Introduction
Sinomenine
(7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinan-6-one) is
a pure alkaloid extracted from the Chinese medicinal plant
Sinomenium acutum. Previous studies demonstrated that the
pharmacological properties of sinomenine included
immunosuppression, anti-inflammation and arthritis amelioration
(1,2). Due to these beneficial effects and
the low incidence of side-effects, sinomenine has been used for the
treatment of various diseases, particularly rheumatoid arthritis
(RA), systemic lupus erythematosus (SLE), renal allograft rejection
and chronic glomerulonephritis (GN) (3–6).
Although sinomenine is effectively used in the clinic and is
gaining international popularity, its exact mechanism of action is
not completely understood.
Observations from inflammatory renal diseases,
including the occurrence of allograft rejection, tubulointerstitial
nephritis and GN, have emphasized the key role of T cells in the
injurious renal immune response (7,8). It
is accepted that signals provided by the T-cell receptor
(TCR)-peptide-major histocompatibility complex (MHC) and
co-stimulatory molecules are required for the optimal activation of
T cells (9,10). Selective manipulation of
co-stimulatory molecules has been demonstrated to be beneficial in
the treatment of autoimmune diseases, tumor and transplantation
rejection (11,12). The programmed death-1
(PD-1)/PD-ligand (PD-L) pathway has been extensively characterized.
Based on results from PD-1−/− mice, PD-1 may be crucial
in maintaining peripheral tolerance (13). PD-L1 and PD-L2 are ligands for
PD-1. The role of PD-L1 in regulating T-cell responses is
controversial. However, overwhelming evidence supports the theory
that PD-L1 is a negative regulator of T-cell response through
either engagement with PD-1 or an unidentified receptor (14,15).
Nevertheless, previous studies indicated that PD-L1 was able to
stimulate T cell activation (16,17).
Whether sinomenine has an immunoregulatory effect on PD-L1 and
PD-L2 has, however, not been investigated.
In the present study, patients with mesangial
proliferative nephritis (MsPGN), one of the most common
pathological types of chronic GN, were examined. To elucidate the
potential immunoregulatory properties of sinomenine, the patients
were treated and then examined to determine the effects of
sinomenine on the expression of PD-L1 and PD-L2 by examining
peripheral blood mononuclear cells (PBMCs).
Subjects and methods
Antibodies and reagents
The mouse anti-human APC-PD-L1 (clone MIH1),
PE-PD-L2 (clone MIH1), PerCP/Cy5.5-CD8a (clone RPA-T8), FITC-CD4
(clone RPA-T4) monoclonal antibodies (mAbs) were purchased from
eBioscience (San Diego, CA, USA). The mouse anti-human PD-L1 and
PD-L2 mAbs were purchased from eBioscience. The Human Erythrocyte
Lysing kit was purchased from R&D Systems (Minneapolis, MN,
USA) and the Real-Time RT-PCR kit was purchased from Takara Bio
Inc. (Shiga, Japan).
Patients
A total of 25 patients with MsPGN that were
hospitalized in the Department of Nephrology, Xinqiao Hospital
(Chongqing, China) between July 2007 and August 2008, were enrolled
in the present study. The average age and gender distribution of
the patients was 35±3.11 years, 10 male/15 female, and that of the
healthy donors was 30.4±0.83 years, 4 male/6 female. No patients
had been treated previously with immunosuppressant or cytotoxic
drugs. All the patients enrolled had been diagnosed with primary GN
with no evidence of systemic disease, including lupus nephritis,
rheumatoid arthritis, other autoimmune diseases, hepatitis B or C
viral infection. The diagnosis of MsPGN was based on light
microscopy findings of increased mesangial cells and matrix levels
[using hematoxylin and eosin (HE), Periodic acid-Schiff (PAS) and
Masson staining]. Healthy donors were randomly selected from
doctors and nurses in the Department of Nephrology, Xinqiao
Hospital (Chongqing, China). The study protocol was conducted in
accordance with procedures approved by the Human Research Ethics
Board of Xinqiao Hospital and following receipt of the subjects’
informed written consent. Enrolled patients received treatment with
sinomenine (240 mg/day, a commonly used therapeutic dose in
clinics, according to pharmacological and clinical research; purity
>99%; obtained from Zhengqing Pharmaceutical Group, Hunan,
China) for ≥3 months unless side-effects occurred.
Blood biochemical parameters and
proteinuria detection
Blood preparations and urine aliquots were collected
at months 0, 1 and 3 during the course of the treatment with
sinomenine. Creatinine, aminotransferase, and albumin levels were
detected by an Olympus AU270 biochemical analyzer (Olympus, Tokyo,
Japan). Complement C3 was detected by immunoturbidimetry using a
Beckman Array 360 analyzer (Beckman Coulter, Miami, FL, USA).
Simultaneously, the level of proteinuria was evaluated with a
Sysmex UF-100 automated urinalysis analyzer (Sysmex, Kobe, Japan)
and was graded semi-quantitatively from 0–3 (0, <0.15 g/l; 1,
0.15–0.3 g/l; 2, 0.3–1.0 g/l; and 3, 1.0–3.0 g/l).
Isolation of human PBMCs
Peripheral whole blood (4 ml) was collected into
heparinized tubes. The human PBMCs were isolated via density
gradient centrifugation using a lymphocyte separating medium
according to the manufacturer’s instructions. The isolated PBMCs
were then lysed with TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA).
RNA extraction and quantitative real-time
RT-PCR
Total RNA was extracted from the lysed cells using
TRIzol reagent, following the manufacturer’s instructions.
Real-time PCR was performed according to the Takara two-step
real-time PCR protocol. Reverse transcription (RT) of the RNA was
performed using PrimeScript™ RTase (Takara). The resulting cDNA was
analyzed by real-time PCR with the ABI PRISM 7500 Fast Real-Time
PCR System (Applied Biosystems, Foster City, CA, USA). The primer
sequences and the product size of each gene are reported in
Table I. Quantification of the
gene expression was performed using the 2−ΔΔCt method
(18). The expression of
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal control to normalize the expression of target genes across
the samples.
 | Table IPrimer sequence used for real-time
RT-PCR. |
Table I
Primer sequence used for real-time
RT-PCR.
| Accession code | Name | Sequence (5′-3′) | Expected length
(bp) |
|---|
| NM_014143 | Human PD-L1 | Forward,
TTTCAATGTGACCAGCAC | 182 |
| | Reverse,
GGCATAATAAGATGGCTC | |
| NM_025239 | Human PD-L2 | Forward,
ATCCAACTTGGCTGCTTC | 162 |
| | Reverse,
CACTGTTCACTTCCCTCT | |
| NM_002046 | Human GAPDH | Forward,
GCACCGTCAAGGCTGAGAAC | 142 |
| | Reverse,
ATGGTGGTGAAGACGCCAGT | |
Flow cytometry
The PD-L1 and PD-L2 expression on the
CD4+ and CD8+ T cells in the PBMCs was
detected by flow cytometry. A total of 100 μl whole blood was
stained with corresponding antibodies and 2 ml of H-lyse buffer was
added to the blood. The surface expression of the immune molecules
on the PBMCs was quantified with a FACScan flow cytometer (Becton
Dickinson, Heidelberg, Germany). The FACS data were analyzed by
using CELLQuestk software (BD Biosciences) and numbers indicated
the mean fluorescence intensity (19). Background fluorescence was measured
in the cells treated with 100 μl staining buffer instead of the
fluorescent antibodies. Appropriate isotype-matched antibodies from
the manufacturer were used as specificity controls.
Immunohistochemistry of the renal
biopsy
The intra-renal levels of PD-L1 and PD-L2 were
determined by performing immunohistochemistry on the 3-μm
paraffin-embedded tissues from the renal biopsies of the 25
patients with MsPGN. Ten specimens obtained from pre-transplant
renal biopsies (collected at Xinqiao Hospital between July 2005 and
August 2008) served as the controls. Sections were incubated with
the anti-PD-L1 (1:100) and anti-PD-L2 (1:100) antibodies overnight
at 4°C. This was followed by subsequent incubation with the
secondary antibody, EnVision™ (EnVision™ system, HRP, mouse/rabbit;
Dako, Denmark) for 30 min. Negative controls were obtained by
omitting the primary antibodies or using irrelevant
immunoglobulins. Reactivity was detected with a DAB Elite kit
(K3465; Dako). PD-L1 and PD-L2 staining was graded
semiquantitatively by noting the percentage of immunoreactive
tubules (grade 0, no reactive tubules; grade 1, <20% of reactive
tubules; grade 2, between 20 and 50% of reactive tubules; and grade
3, >50% of reactive tubules). The immunohistochemical grading
was evaluated by 2 independent observers with good concordance.
Statistical analysis
All data are expressed as the means ± SEM. The
independent samples t-test and χ2 test were used for the
continuous and categorical variables, respectively, in the
comparison between the healthy donors and the MsPGN patients prior
to treatment. A general linear model was used to examine the
repeated measurement data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics and laboratory
data
The clinical diagnosis of MsPGN was made by the
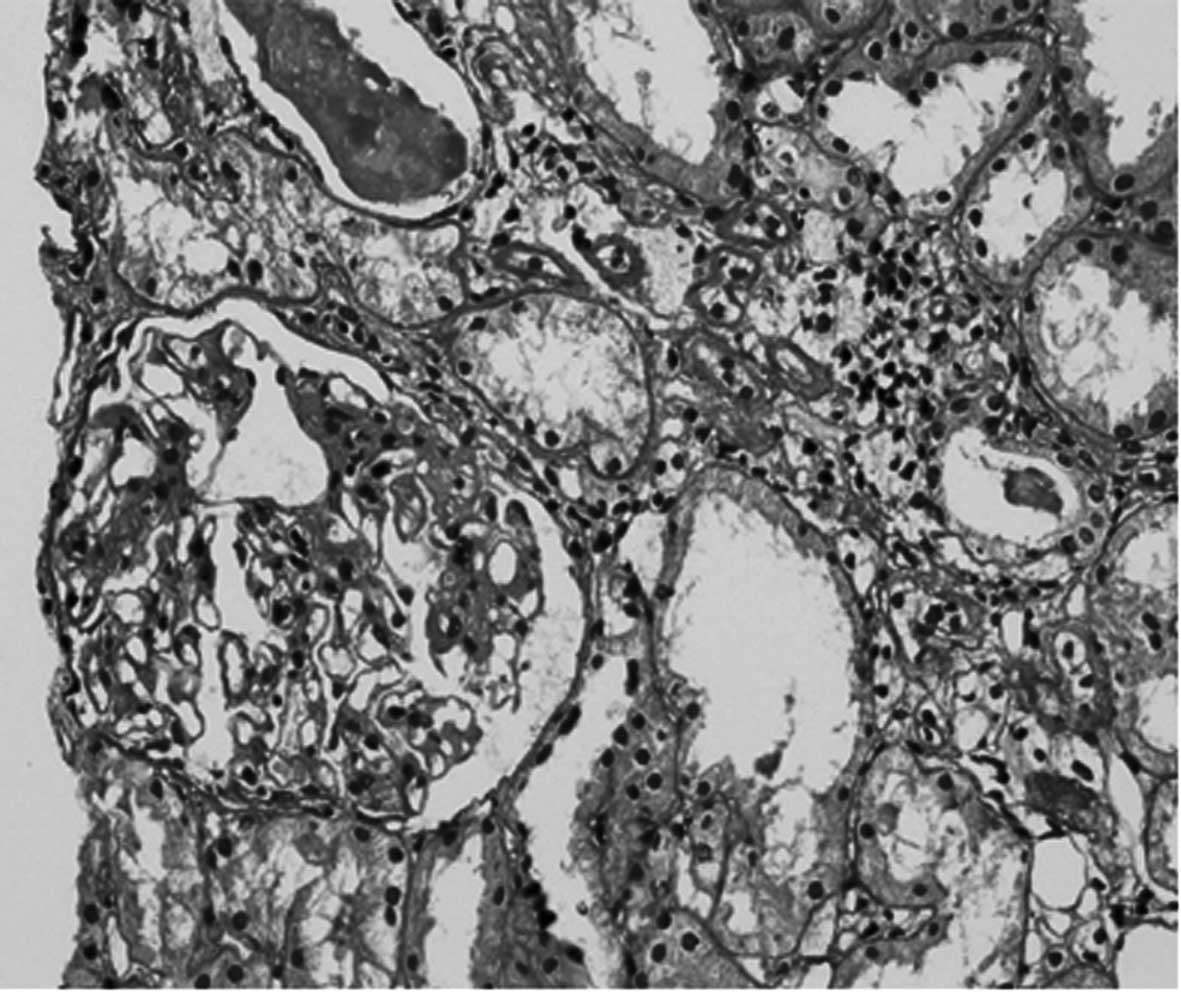
histological examination of each renal biopsy. Fig. 1 shows a patient renal biopsy slide
with glomerular mesangial proliferation stained with PAS. The
patients enrolled in the present study were the same individuals as
in our previous study (20). The
average age and the gender distribution of the patients (35±3.11
years, 10 male/15 female) did not differ significantly from those
of the healthy donors (30.4±0.83 years, 4 male/6 female). There
were no differences in the serum levels of creatinine, urea
nitrogen, albumin or aminotransferase between the patients and
healthy donors. There were also no differences in the values of
hemoglobin and white blood cells (WBCs) between the two groups.
Sinomenine was observed to have no effect on these laboratory data.
In total, three patients had a transient skin rash. Varying levels
of proteinuria were observed in the treated patients (the level of
proteinuria was graded semiquantitatively from 0–3; 3 patients were
grade 3, 13 patients were grade 2 and 9 patients were grade 1 prior
to treatment with sinomenine). At 1 month subsequent to initiation
of the treatment with sinomenine, the proteinuria was ameliorated
(11 patients were grade 2, 11 patients were grade 1 and 3 patients
were grade 0). At 3 months, the amelioration of the proteinuria by
sinomenine was more significant (3 patients were grade 2, 7
patients were grade 1 and 15 patients were grade 0).
Changes in the expression of the PD-L1
and PD-L2 mRNA in the PBMCs
Quantitative real-time RT-PCR was used to measure
the expression levels of the PD-L1 and PD-L2 mRNA in the PBMCs from
the 10 healthy donors and 25 patients with MsPGN, at 0, 1 and 3
months subsequent to initiation of treatment with sinomenine. As
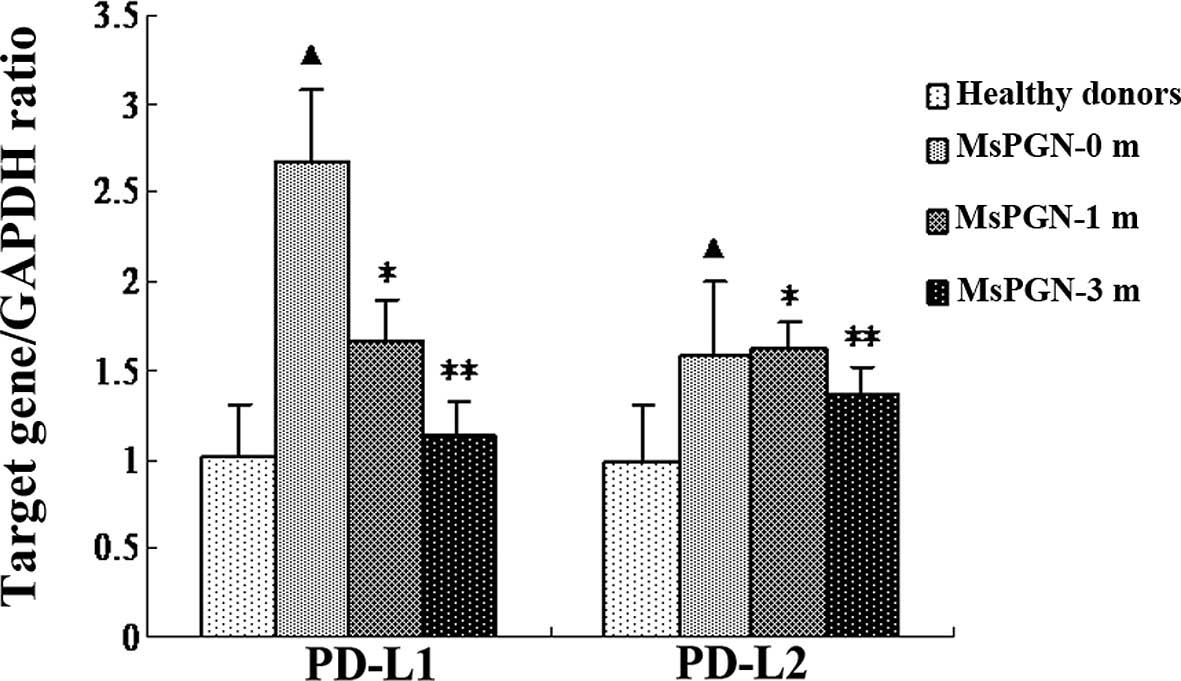
shown in Fig. 2, the PBMCs from
the MsPGN patients expressed high levels of the PD-L1 (P=0.037)
mRNA compared with the controls. There were no significant
differences in the expression of PD-L2 mRNA between the MsPGN
patients and the controls (P=0.627). The expression of the PD-L1
mRNA was suppressed by sinomenine. The decrease in the PD-L1
expression was detected at 1 (P=0.034) and 3 months (P=0.002).
However, sinomenine did not affect the expression of the PD-L2 mRNA
(P=1.000).
Change in the expression of the PD-L1 and
PD-L2 protein in the PBMCs
The expression of the PD-L1 and PD-L2 molecules on
the CD4+ and CD8+ T cells in the PBMCs was
investigated by using flow cytometry analysis. As shown in Fig. 3, the constitutive expression of
PD-L1 was detected on the CD4+ and CD8+ T
cells in the PBMCs from the MsPGN patients and the healthy donors.
However, the expression of PD-L2 was hardly detected on the
CD4+ and CD8+ T cells in the PBMCs from the
two groups. The patients with MsPGN were observed to have an
increased PD-L1 expression on the CD4+ and
CD8+ T cells in the PBMCs compared with the healthy
donors. Overall, treatment with sinomenine significantly inhibited
the high expression of PD-L1 on the CD4+ and
CD8+ T cells in the PBMCs from the MsPGN patients,
whereas it had no effect on the expression of PD-L2.
Intra-renal PD-L1 and PD-L2 protein
expression
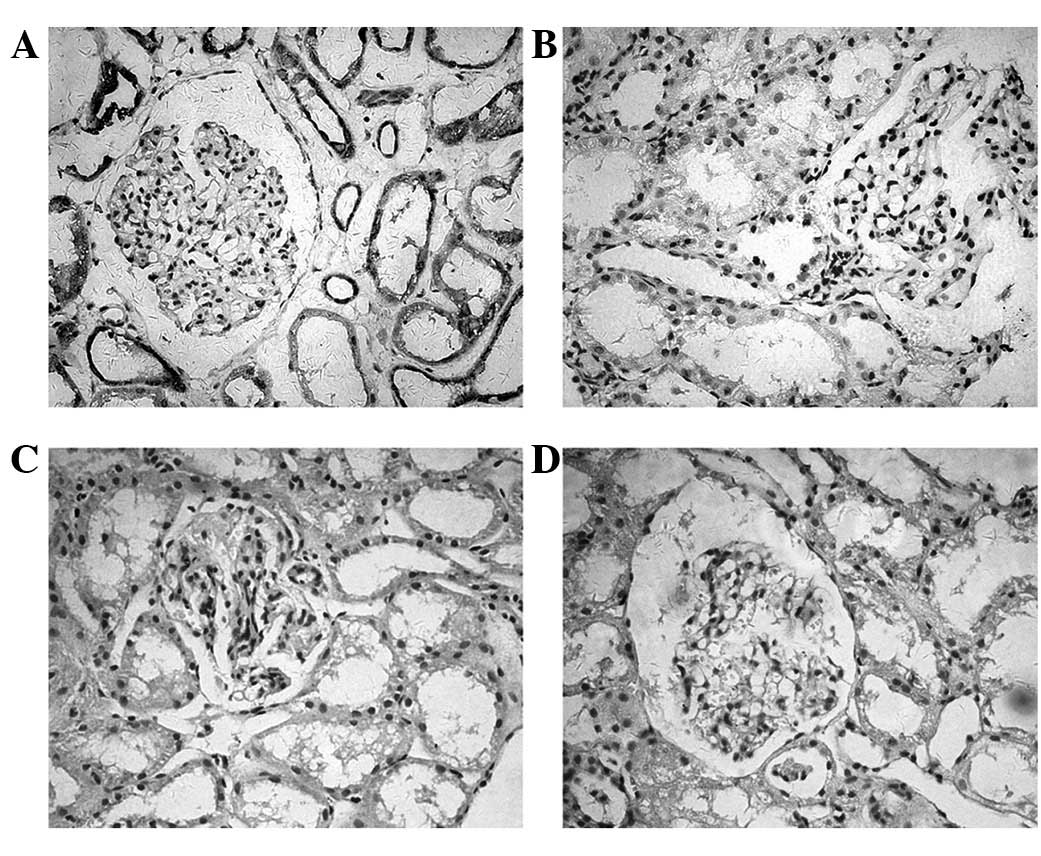
The expression of PD-L1 and PD-L2 in the human renal
biopsy tissue samples was detected by immunohistochemistry. As
shown in Fig. 4, a significant
PD-L1 expression was detected in the tubular epithelium. Positive
staining was detected in the cell membrane or cytoplasm or in the
two together. However, the expression of PD-L1 was not observed in
the glomeruli. Patients with MsPGN were identified as having
increased PD-L1 (P=0.012) expression in the renal biopsy tissues
compared with normal individuals. The intensity levels of the PD-L1
expression in the normal or diseased renal tissues are shown in
Table II. The expression of PD-L2
was not observed in the renal tissues of the MsPGN patients or the
controls.
 | Table IIIntra-renal expression of PD-L1. |
Table II
Intra-renal expression of PD-L1.
| No. of positive
cases |
|---|
|
|
|---|
| Group | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|
| MsPGNa | 0 | 7 | 14 | 4 |
| Normal kidneys | 5 | 4 | 1 | 0 |
Discussion
To elucidate the potential immunoregulatory
properties of sinomenine, its effect on the expression of the
co-stimulatory molecules, the PD-1 ligands, by the PBMCs was
investigated in the present study. The upregulation of PD-L1 was
detected in the PBMCs from the MsPGN patients at the mRNA and
protein levels. In contrast to PD-L1, the expression of PD-L2 in
the PBMCs was hardly detected by flow cytometry in the MsPGN
patients and the healthy donors, although the PD-L2 mRNA expression
was observed. This translational suppression may be related to the
different expression pattern of PD-L2, since the cell-surface
expression of PD-L2 is limited to dendritic cells and macrophages,
whereas PD-L1 is widely distributed in the lymphoid organs and
non-lymphoid tissues (21,22). We previously observed significant
PD-L1 staining in the renal tubules using immunohistochemistry in
diseased kidneys suffering IgA nephropathy, interstitial nephritis
or lupus nephritis (19). In the
present study, the high expression of PD-L1 was also detected in
the tubular epithelium in the renal biopsy tissues from the MsPGN
patients, suggesting that PD-L1 expression is upregulated in
vivo in inflammatory kidneys. However, the expression of PD-L2
was not observed in the kidneys from the MsPGN patients. The
differential expression patterns of PD-L1 and PD-L2 thus suggest
different roles for these molecules in the pathogenesis of
MsPGN.
While PD-L1 is associated with the negative
regulation of T-cell responses via PD-1, several studies have
indicated that PD-L1 was able to co-stimulate T-cell growth and
cytokine production. These outcomes were produced when resting T
cells were stimulated with suboptimal concentrations of anti-CD3
mAb, immobilized PD-L1-Ig moderately enhanced proliferation,
strongly upregulated IL-10 production and modestly upregulated
IFN-γ and GM-CSF production in both human and mouse systems
(15,23). A noteworthy result was recorded in
a study by Kanai et al(24), which observed the expression
profiles of the PD-1 ligands in human inflammatory bowel disease
and a murine chronic colitis model. There was a significantly
increased expression of PD-L1 on the T lymphocytes, B lymphocytes,
macrophage and DCs in the inflamed colons of the inflammatory bowel
disease patients and the colitic mice. However, the administration
of the anti-PD-L1 mAbs suppressed the wasting disease and colitis,
abrogated the leukocyte infiltration and reduced the production of
interferon (IFN)-γ, interleukin (IL)-2, and tumor necrosis factor
(TNF)-α in the lamina propria of the CD4+ T cells. These
data suggested that the blockade of PD-L1 suppressed the
development of chronic intestinal inflammation. In the present
study, the high expression of PD-L1 in the PBMCs and renal biopsy
tissues may correlate with the development of MsPGN.
Sinomenine has been widely used in China for the
treatment of rheumatoid arthritis, renal allograft rejection and
SLE (3–6). In the present study, the treatment
with sinomenine led to significantly reduced proteinuria and
elevated complement C3 levels, which demonstrated that sinomenine
was able to effectively improve the clinical symptoms of the
patients with MsPGN. Previous studies have demonstrated that
sinomenine was able to regulate several immune functions, including
i) reducing the production of TNF-α by activating macrophages; ii)
inhibiting IL-8 and membrane (m)IL-2R and enhancing IL-6 production
on the PBMCs; iii) inhibiting human CD4+ T-cell
proliferation; iv) inducing apoptosis in the synoviocytes; and v)
inhibiting maturation of the monocyte-derived dendritic cells
(25–30).
In conclusion, sinomenine was an effective strategy
for treating MsPGN through the downregulation of PD-L1 expression.
These results suggest that sinomenine may be able to regulate the
T-cell response by the inhibition of T-cell-associated PD-L1
expression.
Acknowledgements
This study is supported by the National Science
Foundation of China (30570870). The authors would like to thank
Senior Technician X.L. Fu from the Institute of Immunology of the
PLA for her assistance in the flow cytometric analysis.
References
|
1
|
Yamasaki H: Pharmacology of sinomenine, an
anti-rheumatic alkaloid from Sinomenium acutum. Acta Med
Okayama. 30:1–20. 1976.PubMed/NCBI
|
|
2
|
Deng ZS, Zhao Y, He CC, Jin J, He YM and
Li JX: pH-dependent, stereoselective dimerization of sinomenine.
Org Lett. 10:3879–3882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou H, Wong YF, Wang J, Cai X and Liu L:
Sinomenine ameliorates arthritis via MMPs, TIMPs, and cytokines in
rats. Biochem Biophys Res Commun. 376:352–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai YB, Huang X, Luo ZG, Li JF, Ou YG,
Yang P and Qin JP: Immunosuppressive effect of Sinomenine on ICAM-1
expression in rat renal allograft rejection. Mod J Integr Tradit
Chin West Med. 12:1358–1363. 2003.
|
|
5
|
Yang P, Yang L and Luo Z: Effects of
sinomenine on T lymphocyte subsets in rat renal allograft
transplantation model. J Clin Urol. 18:620–622. 2003.
|
|
6
|
Feng H, Yamaki K, Takano H, Inoue K,
Yanagisawa R and Yoshino S: Effect of sinomenine on
collagen-induced arthritis in mice. Autoimmunity. 40:532–539. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen RA, Bayliss G, Crispin JC,
Kane-Wanger GF, Van Beek CA, Kyttaris VC, Avalos I, Yu CY, Tsokos
GC and Stillman IE: T cells and in situ cryoglobulin deposition in
the pathogenesis of lupus nephritis. Clin Immunol. 128:1–7. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurts C, Heymann F, Lukacs-Kornek V, Boor
P and Floege J: Role of T cells and dendritic cells in glomerular
immunopathology. Semin Immunopathol. 29:317–335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baxter AG and Hodgkin PD: Activation
rules: the two-signal theories of immune activation. Nat Rev
Immunol. 2:439–446. 2002.PubMed/NCBI
|
|
10
|
Goronzy JJ and Weyand CM: T-cell
co-stimulatory pathways in autoimmunity. Arthritis Res Ther.
10(Suppl 1): S32008. View
Article : Google Scholar
|
|
11
|
Loisel-Meyer S, Foley R and Medin JA:
Immuno-gene therapy approaches for cancer: from in vitro studies to
clinical trials. Front Biosci. 13:3202–3214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korman A, Yellin M and Keler T: Tumor
immunotherapy: preclinical and clinical activity of anti-CTLA4
antibodies. Curr Opin Investig Drugs. 6:582–591. 2005.PubMed/NCBI
|
|
13
|
Nishimura H, Okazaki T, Tanaka Y, Nakatani
K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N
and Honjo T: Autoimmune dilated cardiomyopathy in PD-1
receptor-deficient mice. Science. 291:319–322. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fife BT, Pauken KE, Eagar TN, Obu T, Wu J,
Tang Q, Azuma M, Krummel MF and Bluestone JA: Interactions between
PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop
signal. Nat Immunol. 10:1185–1192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Latchman YE, Liang SC, Wu Y, Chernova T,
Sobel RA, Klemm M, Kuchroo VK, Freeman GJ and Sharpe AH:
PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting
cells, and host tissues negatively regulates T cells. Proc Natl
Acad Sci USA. 101:10691–10696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subudhi SK, Zhou P, Yerian LM, Chin RK, Lo
JC, Anders RA, Sun Y, Chen L, Wang Y, Alegre ML and Fu YX: Local
expression of B7-H1 promotes organ-specific autoimmunity and
transplant rejection. J Clin Invest. 113:694–700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2[-Delta Delta C(T)] method. Methods. 25:402–408. 2001.
|
|
19
|
Chen Y, Zhang J, Li J, Zou L, Zhao T, Tang
Y and Wu Y: Expression of B7-H1 in inflammatory renal tubular
epithelial cells. Nephron Exp Nephrol. 102:81–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Zhang J, Hou W, Wang D, Li F,
Zhang Y and Yuan F: Immunoregulatory effects of sinomenine on the
T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of
mesangial proliferative nephritis. Int Immunopharmacol. 9:894–899.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
del Rio ML, Buhler L, Gibbons C, Tian J
and Rodriguez-Barbosa JI: PD-1/PD-L1, PD-1/PD-L2, and other
co-inhibitory signaling pathways in transplantation. Transpl Int.
21:1015–1028. 2008.PubMed/NCBI
|
|
22
|
Menke J, Lucas JA, Zeller GC, Keir ME,
Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH and Kelley VR:
Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune
kidney disease: distinct roles. J Immunol. 179:7466–7477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamura H, Dong H, Zhu G, Sica GL, Flies
DB, Tamada K and Chen L: B7-H1 costimulation preferentially
enhances CD28-independent T-helper cell function. Blood.
97:1809–1816. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanai T, Totsuka T, Uraushihara K, et al:
Blockade of B7-H1 suppresses the development of chronic intestinal
inflammation. J Immunol. 171:4156–4163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shu L, Yin W, Zhang J, Tang B, Kang YX,
Ding F and Hua ZC: Sinomenine inhibits primary CD4+
T-cell proliferation via apoptosis. Cell Biol Int. 31:784–789.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He X, Wang J, Guo Z, Liu Q, Chen T, Wang X
and Cao X: Requirement for ERK activation in sinomenine-induced
apoptosis of macrophages. Immunol Lett. 98:91–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Fang Y, Huang W, Zhou X, Wang M,
Zhong B and Peng D: Effect of sinomenine on cytokine expression of
macrophages and synoviocytes in adjuvant arthritis rats. J
Ethnopharmacol. 98:37–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu S, Hu Y and Lu F: Effect of Sinomenine
on IL-8, IL-6, IL-2 produced by peripheral blood mononuclear cells.
J Tongji Med Univ. 19:257–259. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang QC, Chen JF, Chen GX, et al: Effect
of sinomenine on apoptosis of synoviocytes in CIA rats. Zhongguo
Lin Chuang Kang Fu. 6:2541–2548. 2002.(In Chinese).
|
|
30
|
Zhao Y, Li J, Yu K, Liu Y and Chen X:
Sinomenine inhibits maturation of monocyte-derived dendritic cells
through blocking activation of NF-kappa B. Int Immunopharmacol.
7:637–645. 2007. View Article : Google Scholar : PubMed/NCBI
|