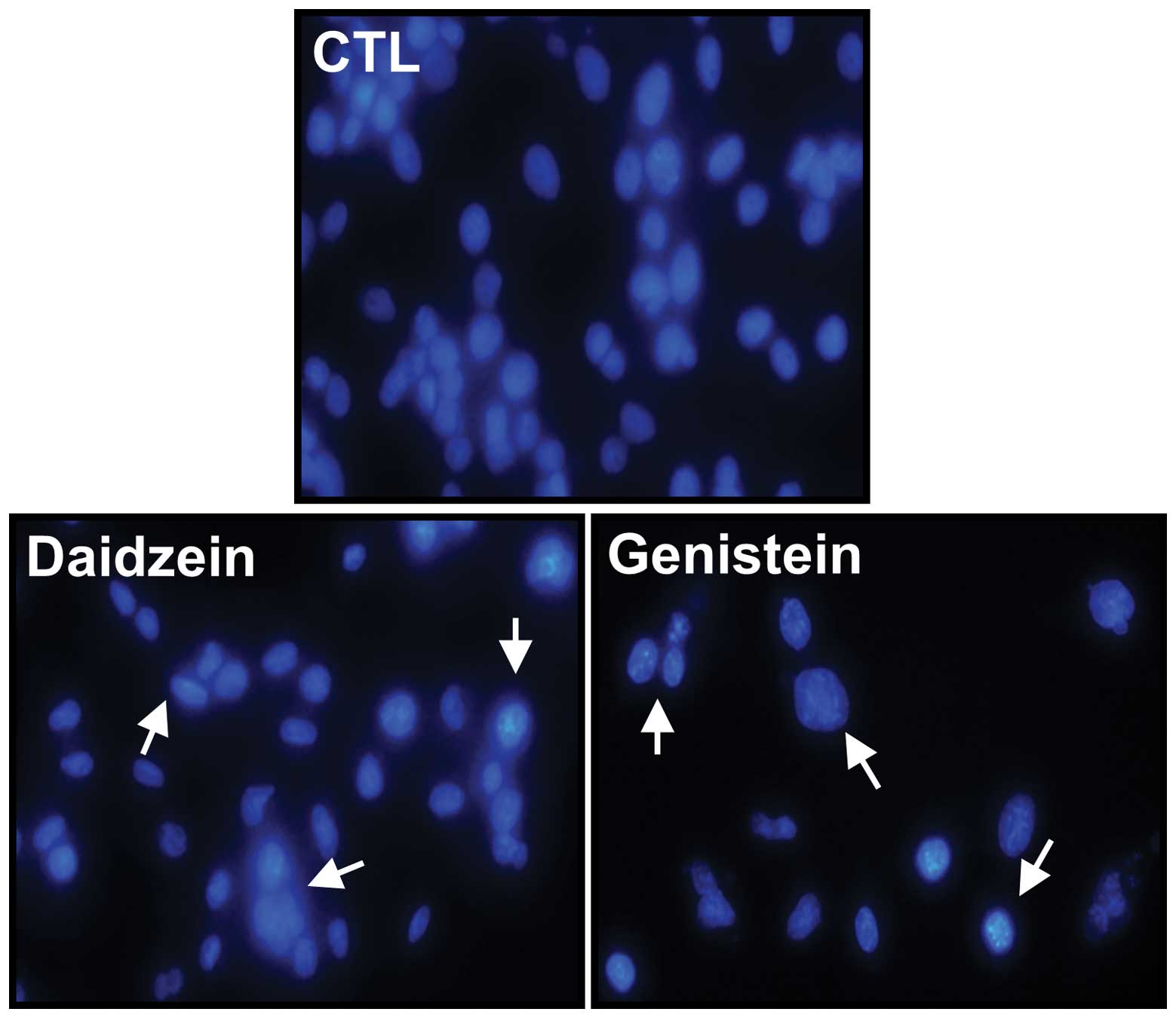
Apoptosis induction
DAPI staining was used to detect apoptotic changes
after exposing ZR-75-1 cells to either daidzein or genistein at a
concentration of 100 μM for 72 h (Fig.
3). Apoptotic morphological features, such as cell shrinkage
and dot-shaped nuclear fragments, were observed following exposure
of cells to daidzein and genistein. Compared with control cells,
exposure to genistein resulted in a marked increase in apoptotic
morphological features.
Discussion
In this study, we investigated the antiproliferative
effects of the isoflavones daidzein and genistein in the following
three breast cancer cell lines: MCF-7 (ERα+), SK-BR-3
(c-erbB-2++) and ZR-75-1 (ERα+ and
c-erbB-2+).
The effects differed greatly according to breast
cancer cell type. Daidzein and genistein exhibited a significant
antiproliferative effect against the three cell lines, however, the
effect on MCF-7 cells was biphasic (inhibitory at high
concentrations and stimulatory at low concentrations). This is
consistent with previous studies that revealed isoflavones exert a
biphasic effect on cell proliferation (15–17),
which may be explained by their estrogen-like activity. Isoflavones
are known as phytoestrogens, which are molecules structurally
similar to 17β-estradiol (18).
This characteristic may cause not only ER-agonistic but also
ER-antagonistic effects and was likely responsible for the biphasic
effect on MCF-7 cell proliferation. This mechanism may prove useful
in the prevention of ER-positive breast cancer. ERα is a member of
the steroid receptor superfamily and regulates growth,
differentiation and other processes in various target cells by
regulating transcriptional processes (19). It is also important in the
development and progression of breast cancer (20) and is expressed in ~70% of breast
cancers (21), which makes it
difficult to obtain a response to cancer drug treatment.
By contrast, daidzein and genistein significantly
inhibited the growth of SK-BR-3 and ZR-75-1 cells in a
dose-dependent manner under the same conditions; however, the
antiproliferative effects of daidzein were relatively weak.
Genistein had a stronger effect on ERα-positive SK-BR-3 cells than
ERα- and c-erbB-2-positive ZR-75-1 cells; the IC50
values were 138.13 and 127.71 μM in SK-BR-3 and ZR-75-1 cells,
respectively. c-erbB-2 (HER2/neu) encodes a 185-kDa transmembrane
glycoprotein that belongs to the epidermal growth factor receptor
family of type I receptor tyrosine kinases (ErbB family). c-erbB-2
overexpression has been reported in ~25–30% of human breast cancers
(22,23). Overall, genistein exhibited an
effect on the three cell lines; the gap between stimulation and
inhibition of proliferation of MCF-7 cells exposed to genistein was
larger than that of daidzein and the antiproliferative effects on
the other cell lines were more marked. This may be due to the
estrogenic activity of genistein, which has a stronger affinity for
ERs than daidzein (12).
Moreover, when cell lines were exposed to daidzein
and genistein under the same conditions, the modulation of ERα and
c-erbB-2 expression by daidzein and genistein reflected their
pattern of antiproliferative activity. Induction of ERα expression
in MCF-7 cells exposed to <10 μM daidzein was unclear; at higher
concentrations, daidzein downregulated the ERα expression. ER
expression in ZR-75-1 cells was also slightly reduced by daidzein,
regardless of dose. Similar to the pattern of MCF-7 cell
proliferation by genistein, its application at a lower
concentration stimulated ERα expression; by contrast, genistein at
a higher concentration markedly reduced this expression. This
reduction was also observed in ZR-75-1 cells exposed to genistein,
even at a lower concentration (1 μM).
c-erbB-2 expression by SK-BR-3 and ZR-75-1 cells was
decreased by daidzein or genistein, with the latter having a
greater effect. It has been reported that the mechanism underlying
the anticancer activity of genistein and daidzein involves
downregulation of c-erbB-2 (24,25)
and enhanced ER-erbB-2 cross-talk in breast cancer cells (26).
The isoflavones daidzein and genistein induced
apoptosis as evidenced by the apoptotic morphological features that
were observed in cells exposed to daidzein and genistein. Several
studies have indicated that anticancer drugs or cancer
chemopreventive agents act through the induction of apoptosis in
various cancer cells (27,28). Recently, the regulation of
apoptosis has been proposed as a promising target for cancer
chemotherapy. Our data suggest that daidzein and genistein may
possess anticancer properties.
Based on our results, although both daidzein and
genistein may be deleterious at lower concentrations by stimulating
proliferation of MCF-7 cells, genistein may offset those effects
and could be beneficial for breast cancer prevention under certain
conditions. Genistein at higher concentrations had a marked
anti-estrogenic effect via ERα-downregulation and the
growth-promotion effect of genistein was attenuated in ERα and
c-erbB-2-coexpressing ZR-75-1 cells via downregulation of
c-erbB-2.
Acknowledgements
This study was supported by the Basic Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education, Science and Technology
(2012-0006811 and 2012-041653).
References
|
1
|
Liggins J, Bluck LJ, Runswick S, et al:
Daidzein and genistein contents of vegetables. Br J Nutr.
84:717–725. 2000.PubMed/NCBI
|
|
2
|
Liggins J, Bluck LJ, Runswick S, et al:
Daidzein and genistein content of fruits and nuts. J Nutr Biochem.
11:326–331. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwasaki M, Inoue M, Otani T, et al: Plasma
isoflavone level and subsequent risk of breast cancer among
Japanese women: a nested case-control study from the Japan Public
Health Center-based prospective study group. J Clin Oncol.
26:1677–1683. 2008. View Article : Google Scholar
|
|
4
|
Lampe JW, Nishino Y, Ray RM, et al: Plasma
isoflavones and fibrocystic breast conditions and breast cancer
among women in Shanghai, China. Cancer Epidem Biomarkers Prev.
16:2579–2586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu LJ, Anderson KE, Grady JJ, et al:
Decreased ovarian hormones during a soya diet: implications for
breast cancer prevention. Cancer Res. 60:4112–4121. 2000.PubMed/NCBI
|
|
6
|
Vissac-Sabatier C, Bignon YJ and
Bernard-Gallon DJ: Effects of the phytoestrogens genistein and
daidzein on BRCA2 tumor suppressor gene expression in breast cell
lines. Nutr Cancer. 45:247–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Lemos ML: Effects of soy phytoestrogens
genistein and daidzein on breast cancer growth. Ann Pharmacother.
35:1118–1121. 2001.PubMed/NCBI
|
|
8
|
Pugazhendhi D, Watson KA, Mills S, et al:
Effect of sulphation on the oestrogen agonist activity of the
phytoestrogens genistein and daidzein in MCF-7 human breast cancer
cells. J Endocrinol. 197:503–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi EJ and Kim GH: Hepatoprotective
effects of daidzein against 7,12-dimetylbenz[a]anthracene-induced
oxidative stress in mice. Int J Mol Med. 23:659–664.
2009.PubMed/NCBI
|
|
10
|
Choi EJ and Lee BH: Evidence for genistein
mediated cytotoxicity and apoptosis in rat brain. Life Sci.
75:499–509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi EJ, Kim T and Lee MS: Pro-apoptotic
effect and cytotoxicity of genistein and genistin in human ovarian
cancer SK-OV-3 cells. Life Sci. 80:1403–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi EJ and Kim GH: Daidzein causes cell
cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7
and MDA-MB-453 cells. Phytomedicine. 15:683–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Breast Cancer Foundation.
http://www.nationalbreastcancer.org/about-breast-cancer/breast-cancer-symptoms.
Accessed, March 2011
|
|
14
|
National Cancer Institute. SEER Cancer
Statistics Review, 1975–2002. http://seer.cancer.gov/csr/1975_2002/.
Accessed, March 2005
|
|
15
|
Guo JM, Xiao BX, Liu DH, et al: Biphasic
effect of daidzein on cell growth of human colon cancer cells. Food
Chem Toxicol. 42:1641–1646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El Touny LH and Banerjee PP:
Identification of a biphasic role for genistein in the regulation
of prostate cancer growth and metastasis. Cancer Res. 69:3695–3703.
2009.PubMed/NCBI
|
|
17
|
Hsu JT, Hung HC, Chen CJ, et al: Effects
of the dietary phytoestrogen biochanin A on cell growth in the
mammary carcinoma cell line MCF-7. J Nutr Biochem. 10:510–517.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sirtori CR, Arnoldi A and Johnson SK:
Phytoestrogens: end of a tale? Ann Med. 37:423–438. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Björnström L and Sjöberg M: Mechanisms of
estrogen receptor signaling: convergence of genomic and nongenomic
actions on target genes. Mol Endocrinol. 19:833–842.
2005.PubMed/NCBI
|
|
20
|
Sperelakis N: Properties of calcium
channels in cardiac muscle and vascular smooth muscle. Mol Cell
Biochem. 99:97–109. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Plaza-Menacho I, Morandi A, Robertson D,
Pancholi S, et al: Targeting the receptor tyrosine kinase RET
sensitizes breast cancer cells to tamoxifen treatment and reveals a
role for RET in endocrine resistance. Oncogene. 29:4648–4657. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slamon DJ, Clark GM, Wong SG, et al: Human
breast cancer: correlation of relapse and survival with
amplification of the HER-2/neu oncogene. Science. 235:177–182.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Bhuiyan M and Sarkar FH: Induction
of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by
genistein. Int J Oncol. 15:525–533. 1999.PubMed/NCBI
|
|
25
|
Kim EJ, Shin HK and Park JH: Genistein
inhibits insulin-like growth factor-I receptor signaling in HT-29
human colon cancer cells: a possible mechanism of the growth
inhibitory effect of Genistein. J Med Food. 8:431–438. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang X, Yang S, McKimmey C, et al:
Genistein induces enhanced growth promotion in
ER-positive/erbB-2-overexpressing breast cancers by ER-erbB-2 cross
talk and p27/kip1 downregulation. Carcinogenesis. 31:695–702. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grimm D, Wehland M, Pietsch J, et al:
Drugs interfering with apoptosis in breast cancer. Curr Pharm Des.
17:272–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin FP and Zhang M: Progress of
experimental researches on Chinese herbal compounds for inducing
tumor cell apoptosis. Chin J Integr Med. 16:565–571. 2010.
View Article : Google Scholar : PubMed/NCBI
|