Introduction
Organ transplantation is one of the greatest
advances in modern medical science, but immune rejection following
organ transplantation may cause tissue damage and even fatality.
Transplant rejection occurs when the lymphocytes of the recipient
recognize the allogenic antigens carried by the donor organ, and
the recipient's immune system starts a process of attacking the
transplanted organ. In the majority of cases, the transplant
rejection response is moderate to severe, and it is almost
inevitable in the allograft. Therefore, researchers are interested
in studying immune suppression following organ transplantation and
induction of immune tolerance prior to organ transplantation.
There are two possible mechanisms of allorecognition
initiated by host T lymphocytes: the direct method (in acute
rejection) and the indirect method (in chronic rejection). IL-2, a
T-lymphocyte growth factor, plays an important role in T
lymphocyte-mediated transplant rejection. The acute rejection is
associated with elevated IL-2 levels in cyclosporine-treated
patients. A significant change in IL-2 mRNA level in transplanted
kidney could be detected 2 days prior to rejection (1–3).
Rimm et al(4) demonstrated
that the IL-2 mRNA level of the graft was correlated with acute
rejection. Moderate rejection, on the contrary, was associated with
decreased IL-2 production. The fixed TCR-β chain caused a defective
response to alloantigen, which was measured as decreased IL-2
generation and utilization, followed by an abnormally decreased
graft-versus-host response (GVHR). Another opposite opinion,
however, deemed that IL-2 induced immune tolerance via regulatory T
cells (5–8).
Immune tolerance related to organ transplantation is
defined as a characterized state induced by the absence of a
specific, deleterious immunological reaction against an allograft.
It can be maintained with immunosuppressant drugs. Certain
researchers (8) observed that
donor antigens were capable of inducing death of lymphocytes in
certain conditions. Transfusion of the donor antigen to the thymus
of the recipient prior to organ transplantation induces immune
tolerance in animals (9–11) and donor-specific transfusion prior
to organ transplantation alleviates immune rejection in clinical
trials (12,13). Therefore, it is reasonable to
propose that donor antigen could inhibit the lymphocytes of the
recipient and even induce their death.
Due to the complicated internal environment, the
observed suppressive effect in vivo is different from that
in vitro, and is also unstable. To resolve the immune
rejection problem ahead of organ transplantation, a lymphocyte
inhibition model was established by co-culture of recipient and
donor lymphocytes in order to induce immune tolerance. We
co-cultured lymphocytes from different individuals in vitro,
who could be regarded as donor and recipient, respectively, and
detected the activities of lymphocytes in order to explore the
mechanism of the activity change.
Materials and methods
Ethics
The study was approved by the Ethics Committee of
Department of Cardiovascular Surgery, the PLA 309 Hospital,
Beijing, China according to the Declaration of Helsinki. Written
informed consent was obtained from the patients.
Cell preparation
Preparation of receptor cells
Blood (from a healthy control) was added to
lymphocyte isolation liquid in a centrifuge tube, centrifuged at
1,000 rpm for 20 min, the mist-like buffy coat in the middle of the
tube was imbibed, washed with RPMI-1640 medium and stained with
trypan blue. More than 95% of the cells were alive, and then the
live cells were counted. The cell concentration was diluted to
1×107 cells/ml with RPMI-1640 containing 20% fetal
bovine serum. The suspension was noted as R.
Preparation of donor (D)
lymphocytes
Another sample of blood (from a healthy control) was
added to lymphocyte isolation liquid in a centrifuge tube,
centrifuged at 1,000 rpm for 20 min, the mist-like buffy coat in
the middle of the tube was imbibed, washed with NS liquid and then
the number of cells was counted. The suspension of the lymphocytes
of the donor, after being inactivated with mitomycin, was noted as
D (Bm). Half of D (Bm), noted as D1 (Bm), was diluted to
1×107 cells/ml with RPMI-1640 medium containing 20%
fetal bovine serum. The other half of D (Bm), noted as D2 (Bm), was
divided into 10 aliquots, and stored at −70°C.
Mixed lymphocyte culture (MLC)
Lymphocyte specimens R and D1 (Bm) were mixed into
6-well plates (10 wells in total), cultured at 37°C in a 5%
CO2 incubator for 72 h, and this was noted as the MLC.
On the fourth day, MLC in the first well was collected, washed with
RPMI-1640 medium and then counted. The cell concentration was
diluted to 1×106 cells/ml with RPMI-1640 containing 20%
fetal bovine serum, and the suspension was noted as PMLC (primed
mixed lymphocyte culture) (14).
An aliquot of frozen D2 (Bm) was washed with RPMI-1640 medium and
then counted. The cell concentration was diluted to
1×106/ml with RPMI-1640 containing 20% fetal bovine
serum.
RPMI-1640 liquid (100 μl) was used as a blank
control, and 50 μl PMLC was used as a control. PMLC and D2 (Bm)
were grouped in the following ways: i) PMLC 50 μl and D2 (Bm) 50
μl; ii) PMLC 50 μl and IL-2 purified neutralizing monoclonal
antibody 15 μl; iii) PMLC 50 μl, D2 (Bm) 50 μl and IL-2 purified
neutralizing monoclonal antibody 15 μl. The mixtures were
respectively divided into 96-well plates and cultured in an
incubator at 37°C in 5% CO2 for 24 h.
IL-2 monoclonal neutralizing antibody was provided
by Professor Boquan Jin (The Fourth Military Medical University,
Xi'an, China).
Wright-Giemsa stain
Following culture and collection, the cells were
diluted to a concentration of 1×106 cells/ml. The cells
were then smeared, fixed with 4% formaldehyde, stained with
Wright-Giemsa for 3–4 min and then washed with distilled water
until the edges showed a faint pinkish red. The smear was made
transparent with xylene, and finally cell morphology was observed
by microscopy.
Electron microscope detection
In order to obtain transmission electron microscope
(TEM) images, cells were harvested from flasks with a cell scraper
and washed twice with PBS prior to fixing with 5% glutaraldehyde
for 1 h at 4°C. The pellet was fixed with 1% osmium tetroxide
(OsO4) and dehydrated in a graded series of cold ethanol
(30, 50, 70, 95 and 100% ethanol, respectively). Finally, pellets
were embedded in 100% pure resin (Durcupan ACM, Fluka, Buchs,
Switzerland) (15). Thick (1.5 μm)
and ultrathin sections (~50 nm) were cut with a Sorvall Porter-Blum
MT2-B ultramicrotome (Porter, USA). Ultrathin sections were
collected on copper grids and stained with uranyl acetate (5%
uranyl acetate in 70% ethanol) and 0.3% lead citrate. Unstained
ultrathin sections were observed with a Philips CM200 TEM to
identify the condition of the lymphocyte membrane, nucleus,
chromatin, nuclear envelope and apoptotic body.
Cell growth and proliferation
assay
Cell growth was determined by the colorimetric
tetrazolium-derived XTT (sodium
3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-
nitro) benzene sulfonic acid hydrate) assay (Roche Applied Science,
Mannheim, Germany) and DNA synthesis of cells was assessed by the
bromodeoxyuridine (BrdU) incorporation assay (Roche Applied
Science). For the cell growth and proliferation assay, at 48 h
after transfection the cells of each group were re-seeded in
96-well plates at a density of 0.3–1×104 cells/well.
After 48 h, XTT and incorporated BrdU were measured
colorimetrically using a microtiter plate reader (Bio-Rad,
Hercules, CA, USA) at a wavelength of 450 nm (16).
Cell viability assay
Cell viability was determined using a CCK-8 cell
viability assay kit (Dojindo Laboratories, Kumamoto, Japan). All
cells (5×103 cells/well) were pretreated according to
the manufacturer's instructions and then incubated with or without
0.1 mM H2O2 for 16 h in a 96-well plate. Cell
viability assay kit solution (10 μl) was added to each well.
Following incubation for 1 h at 37°C in the dark, absorbencies were
measured at 450 nm using a multiwell plate reader (17).
Determination of apoptosis
Apoptotic cells were identified by
fluorescence-activated cell sorting (FACS) using Annexin V-Fluos
(Biolegend, San Diego, CA, USA) according to the manufacturer's
instructions. Apoptosis was verified by detection of activated
caspases and p53.
Antibodies and immunoblotting
Immunoblotting was performed as described previously
(14). The following antibodies
were used for immunoblotting at the indicated dilutions: p53
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA;
1:1,000), caspase-3 (Cell Signaling Technology; 1:1,000) and
β-actin (Santa Cruz Biotechnology, Inc.; 1:1,000). All
affinity-purified and species-specific fluorophore-conjugated
secondary antibodies were obtained from Santa Cruz Biotechnology,
Inc. and used at dilutions between 1:500 and 1:800.
Statistics
All data are shown in the form of the means ± SD.
Significance was determined using the paired Student's t-test and
one-way ANOVA test on the mean values of 3 different experiments.
SNK-q was used to compare between 2 groups. All data were analyzed
with SPSS 10.0 software for Windows™. P<0.05 was considered to
indicate a statistically significant difference.
Results
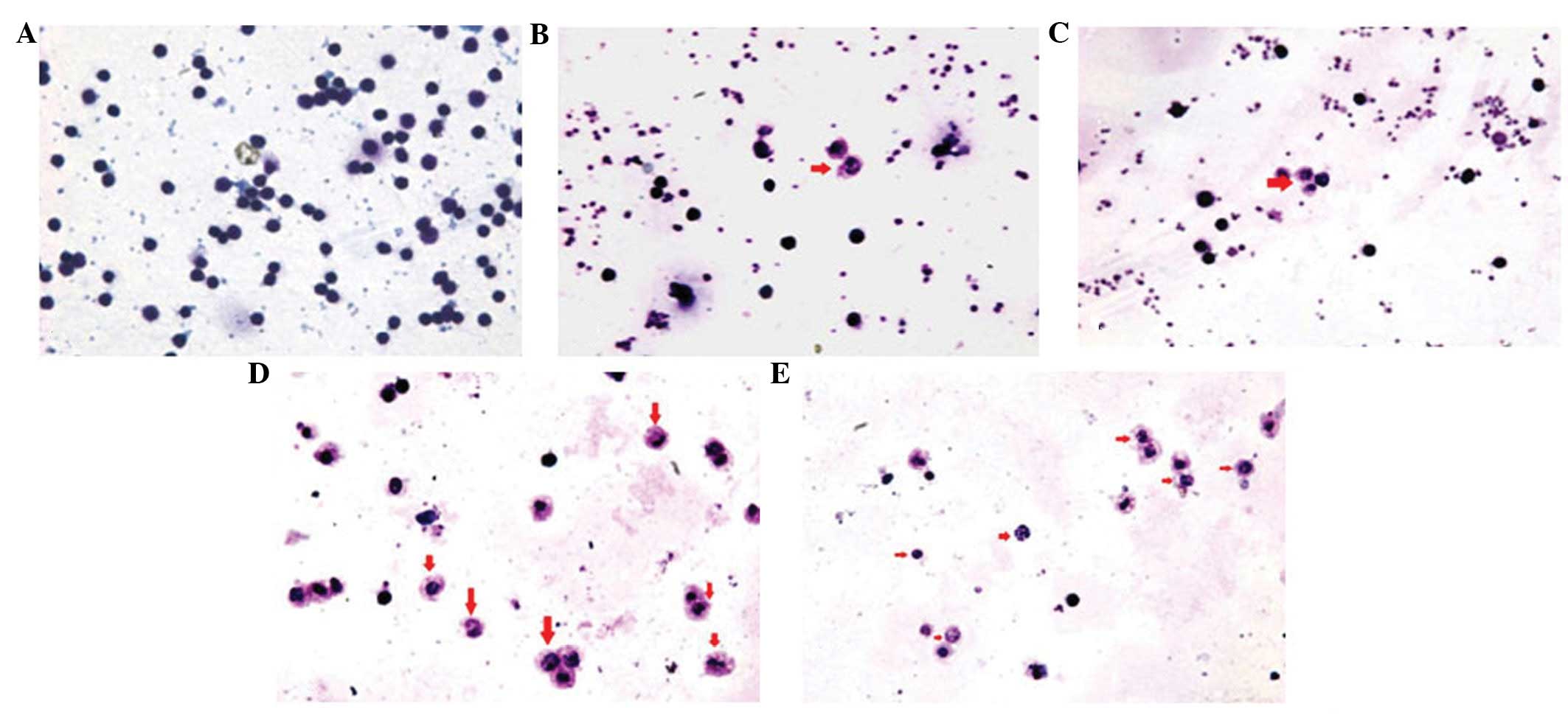
Lymphocytes stained with
Wright-Giemsa
The lymphocytes of each group were stained with
Wright-Giemsa at an appropriate time point. All apoptotic cells
exhibited deep blue karyotin staining, nuclear atypia, chromatin
margination, apoptotic bodies, karyopyknosis and nuclear
schizolysis, but the normal group did not (Fig. 1A). The counts of apoptotic cells in
the PMLC (Fig. 1B) and PMLC + D2
(Bm) groups (Fig. 1C) were
markedly lower than those in the PMLC + anti-IL-2 (Fig. 1D) and PMLC + D2 (Bm) + anti-IL-2
groups (Fig. 1E). In the PMLC + D2
(Bm) + anti-IL-2 group (Fig. 1E),
the counts of lymphocytes were lower than those in the PMLC +
anti-IL-2 group (Fig. 1D).
Apoptosis of lymphocytes observed by
TEM
The lymphocytes were observed by TEM (x10,000).
Nuclear atypia, chromatin margination, karyopyknosis and
schizolysis, as well as disappearance of nuclear envelopes and
integration of cellular membrane, was observed in the images,
particularly in the PMLC + anti-IL-2 group.
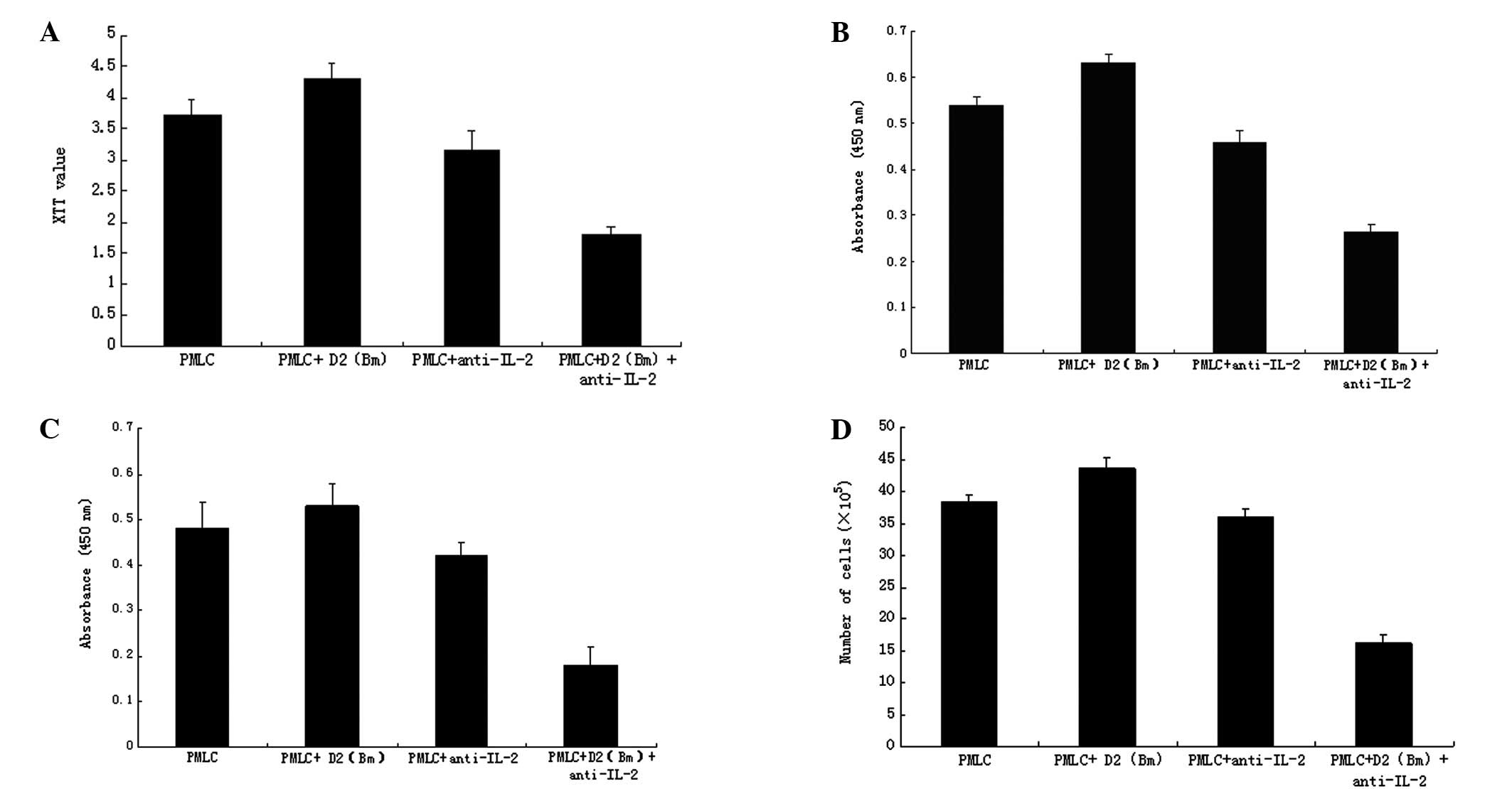
Blockade of IL-2 with anti-IL-2 purified
neutralizing monoclonal antibody inhibits lymphocytes growth and
proliferation
The lymphocyte growth in the PMLC + D2 (Bm) +
anti-IL-2 group was lower than that in the PMLC and PMLC + D2 (Bm)
group (P<0.01) as detected by XTT assay, but not in the PMLC +
anti-IL-2 group (Fig. 2A). The
results of lymphocyte DNA synthesis analyzed with BrdU showed that
the DNA change in the PMLC + D2 (Bm) + anti-IL-2 group was less
than that in the PMLC and PMLC + D2 (Bm) group (P<0.01), but not
in the PMLC + anti-IL-2 group (Fig.
2B). Cell viability was evaluated by CCK-8 assay (Fig. 2C) and lymphocyte proliferation was
evaluated by cell count (Fig. 2D).
The results were similar to those from the XTT assays. The
proliferation of lymphocytes in the PMLC + anti-IL-2 group
(P<0.01) was significantly inhibited, but not in the PMLC + D2
(Bm) and PMLC + anti-IL-2 groups (P>0.05).
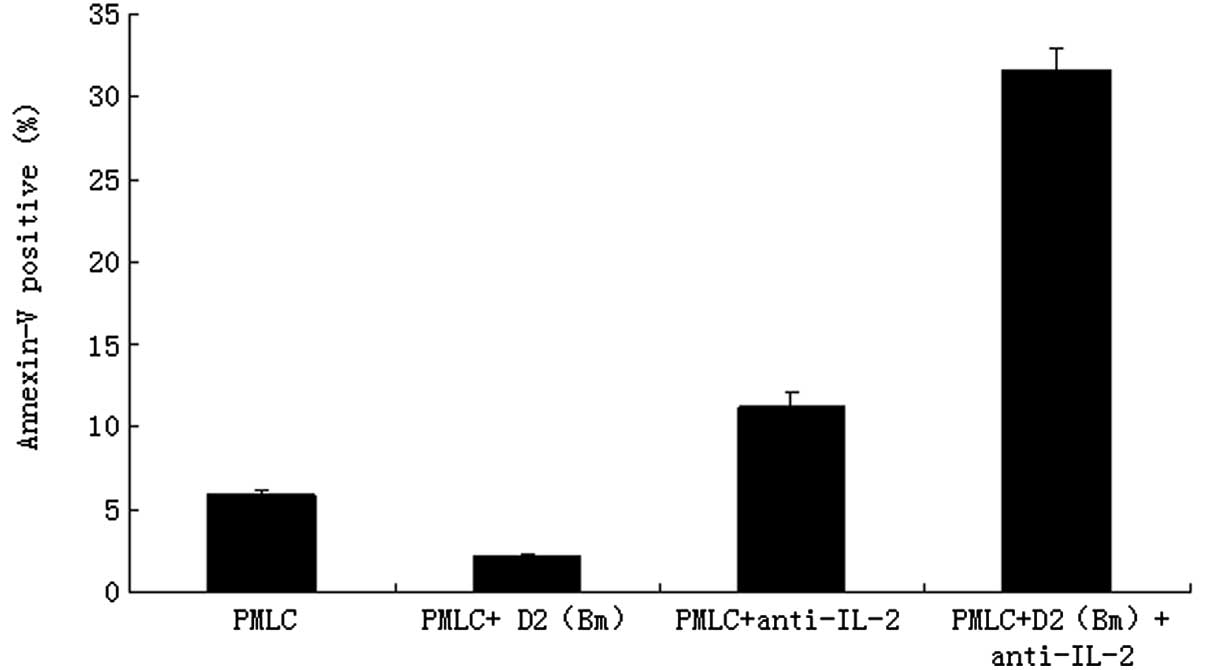
Blockade of IL-2 with anti-IL-2 purified
neutralizing monoclonal antibody promotes lymphocyte apoptosis
The effects of MLC, as well as IL-2 purified
neutralizing monoclonal antibody, on apoptosis distribution were
determined by flow cytometry. The counts of apoptotic lymphocytes
in the PMLC + anti-IL-2 group (11.25±0.78%) and the PMLC + D2 (Bm)
+ anti-IL-2 group (31.65±1.33%) were much higher than in the PMLC
(5.95±0.24%) or PMLC + D2 (Bm) groups (2.15±0.04%), respectively
(P<0.01). The difference between the PMLC + anti-IL-2 and PMLC +
D2 (Bm) + anti-IL-2 groups was also statistically significant
(P<0.01; Fig. 3). The results
revealed that MLC and IL-2 purified neutralizing monoclonal
antibody significantly induced apoptosis of lymphocytes.
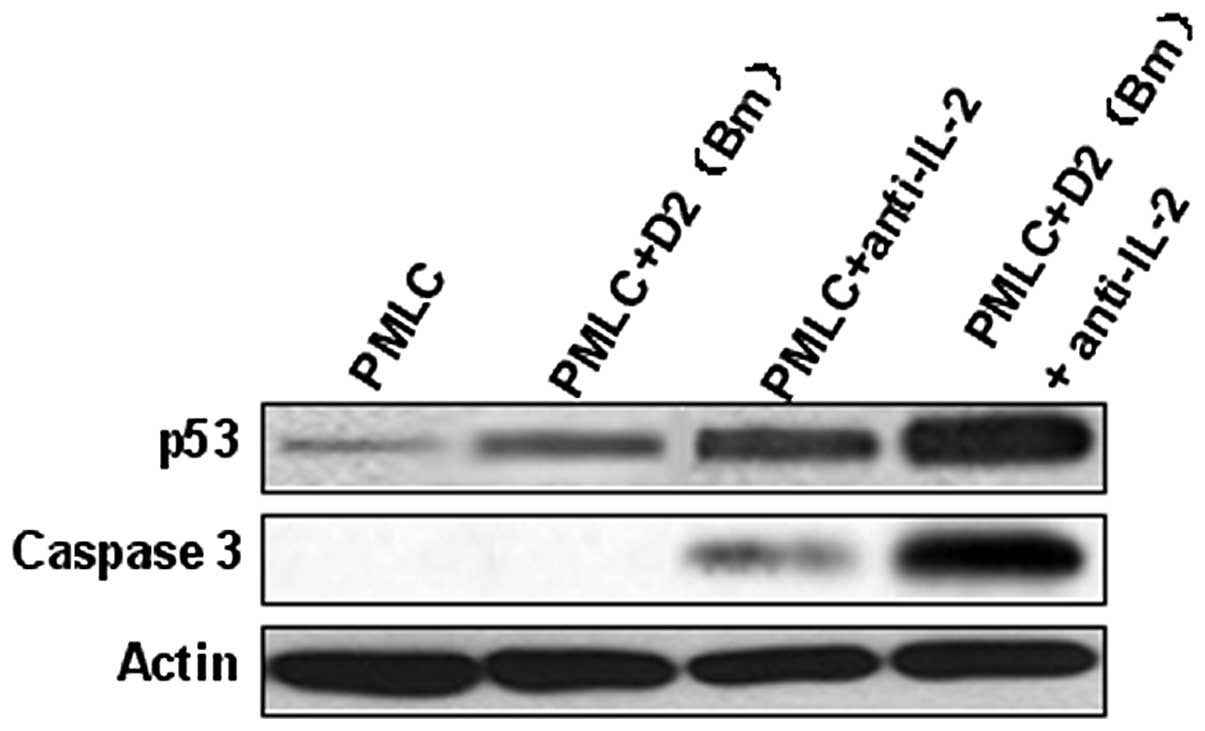
p53 and caspase-3 were involved in
recipient lymphocyte apoptosis
In the PMLC + anti-IL-2 and PMLC + D2 (Bm) +
anti-IL-2 groups, the expression of p53 increased significantly,
particularly in the latter. The evidence that lymphocyte apoptosis
is induced by MLC and IL-2 purified neutralizing monoclonal
antibody, particularly by the latter, and is associated with
caspase-3 activated cleavage products is shown in Fig. 4. Overall, these results indicate
that MLC and IL-2 purified neutralizing monoclonal antibody
promotes lymphocyte apoptosis via p53 and caspase-3 pathways
(Fig. 4).
Discussion
Transplant rejection is a complex and redundant
response to grafted organs. Its major targets are the major
histocompatibility complex (MHC) antigens, which are designated as
human leukocyte antigens (HLAs). T lymphocytes recognize foreign
antigens only when the antigen or an immune peptide is associated
with a self-HLA molecule on the surface of an accessory cell known
as an antigen-presenting cell (APC). To resolve the immune
rejection problem ahead of organ transplantation, we established a
lymphocyte inhibition model by co-culturing mixed lymphocytes to
induce recipient cell apoptosis and immune tolerance.
The main type of inducible cell death is apoptosis.
Apoptosis is a type of programmed cell death (18) and is regulated by a series of
signal cascades in an orderly manner. The caspase cascade response
plays a vital role in the induction, transduction and amplification
of intracellular apoptotic signals (19). Caspase-3 is a key factor in
apoptosis execution, being responsible either partially or
completely for the proteolytic cleavage of numerous key proteins
(20).
In addition, p53 also plays a fundamental regulatory
role in apoptosis, and induces apoptosis by caspase activation
(21). The p53 protein, which is
encoded by the TP53 gene, has a broad range of biological
functions, including regulation of cell cycle, apoptosis,
senescence, DNA metabolism, angiogenesis, cellular differentiation
and the immune response. The key role of p53 is to induce apoptosis
in response to cellular stresses such as DNA damage (22). Numerous studies have described the
mechanism by which p53 induces apoptosis. As p53 functions mainly
as a transcription factor, it is important to explore the genes
regulated by p53 that contribute to the regulation of apoptosis.
Apoptosis occurs through one of two major pathways, described as
either the intrinsic mitochondrial or extrinsic death receptor
pathway. In the mitochondrial pathway, death stimuli target
mitochondria either directly or through transduction by
proapoptotic members of the Bcl-2 family, including Bax and Bak.
The mitochondria then release apoptogenic proteins, ultimately
leading to caspase activation and apoptosis. In the death receptor
pathway, following interactions with their cognate ligands, the
receptors located at the cellular membrane recruit adaptor proteins
such as initiator caspase-8, triggering the activation of caspases
to orchestrate apoptosis. The crosstalk between the pathways is
mediated via Bid, and possibly other factors that mediate cell
death by modulation of the two pathways and perhaps other
unrecognized pathways.
Immune tolerance is important in organ
transplantation. Apoptosis of lymphocytes plays a critical role in
central immune tolerance and peripheral immune tolerance, and is a
type of programmed cell death. It is a well-known fact that the
properly functioning immune system is dependent on programmed cell
death at every developmental stage of the lymphocyte and for its
activity. Activation-induced cell death (AICD) is the process by
which cells undergo apoptosis in a controlled manner through the
interaction of a death factor and its receptor (23,24).
IL-2 plays an important and complex role in the
immune system, serving as growth factor, differentiation factor and
regulator of cell death. The endogenous expression of IL-2 is
increased by the costimulation of previously activated T
lymphocytes, which increases the expression of Bcl-2 and inhibits
AICD of previously activated T lymphocytes (25). However, certain researchers
observed the discrepant phenomenon that IL-2 leads to increased
susceptibility to AICD (26–29).
In our research, by using MLC and IL-2 neutralizing
monoclonal antibody, we successfully developed an in vitro
lymphocyte inhibition model. We demonstrated that antigens and IL-2
neutralizing monoclonal antibody had different inhibitory effects
on the lymphocyte activities in various mixed ways. The results
showed that the lymphocyte activity was very high when adding
antigens alone [in the PMLC + D2 (Bm) group], but was markedly
lowered when adding antigens and IL-2 neutralizing monoclonal
antibody together [in the PMLC + D2 (Bm) + anti-IL-2 group]. Adding
IL-2 neutralizing monoclonal antibody alone (in the PMLC +
anti-IL-2 group) also inhibited lymphocyte activity, but the
depression effect was weaker than adding antigens and IL-2
neutralizing monoclonal antibody together. By combining the TEM
technique, Giemsa-Wright stain and flow cytometry, we found that
the depressed lymphocyte activity was due to the enhancement of
apoptosis. Similar to the results above, we observed various ratios
of apoptotic cells in the different groups. With the exception of
the control group, the ratio of apoptotic cells was highest in the
PMLC + D2 (Bm) + anti-IL-2 group and lowest in the PMLC + D2 (Bm)
group. The presence of IL-2 neutralizing monoclonal antibody was
associated with more apoptotic activated lymphocytes in our
experiment, which suggested that IL-2 may inhibit apoptosis of
lymphocytes.
In our study, we first observed the different level
of apoptosis of lymphocytes among different groups, and
subsequently attempted to find the dominant mechanism related to
apoptosis. As we know, induction of apoptosis is an essential
function of p53 as a tumor suppressor (22,30,31).
The protein p53 can activate its downstream targets in a
sequence-specific manner to induce apoptosis. The majority of
tumor-derived p53 mutants are deficient in transcription activation
as well as apoptosis induction. p53 can activate genes in the
extrinsic and intrinsic pathways through transcription-dependent
mechanisms or induce apoptosis through transcription-independent
mechanisms. Results showed that the expression of p53 and cleaved
caspase-3 increased markedly in the PMLC + D2 (Bm) + anti-IL-2
group, while there was an extremely low level of p53 and cleaved
caspase-3 in the PMLC + D2 (Bm) group, which was in accordance with
the ratio of apoptotic lymphocytes.
According to these results, we can summarize a novel
mechanism of immune rejection: when the IL-2 from activated
lymphocytes is blocked with neutralizing antibody, the activated
lymphocytes undergo p53-induced apoptosis.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30600524).
References
|
1
|
Helderman JH, Hernandez J, Sagalowsky A,
et al: Confirmation of the utility of fine needle aspiration biopsy
of the renal allograft. Kidney Int. 34:376–381. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Von Willebrand E and Hughes D: Fine-needle
aspiration cytology of the transplanted kidney. Kidney
Transplantation. Morris PJ: 4th edition. WB Saunders; Philadelphia,
PA: pp. 3011994
|
|
3
|
Suthanthiran M: Clinical application of
molecular biology: a study of allograft rejection with polymerase
chain reaction. Am J Med Sci. 313:264–267. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rimm IJ, Krenger W, Beland JL, et al:
TCR-beta transgenic mice fail to mediate a GVHR due to defects of
allorecognition and subsequent IL-2 generation. Bone Marrow
Transplant. 17:835–842. 1996.PubMed/NCBI
|
|
5
|
Koreth J, Matsuoka K, Kim HT, et al:
Interleukin-2 and regulatory T cells in graft-versus-host disease.
N Engl J Med. 365:2055–2066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Dai H, Wan N, et al: Manipulating
IL-2 availability amid presentation of donor MHC antigens
suppresses murine alloimmune responses by inducing regulatory T
cells. PLoS One. 5:e87562010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Webster KE, Walters S, Kohler RE, et al:
In vivo expansion of T reg cells with IL-2-mAb complexes: induction
of resistance to EAE and long-term acceptance of islet allografts
without immunosuppression. J Exp Med. 206:751–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang HG, Zhang D, Degauque N, et al:
Effects of cyclosporine on transplant tolerance: the role of IL-2.
Am J Transplant. 7:1907–1916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sebille F, Brouard S, Petzold T, et al:
Tolerance induction in rats, using a combination of anti-CD154 and
donor splenocytes, given once on the day of transplantation.
Transplantation. 75:169–172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Subbotin V, Sun H, Aitouche A, et al:
Abrogation of chronic rejection in a murine model of aortic
allotransplantation by prior induction of donor-specific tolerance.
Transplantation. 64:690–695. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen Z, Mohiuddin M, Goldstein C, et al:
Durability of donor-specific and organ-specific heart transplant
tolerance induced by intrathymic pretreatment with allogeneic
spleen cells. J Thorac Cardiovasc Surg. 111:429–431. 1996.
View Article : Google Scholar
|
|
12
|
Opelz G and Terasaki PI: Poor
kidney-transplant survival in recipients with frozen-blood
transfusions or no transfusions. Lancet. 2:696–698. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sheehy MJ, Sondel PM, Bach ML, et al: HL-A
LD (lymphocyte defined) typing: a rapid assay with primed
lymphocytes. Science. 188:1308–1310. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amicarelli F, Bucciarelli T, Poma A, et
al: Adaptive response of human melanoma cells to methylglyoxal
injury. Carcinogenesis. 19:519–523. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ford J, Jiang M and Milner J:
Cancer-specific functions of SIRT1 enable human epithelial cancer
cell growth and survival. Cancer Res. 65:10457–10463. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamamoto R, Furukawa Y, Morita M, et al:
SMYD3 encodes a histone methyltransferase involved in the
proliferation of cancer cells. Nat Cell Biol. 6:731–740. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: a basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krajewska M, Wang HG, Krajewski S, et al:
Immunohistochemical analysis of in vivo patterns of expression of
CPP32 (Caspase-3), a cell death protease. Cancer Res. 57:1605–1613.
1997.PubMed/NCBI
|
|
21
|
Amaral JD, Castro RE, Sola S, et al: p53
is a key molecular target of ursodeoxycholic acid in regulating
apoptosis. J Biol Chem. 282:34250–34259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amaral JD, Xavier JM, Steer CJ, et al: The
role of p53 in apoptosis. Discov Med. 9:145–152. 2010.
|
|
23
|
Shi YF, Sahai BM and Green DR: Cyclosporin
A inhibits activation-induced cell death in T-cell hybridomas and
thymocytes. Nature. 339:625–626. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lieberman AC, Refojo D, Antunica-Noguerol
M, et al: Underlying mechanisms of cAMP- and
glucocorticoid-mediated inhibition of FasL expression in
activation-induced cell death. Mol Immunol. 50:220–235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pender MP: Activation-induced apoptosis of
autoreactive and alloreactive T lymphocytes in the target organ as
a major mechanism of tolerance. Immunol Cell Biol. 77:216–223.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lawetzky A, Kubbies M and Hünig T: Rat
‘first-wave’ mature thymocytes: cycling lymphoblasts that are
sensitive to activation-induced cell death but rescued by
interleukin 2. Eur J Immunol. 21:2599–2604. 1991.
|
|
27
|
Miller AT and Berg LJ: Defective Fas
ligand expression and activation-induced cell death in the absence
of IL-2-inducible T cell kinase. J Immunol. 168:2163–2172. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maher SG, Condron CE, Bouchier-Hayes DJ
and Toomey DM: Taurine attenuates CD3/interleukin-2-induced T cell
apoptosis in an in vitro model of activation-induced cell death
(AICD). Clin Exp Immunol. 139:279–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Curtale G, Citarella F, Carissimi C, et
al: An emerging player in the adaptive immune response:
microRNA-146a is a modulator of IL-2 expression and
activation-induced cell death in T lymphocytes. Blood. 115:265–273.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liebermann DA, Hoffman B and Vesely D: p52
induced growth arrest versus apoptosis and its modulation by
survival cytokines. Cell Cycle. 6:166–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Olivier M, Petitjean A, Marcel V, et al:
Recent advances in p53 research: an interdisciplinary perspective.
Cancer Gene Ther. 16:1–12. 2009. View Article : Google Scholar
|