Introduction
Osteoarthritis (OA), a public health concern that
causes the most chronic disability in middle-aged and older
individuals in modern society, is a degenerative joint disease that
is characterized by a progressive loss of articular cartilage
(1,2). Chondrocytes, the only types of cell
present in cartilage, play a central role in the equilibrium
between the anabolism and catabolism of the fundamental component
of cartilage, the extracellular matrix (ECM). The stability of
chondrocyte function is evidently extremely significant for
maintaining the normal activity of cartilage (3,4). As
cartilage has a limited capacity to respond to injury and a low
potential for self-repair, strengthening the functions of the
chondrocytes by promotion of their proliferation may be an
effective treatment for OA.
The eukaryotic cell cycle is divided into 4 phases:
G1, S, G2 and M. Of these stages, the
G1 phase, in which DNA synthesis is prepared, is the
director that determines whether the cell is able to continue
through the cycle or withdraw. Only once past the G1/S
transition may the cell continue to proliferate (5). The progression through each phase of
the cell cycle is delicately controlled by the activity of various
cyclin-dependent kinases (CDKs) and their regulatory subunits, the
cyclins (6). During the
G1 phase, cyclin D1 is a key cell cycle regulatory
protein that is associated with CDK4 or CDK6 in the control of cell
cycle progression (7,8). Therefore, cyclin D1/CDK4 or D1/CDK6
complexes may promote cell proliferation via the promotion of the
cell cycle from the G1 to the S phase.
Radix Achyranthes bidentata (AB), a
traditional Chinese medicinal herb, has been extensively used in
Chinese medicinal formulations for the clinical treatment of OA
(9). AB polysaccharides (ABPS)
have a unit composition molecular weight of ~1,400 Da. The ABPS are
purified polysaccharides isolated from AB and composed of fructose
and glucose residues in the molar ratio of 8:1. In addition, ABPS
contain 2,1-linked fructose, 1,2,6-linked fructose, terminal
fructose and terminal glucose residues (10,11).
Previous studies reported that the medical effects of ABPS were
anti-inflammatory, antiviral, immunomodulatory and
antitumoral(12–15). In order to explore the activity of
ABPS in OA treatment, the present study observed their effects on
cultured chondrocytes and attempted to identify the underlying
mechanisms. ABPS were subsequently identified as promoting
chondrocyte proliferation via the upregulation of cyclin D1, CDK4
and CDK6 expression.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM),
trypsin-EDTA, fetal bovine serum (FBS) and penicillin-streptomycin
were purchased from Hyclone Laboratories, Inc. (Logan, UT, USA).
The type II collagenase was purchased from Sigma (St. Louis, MO,
USA) and the cell cycle detection kit was from Nanjing Key Gen
Biotech (Nanjing, Jiangsu, China). The reverse transcription system
was purchased from the Promega Corporation (Madison, WI, USA). The
DNA primers were synthesized by Shengong Biotech (Shanghai, China),
while the rabbit anti-rat cyclin D1, CDK4 and CDK6 antibodies and
the HRP secondary goat anti-rabbit antibodies were provided by
Bioworld Technology Co., Ltd. (Nanjing, China). The study was
approved by the ethics committee of Fujian University of
Traditional Chinese Medicine.
Preparation of ABPS from AB
The dried and sliced AB was refluxed twice with 80%
alcohol for 1 h/time. Subsequent to evaporation to dry the solvent,
the residue was refluxed 3 times with distilled water (100 g/l) for
2 h/time and extracted. All extractions were concentrated to 100 ml
under hypopiesia conditions. The condensed solution was then
purified by being precipitated with anhydrous alcohol whose final
content was 80% in the decoction and being stewed overnight. The
precipitates isolated by centrifugation were then lyophilized. The
crude polysaccharide was dissolved into distilled water (200 g/l),
then the protein was removed by Sevag's method. The polysaccharides
formed a white power; the ABPS were dissolved in DMEM containing
10% FBS at a density of 10 mg/ml, then the mother liquor was
filtered through a 0.22-μm filter and stored at 4°C.
Isolation and culture of the
chondrocytes
Male, 4-week-old, Sprague-Dawley (SD) specific
pathogen-free (SPF) rats were purchased from the Super-BK
Laboratory Animal, Inc. (Shanghai, China). The rats were sacrificed
using cervical dislocation and their knees were stripped and soaked
with 75% ethanol for 15 min. The articular cartilages were cut down
subsequent to the opening of the joint spaces. then they were
transferred to PBS containing penicillin and streptomycin and
washed 3 times. The cartilages were cut into 1-mm3
sections and subsequently digested with 0.2% type II collagenase.
The isolated cells were collected every 2 h and cultured in 50-ml
culture flasks in 4 ml DMEM containing 10% FBS at 37°C and 5%
CO2. The culture media were changed every 2 days and the
cells were subcultured at 90% confluency.
Evaluation of cell viability by MTT
assay
The passage 2 chondrocytes were seeded into 96-well
plates at a density of 1.0×104/ml and cultured for 24 h.
The cells were treated with varying concentrations of ABPS for 24,
48 and 72 h. At the end of the treatment, 100 μl MTT (1 mg/ml in
PBS) was added into each well and subsequent to a 4-h incubation at
37°C, the supernatant was removed and 150 μl DMSO was added to
dissolve the formazane. The solution was agitated for 10 min and
the OD490 was analyzed using an ELISA reader.
Observation of morphological changes
The passage 2 chondrocytes were seeded into 50-ml
culture flasks at a density of 1×104/ml in 4 ml medium
and cultured for 24 h. The cells were treated with the varying
final concentrations of ABPS (0, 50, 100 and 150 μg/ml) for 48 h.
The cell morphology was observed and images were captured by
phase-contrast microscopy (x100 magnification).
Detection of cell cycle by flow
cytometry
The passage 2 chondrocytes were seeded into 6-well
plates at a density of 1×104/ml and cultured to a
logarithmic growth phase. The cells were treated with varying
concentrations of ABPS for 48 h, collected and the cell density
adjusted to 1×105/ml. The suspension was incubated with
A, B, C solutions from the cell cycle detection kit (Nanjing Key
Gen Biotech) according to the manufacturer's instructions. The
percentage of cells in each phase was calculated by ModFit software
and the cell numbers from the G0/G1, S and
G2/M transition phases were obtained.
RNA extraction and RT-PCR analysis
The total RNA of the chondrocytes treated with the
varying concentrations of ABPS for 48 h was extracted with TRIzol
reagent and the RNA (2 μg) was reverse transcribed into cDNA. The
obtained cDNA was used in PCR to determine the amount of cyclin D1,
CDK4 and CDK6 mRNA. β-actin was used as an internal control. The
primers used for the amplification of the cyclin D1, CDK4, CDK6 and
β-actin transcripts were as follows: cyclin D1 forward, 5′-AAT GCC
AGA GGC GGA TGA GA-3′ and reverse, 5′-GCT TGT GCG GTA GCA GGA
GA-3′; CDK4 forward, 5′-GAA GAC GAC TGG CCT CGA GA-3′ and reverse,
5′-ACT GCG CTC CAG ATT CCT CC-3′; CDK6 forward, 5′-TTG TGA CAG ACA
TCG ACG AG-3′ and reverse, 5′-GAC AGG TGA GAA TGC AGG TT-3′;
β-actin forward, 5′-CGT TGA CAT CCG TAA AGA CC-3′ and reverse,
5′-GGA GCC AGG GCA GTA ATC T-3′. The DNA bands were examined using
a Gel Documentation system.
Western blot analysis
The passage 2 chondrocytes were seeded into 50-ml
culture flasks at a density of 1×104/ml in 4 ml medium
and cultured for 24 h. Subsequent to treatment with the varying
concentrations of ABPS for 48 h, the cells were lysed and the
protein concentrations were determined by the BCA assay. The assay
proteins were separated by electrophoresis on 12%
SDS-polyacrylamide gels and then transferred onto PVDF membranes.
The membranes were blocked for 2 h with agitation at room
temperature in 5% skimmed milk powder dissolved in TBST. The
membranes were washed in TBST and then incubated with the primary
antibody solution (1:1,000) at 4°C overnight. Once the membranes
had been washed in TBST, the secondary antibody solution (1:1,500)
was added for 1 h at room temperature and then the membranes were
washed again in TBST. Finally, the antibody-bound protein bands
were detected with ECL and images were captured using a Kodak image
station 400R (Kodak, Rochester, NY, USA).
Statistical analysis
The data were analyzed with SPSS 16.0 and expressed
as the mean ± standard deviation (SD). A statistical analysis of
the data was conducted using a Student's t-test and ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
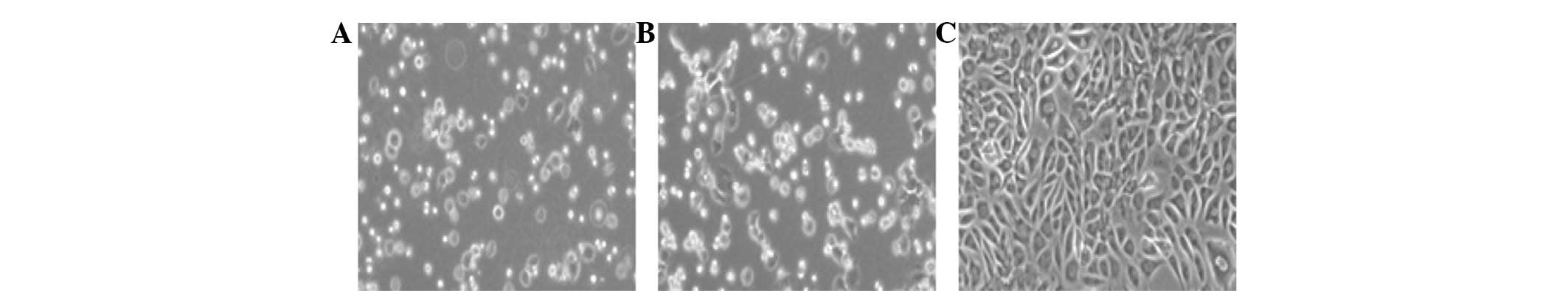
Morphology and characteristics of the
chondrocytes
The newly isolated chondrocytes were small, round
cells initially grown as a suspension culture. Subsequent to 24 h
of proliferation, the cells had gradually attached themselves to
the culture flask and formed into halo-like shapes (Fig. 1A). Subsequent to 3 days of
proliferation, a number of cells showed an irregular flagstone
stereo shape growth and certain cells exhibited a fibroblast-like
morphology (Fig. 1B). Subsequent
to 8 days of proliferation, the cells spread across the flask in
long spindle lines and demonstrated clear boundaries and distinct
nuclei (Fig. 1C). In the
subculture, chondrocytes proliferated markedly faster than the
primary generation and usually reached 80 or 90% density in ~5
days.
ABPS promote the proliferation of the
chondrocytes
The effect of ABPS on the viability of the
chondrocytes was measured by MTT assay. As shown in Fig. 2A, treatment with 50, 100, 200, 400
and 800 μg/ml ABPS for 48 h increased cell viability by 24.82±2.11,
34.14±2.50, 23.21±2.62, 11.92±3.08 and 11.73±1.92%, respectively,
compared with the 0-μg/ml group (P<0.01). The data in Fig. 2B showed that treatment with 100
μg/ml ABPS for 24, 48 and 72 h increased cell viability by
23.44±2.41 (P<0.01), 34.09±4.53 (P<0.01) and 5.23±4.24%
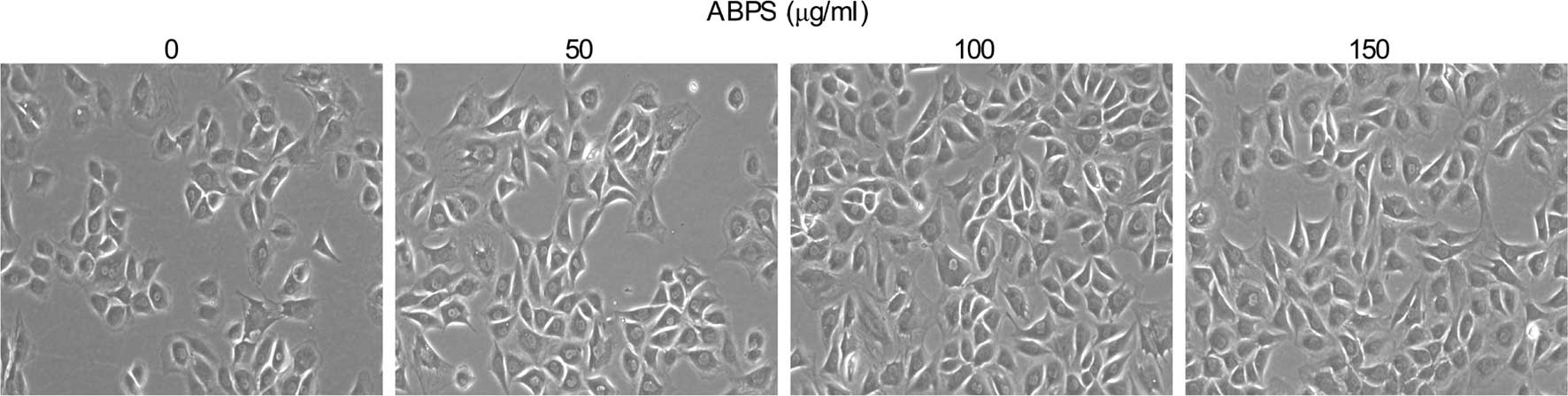
(P=0.085), respectively. As shown in Fig. 3, when compared with the 0-μg/ml
group, there were no significant differences in the morphological
changes of the treated groups, but the number of chondrocytes in
the ABPS-treated groups was markedly greater and with an evident
time dependence. Taken together, it may be suggested that ABPS
treatment promotes the growth of chondrocytes in a dose- and
time-dependent manner within appropriate ranges.
Effects of ABPS on the chondrocyte cell
cycle
Prior to treatment with ABPS, all cells were
cultured in DMEM without FBS for 24 h to synchronize the cell cycle
stage. As shown in Fig. 4, the
percentage proportion of cells in the G0/G1
phase was lower in all the ABPS-treated groups (74.88±6.20,
67.48±2.63 and 74.89±3.48; 50, 100, 150 μg/ml, respectively), with
levels in the 100-μg/ml group significantly decreased compared with
the 0-μg/ml group (77.78±5.59, P<0.05). The percentages of cells
in the S phase from the ABPS-treated groups were 16.28±2.81,
24.72±3.88 and 16.78±3.01% (50, 100, 150 μg/ml, respectively), with
levels in the 100-μg/ml group significantly higher than in the
0-μg/ml group (13.90±3.05, P<0.01). This showed an opposite
trend to the G0/G1 phase. These results
suggested that ABPS treatment is able to promote the progression of
the cell cycle in the transition from G1 to S phase.
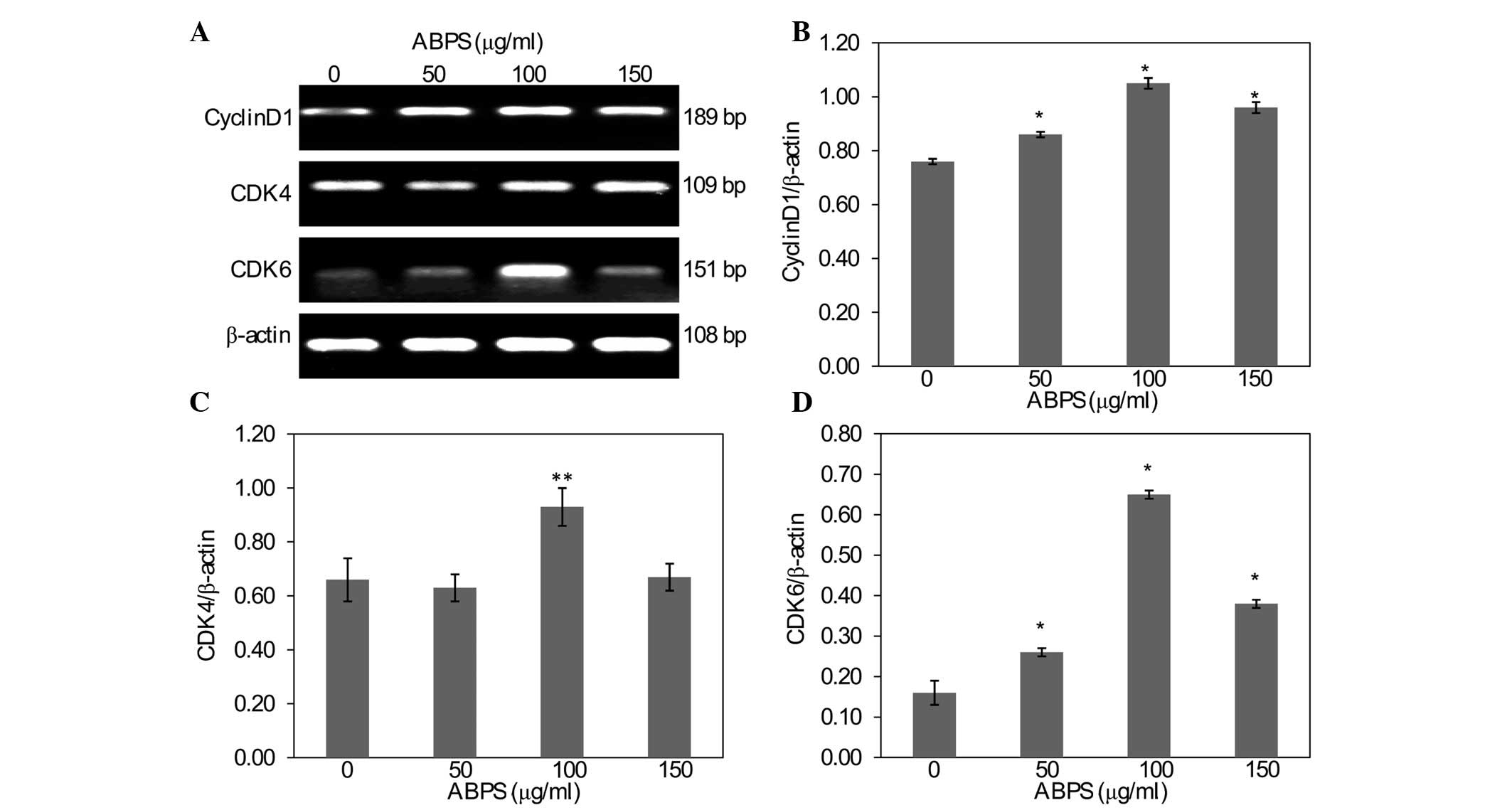
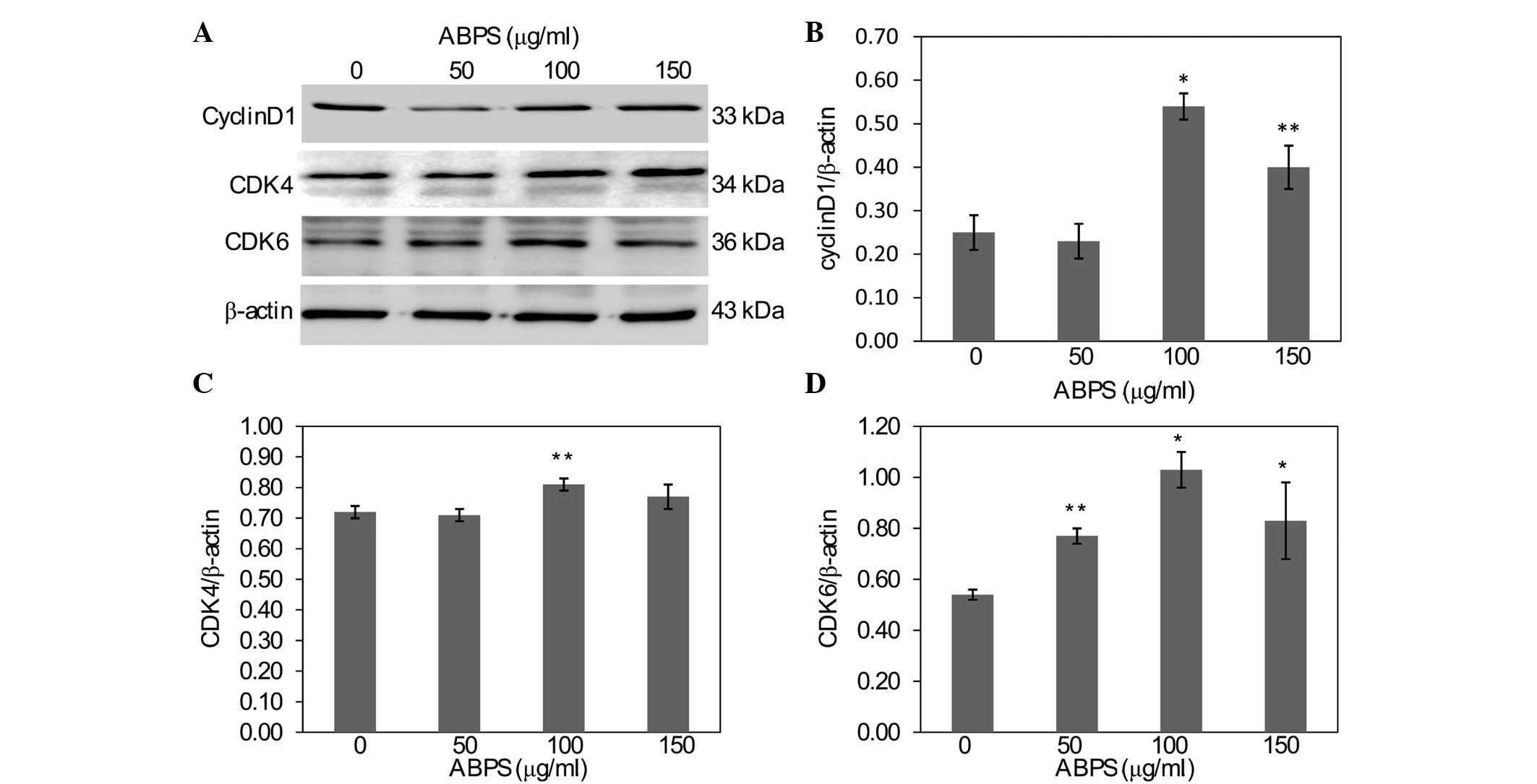
ABPS upregulate the expression of cyclin
D1, CDK4 and CDK6
In order to further explore the mechanism of the
ABPS-induced promotion of chondrocyte proliferation, RT-PCR and
western blot analysis were used to examine the mRNA and protein
expression levels of cyclin D1, CDK4 and CDK6. As shown in Fig. 5, compared with the 0-μg/ml group,
the cells that underwent ABPS treatment had significantly increased
levels of cyclin D1 and CDK6 mRNA expression (P<0.01), while the
mRNA expression of CDK4 in the 100 μg/ml group was also
significantly increased (P<0.05). The protein expression of
cyclin D1, CDK4 and CDK6 was similar to their respective mRNA
levels (Fig. 6).
Discussion
Polysaccharides are high molecular weight compounds
formed from repeating sub-units of sugars and are widely
distributed in Chinese herbs. Polysaccharides are one of the four
basic materials that compose life, with the ability to store
bioenergy and support the role of the structure components
(16). To further investigate the
underlying mechanisms behind the effects of ABPS on chondrocytic
functions, chondrocytes with varying concentrations of ABPS using
differing times in vitro were observed. According to the
results from the MTT assay, cell viability was enhanced when using
100 μg/ml ABPS treatment for 48 h, therefore 0, 50, 100 and 150 μg
/ml ABPS and 48 h were set as variables for the further
experimental program. The present study demonstrated that ABPS
treatment promotes chondrocyte proliferation via promotion of the
transition from G1 to S phase.
As the principle function of cartilage is to provide
a low friction load-bearing surface that facilities free movement
of the joints, the generation of cartilage is a significant cause
of OA (17). Chondrocytes form the
essential composition of articular cartilage, therefore their
functional changes play an extremely significant role in the damage
of cartilage, and the proliferation of chondrocytes is necessary in
maintaining cellular functions (18).
The cell cycle, a set of events that are responsible
for the duplication of the cell, is composed of 4 stages:
G1, the preparation for DNA synthesis; S, DNA synthesis;
G2, the preparation for mitosis; and M, mitosis. The S
and M phases are the two most important processes. Between these
phases, there are two gaps, G1 prior to the S phase and
G2 prior to the M phase. The G1/S and
G2/M transitions are the two checkpoints regulating
stage transition and cell cycle progression (7,19).
MTT data from the present study showed that ABPS treatment promoted
chondrocyte viability within certain doses and times. To further
explore the mechanism of ABPS activity, flow cytometry was used to
examine the changes in the chondrocyte cell cycle brought about by
treatment with ABPS; the results showed that the percentage of
chondrocytes in the G0/G1 phase was reduced
and that the percentage of chondrocytes in the S phase was
significantly increased, demonstrating that ABPS treatment promotes
chondrocyte proliferation via the promotion of cell cycle
progression.
The CDKs and the cyclins are two basic protein
families of the cell cycle control system that associate with each
other as CDK/cyclin complexes to regulate the progress of the cell
cycle. The complex that regulates the progression of each phase of
the cell cycle varies, for example, cyclin D associates with CDK4
and CDK6 during early G1 phase, cyclin E binds to CDK2
during G1 to S phase transition and cyclin A activates
CDK2 during the S phase and the S to M phase transition. CDKs,
which allow progression through the phases of the cell cycle by
phosphorylating substrates, have kinase activity which is dependent
on the presence of their activating subunits, the cyclins. Only
when the specific CDK/cyclin complexes are activated does their
phosphorylation of particular proteins permit cell cycle
progression to continue (20–23).
In the present study, the results showed that ABPS treatment
enhances the mRNA and protein expression of cyclin D1, CDK4 and
CDK6, suggesting that ABPS treatment promotes the progression of
chondrocytes from the G1 to the S phase by regulating
cyclin D1, CDK4 and CDK6.
In conclusion, the data demonstrated that ABPS
effectively promote proliferation via the promotion of the
G1/S cell cycle transition and upregulation of the
expression of cyclin D1, CDK4 and CDK6. This suggests that ABPS may
be potential novel therapeutic agents for the treatment of OA.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81102609), the Key
Project of Fujian Provincial Department of Science and Technology
(grant no. 2012Y0046), the Natural Science Foundation of Fujian
Province (grant no. 2011J05074) and the Developmental Fund of Chen
Keji Integrative Medicine (grant no. CKJ20110003).
References
|
1
|
Iliopoulos D, Gkretsi V and Tsezou A:
Proteomics of osteoarthritic chondrocytes and cartilage. Expert Rev
Proteomics. 7:749–760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lotz MK and Caramés B: Autophagy and
cartilage homeostasis mechanisms in joint health, aging and OA. Nat
Rev Rheumatol. 7:579–587. 2011.PubMed/NCBI
|
|
3
|
Clouet J, Vinatier C, Merceron C,
Pot-vaucel M, Maugars Y, Weiss P, Grimandi G and Guicheux J: From
osteoarthritis treatments to future regenerative therapies for
cartilage. Drug Discov Today. 14:913–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schroeppel JP, Crist JD, Anderson HC and
Wang J: Molecular regulation of articular chondrocyte function and
its significance in osteoarthritis. Histol Histopathol. 26:377–394.
2011.PubMed/NCBI
|
|
5
|
Zhang M, Xie R, Hou W, Wang B, Shen R,
Wang X, Wang Q, Zhu T, Jonason JH and Chen D: PTHrP prevents
chondrocyte premature hypertrophy by inducing cyclin-D1-dependent
Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal
degradation. J Cell Sci. 122:1382–1389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Golias CH, Charalabopoulos A and
Charalabopoulos K: Cell proliferation and cell cycle control: a
mini review. Int J Clin Pract. 58:1134–1141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blagosklonny MV and Pardee AB: The
restriction point of the cell cycle. Cell Cycle. 1:103–110. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Ye H, Yu F, et al: Millimeter wave
treatment promotes chondrocytes proliferation via G1/S
cell cycle transition. Int J Mol Med. 29:823–831. 2012.PubMed/NCBI
|
|
9
|
Chen Q, Liu Z and He J: Achyranthes
bidentata polysaccharide enhances immune response in weaned
piglets. Immunopharmacol Immunotoxicol. 31:253–260. 2009.
View Article : Google Scholar
|
|
10
|
Xue JP and Shi JY: Primary study on
suspension cell culture and polysaccharides content of Achyranthes
bidentata. Zhongguo Zhong Yao Za Zhi. 33:2467–2469. 2008.(In
Chinese).
|
|
11
|
Chen XM, Xu YJ and Tian GY:
Physical-chemical properties and structure elucidation of abPS
isolated from the root of Achyranthes bidentata. Yao Xue Xue
Bao. 40:32–35. 2005.(In Chinese).
|
|
12
|
Peng ZG, Chen HS, Guo ZM, Dong B, Tian GY
and Wang GQ: Anti-HIV activites of Achyranthes bidentata
polysaccharide sulfate in vitro and in vivo. Yao Xue Xue Bao.
43:702–706. 2008.(In Chinese).
|
|
13
|
Zhu X, Pan Y, Zheng L, Cui L and Cao Y:
Polysaccharides from the Chinese medicine herb Achyranthes
bidentata enhance anti-malarial immunity during Plasmodium
yoelii 17XL infection in mice. Malar J. 11:492012.
|
|
14
|
Zou Y, Meng J, Chen W, Liu J, Li X, Li W,
Lu C and Shan F: Modulation of phenotypic and functional maturation
of murine dendritic cells (DCs) by purified Achyranthes
bidentata polysaccharide (ABP). Int Immunopharmacol.
11:1103–1108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin J, Zhang Z and Shan Y: Effect of
Achyranthes bidentata polysaccharides on the expression of
BCL-2 and bax in hepatic tissues after exhaustive exercise in rats.
Afr J Tradit Complement Altern Med. 7:307–314. 2010.
|
|
16
|
Boddohi S and Kipper MJ: Engineering
nanoassemblies of polysaccharides. Adv Mater. 22:2998–3016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris JD, Siston RA, Pan X and Flanigan
DC: Autologous chondrocyte implantation: a systematic review. J
Bone Joint Surg Am. 92:2220–2233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan BY, Fuller ES, Russell AK, et al:
Increased chondrocyte sclerostin may protect against cartilage
degradation in osteoarthritis. Osteoarthritis Cartilage.
19:874–885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Onumah OE, Jules GE, Zhao Y, Zhou L, Yang
H and Guo Z: Overexpression of catalase delays
G0/G1-to S-phase transition during cell cycle
progression in mouse aortic endothelial cells. Free Radic Biol Med.
46:1658–1667. 2009.PubMed/NCBI
|
|
20
|
Pietras EM, Warr MR and Passegué E: Cell
cycle regulation in hematopoietic stem cells. J Cell Biol.
195:709–720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogasawara T, Mori Y, Abe M, et al: Role of
cyclin-dependent kinase (Cdk)6 in osteoblast, osteoclast, and
chondrocyte differentiation and its potential as a target of bone
regenerative medicine. Oral Science International. 8:2–6. 2011.
View Article : Google Scholar
|
|
22
|
Echalier A, Endicott JA and Noble ME:
Recent developments in cyclin-dependent kinase biochemical and
structural studies. Biochim Biophys Acta. 1804:511–519. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inagaki S and Umeda M: Cell-cycle control
and plant development. Int Rev Cell Mol Biol. 291:227–261. 2011.
View Article : Google Scholar : PubMed/NCBI
|