Introduction
Propofol is commonly used for the induction of
anesthesia in surgical procedures worldwide. Its anesthetic action
is believed to occur through potentiation of GABAA receptor
activity. Propofol has also been widely used in pediatric
anesthesia and in the pediatric critical care unit. It has been
shown that repeated exposure of the developing brain to propofol
results in neuronal cell apoptosis (1). Cattano et al reported that
injection of propofol over a dose of 50 mg/kg into Day 5–7 C57BL6
rat neonates led to marked neuronal cell apoptosis (2). A similar result was observed by
Fredriksson et al(3). It
has been found that propofol or modulators of GABAA regulate
intracellular Ca++ concentration which contributes in
part to cell apoptosis (4).
However, the molecular mechanism by which propofol induces neuronal
cell apoptosis remains largely to be elucidated.
Neural stem cells were first identified in the
embryonic Day 13.5–14.5 rat forebrain in 1989 (5). Since then, they have been isolated
from various areas of the adult brain (6,7).
Neural stem cells are multipotent, self-renewing cells, capable of
differentiating into different phenotypes of the nervous system. In
this study, we isolated and cultured rat embryonic neural stem
cells and investigated in vitro propofol-induced stem cell
apoptosis. Furthermore, using western blot analysis, we examined
the effect of propofol in activating several notable apoptotic
proteins, i.e., cytochrome C, caspase-3, caspase-8 and caspase-9.
It is known that caspase-3 and -8 regulate the extrinsic apoptotic
pathway while cytochrome C and caspase-9 regulate the intrinsic
apoptotic pathway (8–11). We believe that the identification
of key players controlling the process of neuronal cell apoptosis
induced by propofol may lead to the development of new methods to
reduce the side-effects caused by propofol, particularly in
pediatric patients.
Materials and methods
Ethics
Ethical approval for this study was provided by the
Ethical Committee of Zhongshan Hospital of Traditional Chinese
Medicine, Guangdong, China (Professor Zhong-Qing Wu) on 9 March,
2010.
Isolation and culture of rat embryonic
neural stem cells
Wistar rat embryos were obtained at 14–16 days of
gestation by routine surgical procedure. The cerebral cortex was
removed and immersed in D-Hanks solution. Blood vessels and
meninges were removed followed by centrifugation at 1,000 rpm for 3
min, then the cerebral cortex was minced and treated with trypsin
(0.125%) and EDTA (0.102%). Digestion with trypsin/EDTA was
neutralized by addition of culture medium Dulbecco’s modified
Eagle’s medium (DMEM)/F12 containing 10% FBS, 2% B27 and 1% N2
supplements, 20 ng/ml EGF, 20 ng/ml FGF, 200 IU/l penicillin and
100 IU/l streptomycin. Cells were then dispersed by pipetting up
and down several times and pelleted with centrifugation. After
re-suspension with culture medium, cells were transferred into T25
cell culture flasks (0.5×106 cells/flask) previously
coated with poly-ornithine and laminin and cultured at 37°C in a
moist atmosphere containing 5% CO2.
Immunofluorescence cell staining
Immunofluorescence cell staining was applied to
characterize the rat embryonic neural stem cells using an
anti-Nestin antibody. Cells (at passage 2–3) cultured in a 4-well
chamber slide were washed once with phosphate-buffered saline (PBS)
and fixed with 4% paraformaldehyde for 30 min at room temperature.
After permeabilization with 0.5% Triton X-100 and blocking with 5%
goat serum, cells were washed three times with PBS. An anti-Nestin
antibody was added to cells (an isotype was used as a control) and
incubated overnight at 4°C followed by three washes with PBS. Cells
were then incubated with a TRITC-conjugated goat anti-rabbit IgG
secondary antibody for 60 min in the dark followed by three washes
with PBS. Cell nuclei were stained with DAPI for 3 min. After three
washes with PBS, cells were mounted with fluorescent mounting
medium and studied under a fluorescent microscope.
MTT assay
MTT assay was employed to examine the effect of
propofol on cell growth. Rat embryonic neural stem cells at passage
2 were seeded into a 96-well plate previously coated with
poly-ornithine and laminin (4×104 cells/well) and
cultured at 37°C for 24 h in a moist atmosphere containing 5%
CO2. Cells were washed once with PBS and propofol was
added at different concentrations (5, 25, 50 and 100 μM) with some
wells of cells treated with vehicle (intralipid) or left untreated.
Each sample was duplicated. After culture for 12 h, 20 μl 0.5% MTT
was added into each well and cells were maintained in culture for
another 4 h. Cell medium was then aspirated and 100 μl 10% DMSO was
added to lyse cells for the release of MTT. The optical density of
each well at a wavelength of 570 nm was measured and recorded with
a standard ELISA microplate reader.
Flow cytometric analysis of cell
apoptosis
Rat embryonic neural stem cells at passage 2 were
seeded in 100-mm cell culture dishes previously coated with 0.1%
poly-L-lysine (1.2×106 cells/dish) and cultured at 37°C
for 24 h in a moist atmosphere containing 5% CO2. Cells
were then treated with propofol (5, 25, 50 and 100 μM), intralipid
or 50 μM propofol + 2 μM caspase-3 inhibitor Z-DEVD-FMK for 12 h.
After detachment with PBS/1 mM EDTA and washing twice with PBS,
cells were re-suspended in 400 μl binding buffer and mixed with 5
μl FITC-conjugated Annexin V, then 5 μl 7-amino-actinomycin was
added to cells and incubated in the dark for 10 min. Annexin
V-FITC-positive cells (apoptotic cells) were analyzed by a flow
cytometer.
Western blot analysis
Rat embryonic neural stem cells were treated with 50
μM propofol for 12 h. Total protein was extracted from cells using
RIPA lysis buffer (0.1% Nonidet P-40, 0.5% sodium deoxycholate,
0.1% SDS, 1 mM Tris-HCl, pH 7.6, 50 mM NaF, 200 mM NaCl, 1 mM EDTA,
1 mM sodium orthovanadate and 1 mM benzamidine) containing a
proteinase inhibitor cocktail (1:100 dilution; Sigma, St. Louis,
MO, USA). After quantification with a protein assay kit (Bio-Rad
Laboratories, Hercules, CA, USA), 100 μg protein in 1X Laemmli
buffer was boiled for 5 min, separated by SDS-PAGE and electrically
transferred onto PVDF membrane. The membranes were blocked at room
temperature in TBS-T buffer (50 mM Tris-HCl, 150 mM NaCl, pH 7.5,
0.1% Tween-20) containing 5% skimmed milk for 1 h followed by
incubation with primary antibodies diluted in blocking buffer for 2
h. All antibodies detected the active forms of proteins and were
used as follows: anti-caspase-3 antibody (1:1,000), anti-cytochrome
C antibody (1:2,000), anti-caspase-9 antibody (1:1,000) and
anti-caspase-8 antibody (1:1,000). Following incubation with the
primary antibody, the membranes were washed three times (15 min ×
3) with TBS-T buffer followed by incubation with HRP-conjugated
anti-mouse or anti-rabbit secondary antibodies (both at a dilution
of 1:5,000; Promega, Madison, WI, USA) for 1 h. The membranes were
washed as above with TBS-T buffer and the specific bands were
visualized using an ECL western blot detection reagent kit
(Amersham Biosciences, Piscataway, NJ, USA), scanned and
densitometrically analyzed with Molecular Analysis Program (Bio-Rad
Laboratories, Shanghai, China). Relative quantification of each
protein was performed following normalization against β-actin.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean (SEM). Statistical analysis was performed using SPSS
13.0 software. One-way ANOVA and the least significant difference
test were performed to compare differences among the groups.
P<0.05 was considered to indicate a statistically significant
result.
Results
Isolation, culture and characterization
of rat embryonic neural stem cells
Rat embryonic neural stem cells were isolated from
E14–16 embryos and seeded in T25 flasks containing neural stem cell
culture medium. Second day suspended stem cell colonies were
observed. The cell colonies were dispersed and passaged, cells then
grew and became adherent. For cell characterization, Nestin protein
production was assayed by immunofluorescent staining using an
anti-Nestin antibody. Positively stained cells were observed with
fluorescence microscopy (Fig. 1B)
and counted. The percentage of Nestin-positive cells was calculated
from five different fields. The results from a total of four
isolations are shown in the right panel of Fig. 1. The percentage of Nestin-positive
cells ranged from 90.36 to 93.90% in each isolation.
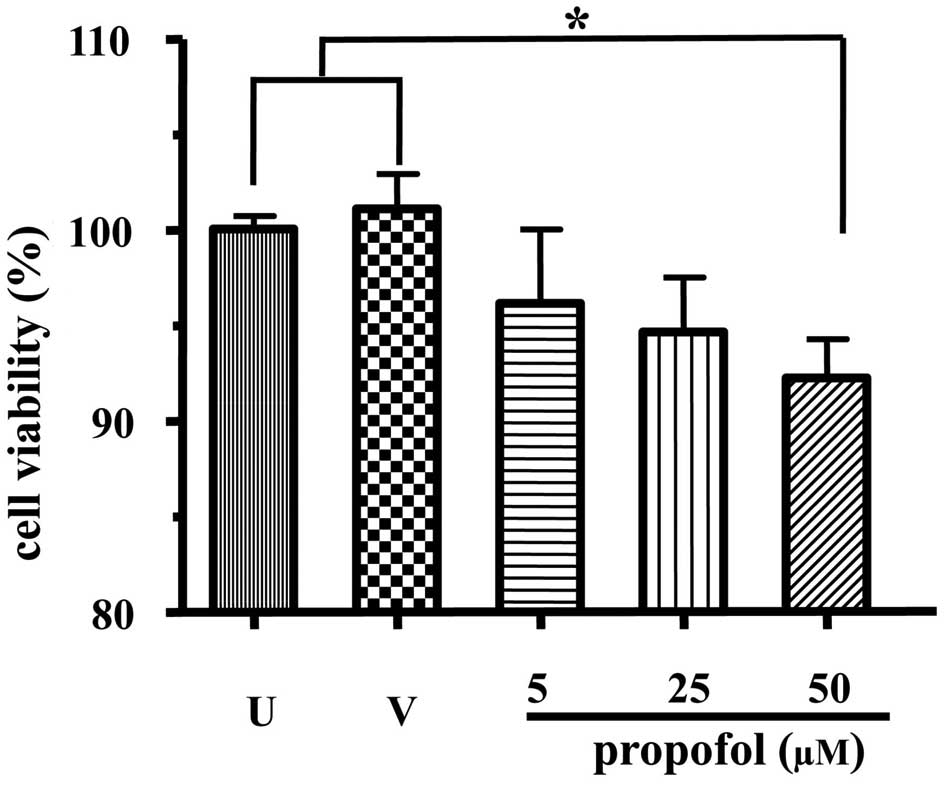
Inhibitory effect of propofol on rat
embryonic neural stem cell growth
First, the effect of propofol on stem cell growth
was examined. The MTT assay was employed for this purpose. As shown
in Fig. 2, there was no
significant difference in the uptake of MTT between the
intralipid-treated and non-treated cells. However, propofol at 50
μM significantly reduced the uptake of MTT by embryonic neural stem
cells (P<0.05, compared with untreated or intralipid-treated
cells), indicating that propofol inhibited stem cell growth.
Propofol induces apoptosis of rat
embryonic neural stem cells
Next, we studied whether propofol induced rat
embryonic neural stem cell apoptosis. Cells were treated with
different doses of propofol as indicated. At different time points
(6, 12 and 24 h) post-treatment, cell apoptosis was assayed with
flow cytometric analysis. We found that significant apoptosis
occurred after cells were treated for 12 h (data not shown). We
therefore used this time point for the apoptosis assay. As shown in
Fig. 3, no significant difference
was observed in cell apoptosis between intralipid-treated and
untreated cells (both at ~3%). By contrast, propofol treatment
starting at 5 μM induced cell apoptosis, doubling that of untreated
cells. With the increase of propofol concentration, the cell
apoptosis increased accordingly in a dose-dependant manner. When
treated with 50 μM propofol, cell apoptosis reached ~30%,
significantly higher than the untreated cells (P<0.001). The
addition of caspase-3 inhibitor Z-DEVD-FMK resulted in a
significant reduction in cell apoptosis (from ~30 to 19%,
P<0.001) in the 50 μM group.
Propofol activates apoptotic proteins in
rat embryonic neural stem cells
To investigate the mechanisms controlling stem cell
apoptosis induced by propofol, we detected cellular levels of
active caspases-3, -8, -9 and cytochrome C in rat embryonic neural
stem cells following treatment with propofol. Western blot analysis
showed that there was no difference in the levels of all active
proteins between untreated and vehicle (intralipid)-treated cells.
However, propofol treatment resulted in a significant augmentation
of all active proteins assayed (Fig.
4), i.e., active caspase-3 from 99.9±5.2 to 216.2±12.1
(P<0.001), active caspase-8 from 100.0±6.5 to 183.6±17.8
(P<0.01), active caspase-9 from 99.9±1.6 to 341.2±18.5
(P<0.0001) and cytochrome C from 99.9±2.5 to 218.5±6.6
(P<0.001).
Discussion
Anesthetic drugs are used frequently in pediatric
medicine for surgery as well as for other procedures such as
angiography, central line catheterization, endoscopy and
intubation. It is known that many of these agents cause damage to
the developing brain. Several studies demonstrated that
administration of isoflurane, ketamine, nitric oxide, midazolam,
thiopental and sevoflurane results in neuronal apoptosis in the
developing mouse brain (3,12,13).
In the present study, we investigated the effect of propofol on rat
embryonic neural stem cells and found that i) >90% rat neuronal
stem cells isolated from E14–16 rat embryos expressed Nestin
protein, ii) propofol inhibited stem cell growth and induced cell
apoptosis in a dose-dependent manner, and iii) propofol activated
caspase-3, -8 and -9 and cytochrome C, which are key players in
regulating extrinsic or intrinsic cell apoptosis pathways.
Studies measuring the brain uptake of propofol in
humans following intravenous administration revealed that the
concentration of propofol in the brain varied from patient to
patient, ranging from 22 to 73 μM (14). In the present study we used 5–100
μM propofol, relative to clinical application, to study its effect
on neural stem cells. Rat embryonic neural stem cells were isolated
from E14–16 embryos when the rat embryonic neural stem cells are
most robust in differentiation and proliferation. The expression of
Nestin protein, a widely used marker for the characterization of
neural stem cells, was detected in the isolated cells (15). Using different assays, propofol was
found to inhibit stem cell growth and induce cell apoptosis in a
dose-dependent manner, which is in agreement with the results from
in vivo studies showing that propofol induced neuronal cell
apoptosis in the developing brain (2,3).
There are two apoptotic pathways, i.e., the
intrinsic and extrinsic pathways, both requiring caspase activation
(9). The extrinsic pathway is
triggered by extracellular ligands such as FasL and TNF-α super
family members, that bind the extracellular domains of
transmembrane receptors, through which the signal is transmitted to
the cytosol, leading to the activation of caspase-8 and caspase-3
(11). Cell apoptosis through the
intrinsic pathway requires activation of caspase-9. Downregulation
of anti-apoptotic Bcl-2 family members such as Bcl-xL, increase of
mitochondria permeability and the release of cytochrome C from the
mitochondria are associated with the intrinsic pathway (11). In the present study, we found that
propofol treatment of rat embryonic neuronal stem cells resulted in
the elevation of active proteins regulating the intrinsic apoptotic
pathway (cytochrome C and caspase-9) as well as the extrinsic
pathway (caspase-8 and -3), suggesting that propofol may induce
neuronal stem cell apoptosis through both pathways. However, these
two pathways are not independent of each other. For example,
caspase-8 activated by the extrinsic pathway cleaves Bid to produce
tBid. tBid transferred into mitochondria stimulates the release of
cytochrome C which together with the cytosolic protein Apaf-1
activates caspase-9, amplifying the apoptotic effect of the
extrinsic pathway. The addition of caspase-3 inhibitor Z-DEVD-FMK
partially abolished the apoptotic effect of propofol, indicating
that the induction cell apoptosis by propofol is
caspase-dependent.
Mechanisms of cell survival also modulate cell
apoptosis. It has been revealed that general anesthesia inhibits
the production of neuronal growth factors, such as brain-derived
neurotrophic factor (BDNF), nerve growth factor (NGF) and
neurotrophin-3 (NT-3) (16).
Neuronal growth factors activate Trk and p75 NTR receptors, leading
to the phosphorylation of Akt, an important kinase controlling cell
survival and growth (17,18). Lu et al reported that the
exposure of neonatal mice to midazolam, isoflurane and nitrous
oxide resulted in neuroapoptosis, which is attributed, at least in
part, to the reduced level of BDNF (19). Synaptic proteins are essential for
the formation of synapses. Nikizad et al showed that levels
of several synaptic proteins such as synaptophysin, synaptobrevin
and amphiphysin in the cerebral cortex and the thalamus regions
were decreased following exposure to general anesthesia (midazolam,
nitrous oxide and isoflurane) in 7-day-old rat pups (20). Recently, Pesić et al studied
the mechanism of propofol inducing neuronal cell death and found
that propofol inhibited NGF production, which contributed to cell
death (21). In this study, the
effects of propofol on the expression of neuronal growth factors in
rat embryonic neural stem cells were not explored, and therefore
remain to be investigated.
Increasing evidence has shown that anesthetic drugs
cause neuronal cell apoptosis in the brain, and that the developing
brain is particularly sensitive to the drug. The timing and
duration of exposure determine the severity of cell damage
(22–25). Neural stem cells play a significant
role during development, producing the enormous diversity of cells
in the brain, including neurons. The present study, to the best of
our knowledge, is the first to explore the effect of propofol on
neural stem cells. Our results show that propofol induces
significant neural stem cell apoptosis, adding new evidence of the
detrimental effect of anesthetic drugs on the developing brain,
suggesting that clinical application of propofol should be
carefully considered in pediatric patients, and if inevitable,
large dosage and repeated use of propofol should be avoided.
Acknowledgements
The authors thank Dr Shu-Ji Li (Laboratory of
Medical Neurobiology, School of Basic Medical Science, Southern
Medical University, China) for his experienced advice. The Bureau
of Science and Technology Development (Zhongshan City, Guangdong,
China; grant number 20102A113) fully financed the study.
References
|
1
|
Bercker S, Bert B, Bittigau P, et al:
Neurodegeneration in newborn rats following propofol and
sevoflurane anesthesia. Neurotox Res. 16:140–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cattano D, Young C, Straiko MM and Olney
JW: Subanesthetic doses of propofol induce neuroapoptosis in the
infant mouse brain. Anesth Analg. 106:1712–1714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fredriksson A, Pontén E, Gordh T and
Eriksson P: Neonatal exposure to a combination of
N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor
anesthetic agents potentiates apoptotic neurodegeneration and
persistent behavioral deficits. Anesthesiology. 107:427–436. 2007.
View Article : Google Scholar
|
|
4
|
Kahraman S, Zup SL, McCarthy MM and Fiskum
G: GABAergic mechanism of propofol toxicity in immature neurons. J
Neurosurg Anesthesiol. 20:233–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Temple S: Division and differentiation of
isolated CNS blast cells in microculture. Nature. 340:471–473.
1989. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 55:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taupin P and Gage FH: Adult neurogenesis
and neural stem cells of the central nervous system in mammals. J
Neurosci Res. 69:745–749. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meier P and Vousden KH: Lucifer’s
labyrinth - ten years of path finding in cell death. Mol Cell.
28:746–754. 2007.
|
|
10
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riedl SJ and Salvesen GS: The apoptosome:
signalling platform of cell death. Nat Rev Mol Cell Biol.
8:405–413. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Xue Z and Sun A: Subclinical
concentration of sevoflurane potentiates neuronal apoptosis in the
developing C57BL/6 mouse brain. Neurosci Lett. 447:109–114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Young C, Jevtovic-Todorovic V, Qin YQ, et
al: Potential of ketamine and midazolam, individually or in
combination, to induce apoptotic neurodegeneration in the infant
mouse brain. Br J Pharmacol. 146:189–197. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ludbrook GL, Visco E and Lam AM: Propofol:
relation between brain concentrations, electroencephalogram, middle
cerebral artery blood flow velocity, and cerebral oxygen extraction
during induction of anesthesia. Anesthesiology. 97:1363–1370. 2002.
View Article : Google Scholar
|
|
15
|
Johansson CB, Momma S, Clarke DL, et al:
Identification of a neural stem cell in the adult mammalian central
nervous system. Cell. 96:25–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bibel M and Barde YA: Neurotrophins: key
regulators of cell fate and cell shape in the vertebrate nervous
system. Genes Dev. 14:2919–2937. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dudek H, Datta SR, Franke TF, et al:
Regulation of neuronal survival by the serine-threonine protein
kinase Akt. Science. 275:661–665. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bittigau P, Sifringer M, Genz K, et al:
Antiepileptic drugs and apoptotic neurodegeneration in the
developing brain. Proc Natl Acad Sci USA. 99:15089–15094. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu LX, Yon JH, Carter LB and
Jevtovic-Todorovic V: General anesthesia activates BDNF-dependent
neuroapoptosis in the developing rat brain. Apoptosis.
11:1603–1615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nikizad H, Yon JH, Carter LB and
Jevtovic-Todorovic V: Early exposure to general anesthesia causes
significant neuronal deletion in the developing rat brain. Ann NY
Acad Sci. 1122:69–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pesić V, Milanović D, Tanić N, et al:
Potential mechanism of cell death in the developing rat brain
induced by propofol anesthesia. Int J Dev Neurosci. 27:279–287.
2009.PubMed/NCBI
|
|
22
|
Rizzi S, Ori C and Jevtovic-Todorovic V:
Timing versus duration: determinants of anesthesia-induced
developmental apoptosis in the young mammalian brain. Ann NY Acad
Sci. 1199:43–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rizzi S, Carter LB, Ori C and
Jevtovic-Todorovic V: Clinical anesthesia causes permanent damage
to the fetal guinea pig brain. Brain Pathol. 18:198–210. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slikker W Jr, Zou X, Hotchkiss CE, et al:
Ketamine-induced neuronal cell death in the perinatal rhesus
monkey. Toxicol Sci. 98:145–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yon JH, Daniel-Johnson J, Carter LB and
Jevtovic-Todorovic V: Anesthesia induces neuronal cell death in the
developing rat brain via the intrinsic and extrinsic apoptotic
pathways. Neuroscience. 135:815–827. 2005. View Article : Google Scholar : PubMed/NCBI
|