Introduction
The N-methyl-D-aspartate receptor (NMDAR) is a
subtype of ion-type glutamic acid receptor and is associated with a
variety of physiological processes, including excitatory synaptic
transmission, learning and memory and neural development. In
particular, NMDA is important in the development of the nervous
system via the regulation of neuronal migration and synaptic
plasticity (1). NMDAR is a
membrane ionophorous protein composed of NR1, the functional
subunit constituting the ion channel and NR2, the regulatory
subunit (2). NMDAR is a voltage
and ligand doubly gated channel and Mg2+ not only blocks
the NMDA channel in a voltage-dependent manner but also potentiates
NMDA-induced responses at positive membrane potentials. Magnesium
glycinate and magnesium taurinate treatment have been utilized to
provide rapid recovery from depression (3). When the postsynaptic membrane
depolarizes, channels open and large quantities of Ca2+
enter the cells to function as second messengers to activate
calmodulin kinase IV (CaMK IV), which then phosphorylates cAMP
response element binding protein (CREB) on Ser133 in the active
site. Following phosphorylation, CREB interacts with CBP
(CREB-binding protein) to activate transcription of downstream
genes.
A number of previous studies have identified a role
for CREB in vision plasticity (4,5).
However, the correlation between NMDAR and CREB expression and
vision plasticity remains unclear. In the present study, expression
levels of NMDAR and CREB were detected in the visual cortex of
monocularly-deprived (MD) animal models. Results of the present
study are likely to provide insight into the molecular mechanism of
vision plasticity.
Materials and methods
Animal groups
Eighty Sprague Dawley pedo-rats (2 weeks old) were
obtained from the animal department of the China Medical University
(Shenyang, China) and divided into 4 groups (n=20). The normal
group received no treatment. The MD group was anesthetized by
peritoneal injection of 5% chloral hydrate (3 ml/kg). Following
this, the pelage surrounding the palpebral margin was removed, the
area was sterilized with iodophors and the superior and inferior
palpebral margins were cut 1–1.5 mm from the inner to external
canthus. Experimental eyelids were sutured closed using the
mattress suture with 6-0 suture silk to adhere the superior and
inferior eyelids to form MD (right eye-deprived). The saline group
consisted of MD rats injected with saline into the archae-visual
cortex ocellanae region. The KN-93 group consisted of MD rats
injected with the calmodulin kinase IV inhibitor, KN-93 (dissolved
in saline as 1 μg/4 μl) into archae-visual cortex ocellanae
regions. Rats in each group were reared in the same naturally lit
environment for 45 days. The experimental protocol was approved by
the Ethics Committee of the China Medical University.
Immunohistochemistry
Hibateral visual cortices were removed from 10 rats
in each group, fixed with 4% paraformaldehyde and embedded in
mineral wax. Following this, slides were treated with dimethyl
benzene and subjected to hydration with sequential ethanol, washed
with 0.01 mol/l PBS (3 times for 5 min), then incubated with 0.5%
parenzyme for 30 min at 37°C. Next, slides were washed with PBS (as
described), incubated with 3% H2O2 for 15 min
at room temperature to block endogenous peroxidase, washed with PBS
(as described) and incubated with normal goat serum for 15 min at
room temperature. Slides were then incubated overnight at 4°C with
primary antibody against NMDAR1 (1:1,000; Pharmigen, San Diego, CA,
USA) and pCREB (1:50, Cell Signaling, Danvers, MA, USA), followed
by incubation with HRP-labeled secondary antibody for 30 min at
37°C and DAB chromogenic reaction. Slides were counterstained with
hematoxylin and observed under a microscope.
Western blot analysis
Hibateral visual cortices were removed from 10 rats
of each group and lysed in RIPA buffer by homogenization on ice.
Lysates were collected following centrifugation at 12,000 rpm for
10 min at 4°C. Protein levels were quantified using the
bicinchoninic acid method. Equivalent amounts of protein (30
μg/lane) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to PVDF
membranes. Membranes were blocked in PBS containing 5% non-fat dry
milk (w/v) for 4 h at 37°C and then incubated with primary
antibodies against NMDAR1 (1:1,000) and pCREB (1:50) overnight at
4°C. Membranes were then incubated with HRP-conjugated secondary
antibodies at room temperature for 1 h, developed using enhanced
chemiluminescence reagent and exposed to X-ray film in a dark room.
β-actin was used as loading control. Gelpro32 gel image analysis
software was used to perform densitometric analysis of the blots
and the ratio of values obtained for NMDAR1 and pCREB to β-actin in
each group was considered to represent the relative amount of
target protein for each sample.
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analysis was performed using the SPSS software 13.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of NMDAR1 and pCREB levels in
the visual cortex of rats in MD and normal groups
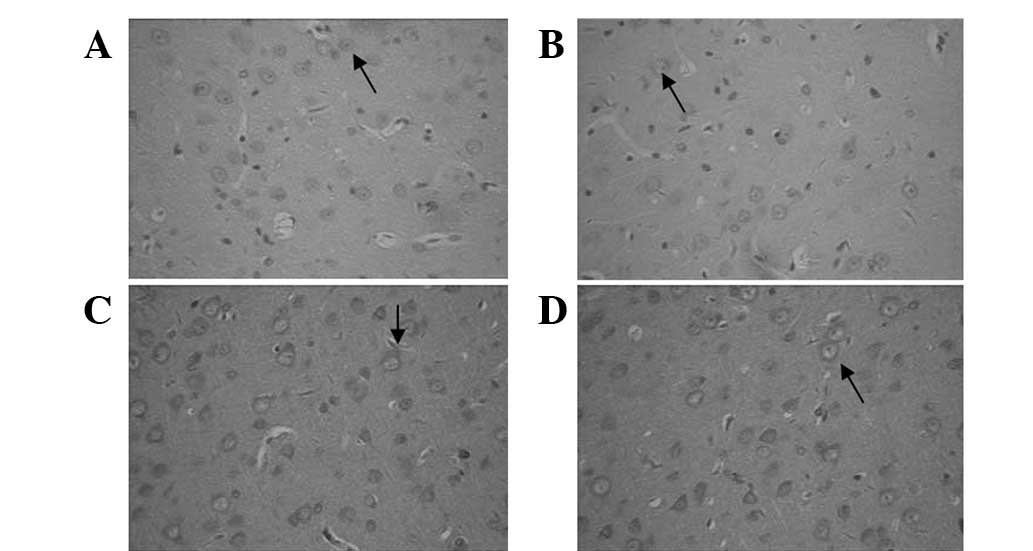
Immunohistochemical staining identified that
compared with normal rats, the number of NMDAR1-positive cells was
increased in the deprived but decreased in the non-deprived-side
visual cortex in MD rats (Fig. 1).
Similarly, the number of pCREB-positive cells was increased in the
deprived- but decreased in the non-deprived-side visual cortex in
MD, compared with the normal rat group (Fig. 2).
Western blot analysis demonstrated that relative
protein expression of NMDAR1 was significantly higher in the
deprived-side visual cortex of MD (0.498±0.05) compared with the
normal group (0.277±0.03; Fig. 3A
and Table I, t=11.985, P<0.05).
Similarly, pCREB protein levels were observed to be significantly
higher in the deprived-side visual cortex of MD (0.652±0.03)
compared with the normal group (0.433±0.03; Fig. 3B and Table I, t=16.323, P<0.05). However,
NMDAR1 protein expression was found to be significantly lower in
the non-deprived-side visual cortex of MD (0.132±0.01) compared
with the normal group (0.249±0.03; Fig. 3A, Table I, t=11.700, P<0.05) and pCREB
protein expression was significantly lower in the non-deprived-side
visual cortex of MD (0.309±0.05)compared with the normal group
(0.391±0.03; Fig. 3B, Table I, t=4.447, P<0.05). Results are
consistent with immunohistochemical analysis.
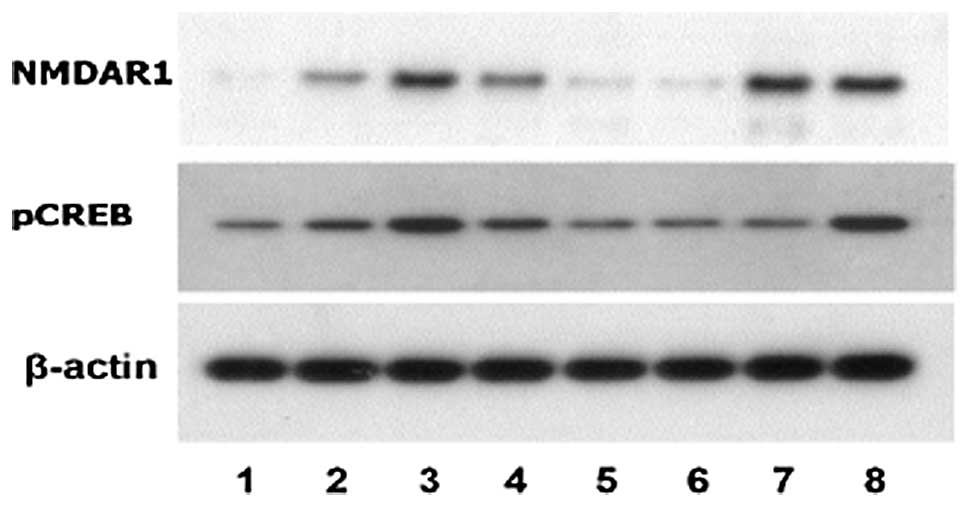 | Figure 3Western blot analysis of NMDAR1 and
pCREB levels in the visual cortex of rat groups. 1, non-deprived
side in MD; 2, non-deprived side in normal; 3, deprived side in MD;
4, deprived side in normal; 5, non-deprived side in KN-93; 6,
non-deprived side in NS; 7, deprived side in KN-93; and 8, deprived
side in NS groups. β-actin served as a loading control.
Representative blots from three independent experiments are
presented. No monocular eyelid suture in normal group was
performed, therefore the non-deprived side of normal was in
homonymy with the non-deprived side of MD. NMDAR1,
N-methyl-D-aspartate receptor subunit 1; pCREB, phosphorylated cAMP
response element binding protein; MD, monocularly-deprived. |
 | Table INMDAR1 and pCREB protein expression
levels in non-deprived and deprived-side visual cortex in MD and
normal groups (mean ± SD). |
Table I
NMDAR1 and pCREB protein expression
levels in non-deprived and deprived-side visual cortex in MD and
normal groups (mean ± SD).
| | NMDAR1 | pCREB |
|---|
| |
|
|
|---|
| Group | n | Non-deprived | Deprived | Non-deprived | Deprived |
|---|
| MD | 10 | 0.132±0.01 | 0.498±0.05 | 0.309±0.05 | 0.652±0.03 |
| Normal | 10 | 0.249±0.03 | 0.277±0.03 | 0.391±0.03 | 0.433±0.03 |
| t | | 11.700 | 11.985 | 4.447 | 16.323 |
| P-value | | <0.05 | <0.05 | <0.05 | <0.05 |
Comparison of NMDAR1 and pCREB levels in
the visual cortex of rats between KN-93 and saline groups
Immunohistochemical staining revealed that the
number of NMDAR1-positive cells was not significantly different in
the deprived- and non-deprived-side visual cortices between the
KN-93 and saline groups (Fig. 4).
However, the number of pCREB-positive cells was reduced in the
deprived-side visual cortex of KN-93 compared with that of the
saline group (Fig. 5). In
addition, the number of pCREB-positive cells was not found to be
significantly different in the non-deprived-side visual cortex of
KN-93 compared with the saline group (Fig. 5).
Western blot analysis demonstrated that NMDAR1
protein levels were not significantly different between the
deprived-side visual cortex of KN-93 (0.485±0.08) and the NS group
(0.498±0.05; Fig. 3A, Table II, t=0.536, P>0.05). However,
pCREB protein levels were observed to be significantly lower in the
deprived-side visual cortex of KN-93 (0.331±0.03) compared with the
saline group (0.666±0.05; Fig. 3B,
Table II, t=18.168, P<0.05).
However, NMDAR1 protein expression was significantly lower
(0.132±0.01) in the non-deprived-side visual cortex of MD compared
with the normal group (0.249±0.03; Fig. 3A, t=11.700, P<0.05) and pCREB
protein expression was observed to be significantly lower
(0.309±0.05) in the non-deprived-side visual cortex of MD rats
compared with the normal group (0.391±0.03; Fig. 3B, t=4.447, P<0.05).
 | Table IINMDAR1 and pCREB protein expression
levels in non-deprived and deprived-side visual cortex in KN-93 and
saline groups (mean± SD). |
Table II
NMDAR1 and pCREB protein expression
levels in non-deprived and deprived-side visual cortex in KN-93 and
saline groups (mean± SD).
| | NMDAR1 | pCREB |
|---|
| |
|
|
|---|
| Group | n | Non-deprived | Deprived | Non-deprived | Deprived |
|---|
| KN-93 | 10 | 0.1457±0.02 | 0.501±0.05 | 0.326±0.05 | 0.331±0.03 |
| NS | 10 | 0.1457±0.02 | 0.485±0.08 | 0.315±0.07 | 0.666±0.05 |
| t | | 0.0175 | 0.536 | 0.404 | 18.168 |
| P-value | | >0.05 | >0.05 | >0.05 | <0.05 |
In the non-deprived-side visual cortex, western blot
analysis revealed that NMDAR1 protein levels were 0.1457±0.02 in
KN-93 and 0.1459±0.03 in saline groups. No statistically
significant difference was identified between the groups (Fig. 3 and Table I, t=0.0175, P>0.05). Similarly,
pCREB protein levels were 0.326±0.05 in KN-93 and 0.315±0.07 in the
saline groups. No statistically significant difference was found
between the groups (Fig. 3 and
Table II, t=0.404, P>0.05).
Results are consistent with those of immunohistochemical
analysis.
Discussion
CREB is a nuclear factor with important biological
functions, including regulation of learning and memory (6,7). The
transcriptional activity of CREB mainly relies on the
phosphorylation of Ser133, catalyzed by CaMK IV. NMDAR is an
excitation pattern ionophorous protein residing in the postsynaptic
membrane of neurons. NMDA induced Ca2+ overload is
important for activation of the downstream cascades (8).
The NMDAR-CREB signaling cascade has been
demonstrated to protect against extrasynaptic NMDAR-induced
neuronal cell death and ischemic brain damage (9). However, the role of the NMDAR-CREB
signaling cascade in vision plasticity remains unclear.
A number of studies have identified that NMDAR1
expression is altered in the visual cortex under various conditions
(10–12). However, studies have yet to examine
the expression and activation of CREB under these conditions and
determine the correlation between NMDAR1 expression and CREB
expression and activation. To the best of our knowledge, this is
the first study to examine this correlation in MD rats. In
addition, previous studies have only compared NMDAR1 expression
between the deprived and non-deprived-side visual cortex of model
rats. In the present study NMDAR1 expression was compared in the
deprived or non-deprived side to that in corresponding sides of
normal control rats, a more appropriate method to identify the
competition mechanism of reciprocal growth and decline in the
projection of binocular retinal ganglial cells to the visual
cortex. Using immunohistochemical staining and western blot
analysis, distinct alterations in NMDAR1 expression in the
hibateral visual cortex were observed following monocular
deprivation. NMDAR1 expression was reduced in the non-deprived-side
visual cortex which received the projection of ganglion cells of
the deprived side, but was increased in the deprived-side visual
cortex which received the projection of ganglion cells of the
non-deprived side.
A limited number of studies have examined the
expression of pCREB during vision plasticity. In the present study,
immunohistochemistry and western blot analysis revealed that pCREB
levels were increased in the deprived and reduced in the
non-deprived-side visual cortex compared with the normal group.
Results demonstrate that altered pCREB expression correlates with
NMDAR1 expression in the deprived and non-deprived-side visual
cortex, indicating that the NMDAR-CREB cascade may play a role in
the formation process of vision plasticity.
To test this hypothesis, cells were treated with
KN-93, a specific inhibitor of CaMK IV which catalyzes the
phosphorylation of CREB and promotes the activation of CREB target
genes. The results of immunohistochemistry and western blot
analysis found no significant difference in NMDAR1 expression in
the visual cortex between the KN-93 and saline groups, indicating
that inhibition of CaMK IV does not affect NMDAR1 expression, which
lies upstream of the NMDAR-CREB cascade. However, as expected,
pCREB levels in the deprived-side visual cortex in KN-93 were lower
than those in the saline group. Collectively, results indicate that
CaMK IV functions upstream of CREB but downstream of NMDAR to
modulate vision plasticity.
In summary, results of the present study demonstrate
that NMDAR1 and pCREB levels in the visual cortex of MD rats were
higher in the deprived and lower in the non-deprived sides compared
with the normal group, which implies that NMDAR1 and CREB are
involved in the ocular dominance forming process during vision
development. CaMK IV inhibitor reduced pCREB but not NMDAR1 protein
levels in the deprived and non-deprived sides, demonstrating that
the NMDAR-CaMK IV-CREB axis is associated with regulation of vision
plasticity.
Acknowledgements
The authors thank Dr Shaoli Zhou and Dr Jiangyue
Zhao for their assistance with this study.
References
|
1
|
Li F and Tsien JZ: Memory and the NMDA
receptors. N Engl J Med. 361:302–303. 2009. View Article : Google Scholar
|
|
2
|
Paoletti P and Neyton J: NMDA receptor
subunits: function and pharmacology. Curr Opin Pharmacol. 7:39–47.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eby GA and Eby KL: Rapid recovery from
major depression using magnesium treatment. Medical hypotheses.
67:362–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mower AF, Liao DS, Nestler EJ, Neve RL and
Ramoa AS: cAMP/Ca2+ response element-binding protein
function is essential for ocular dominance plasticity. J Neurosci.
22:2237–2245. 2002.PubMed/NCBI
|
|
5
|
Pham TA, Graham SJ, Suzuki S, Barco A,
Kandel ER, Gordon B and Lickey ME: A semi-persistent adult ocular
dominance plasticity in visual cortex is stabilized by activated
CREB. Learn Mem. 11:738–747. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giachino C, De Marchis S, Giampetro C,
Parlato R, Perroteau I, Schütz G, Fasolo A and Peretto P: cAMP
response element-binding protein regulates differentiation and
survival of newborn neurons in the olfactory bulb. J Neurosci.
25:10105–10118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peltier J, O’Neill A and Schaffer DV:
PI3K/Akt and CREB regulate adult neural hippocampal progenitor
proliferation and differentiation. Dev Neurobiol. 67:1348–1361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun XM, Lu JH, Qiu YH, Liu Z, Wang XQ and
Peng YP: Interleukin-6 reduces NMDA-induced Ca2+
overload via prevention of Ca2+ release from
intracellular store. Int J Neurosci. 121:423–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang SJ, Buchthal B and Lau D: A
signaling cascade of nuclear calcium-CREB-ATF3 activated by
synaptic NMDA receptors defines a gene repression module that
protects against extrasynaptic NMDA receptors-induced neuronal cell
death and ischemic brain damage. J Neurosci. 31:4978–4990. 2011.
View Article : Google Scholar
|
|
10
|
Aoki C, Venkatesan C and Go CG: Cellular
and subcellular localization of NMDA-R1 subunit immunoreactivity in
the visual cortex of adult and neonatal rats. J Neurosci.
14:5202–5222. 1994.PubMed/NCBI
|
|
11
|
Yin ZQ, Deng ZM and Crewther SG: Altered
expression of alternatively spliced isoforms of the mRNA NMDAR1
receptor in the visual cortex of strabismic cats. Mol Vis.
20:271–276. 2001.PubMed/NCBI
|
|
12
|
Murphy KM, Duffy KR and Jones DG:
Experience-dependent changes in NMDAR1 expression in the visual
cortex of an animal model for amblyopia. Vis Neurosci. 21:653–670.
2004. View Article : Google Scholar : PubMed/NCBI
|