Introduction
Adipose-derived stem cells (ASCs) are considered to
be an ideal source for tissue regeneration due to their high
proliferative capability and their multi-lineage differentiation
potential, such as adipogenic, osteogenic and chondrogenic lineage
differentiations (1,2). Hair follicles are unique organs in
mammals, containing multiple tissue types. Previous studies have
revealed a close correlation between subcutaneous adipose tissue
and hair follicle formation and function (3,4).
However, it is not clear whether ASCs are capable of
directly participating in hair follicle morphogenesis. In fact,
several studies have revealed that bone marrow-derived stem cells
(BMSCs) participate in skin appendage formation. Kataoka et
al(5) have demonstrated that
murine BMSCs are capable of differentiating and participating in
hair follicle formation when implanted with embryonic mouse skin
cells (at gestational day 17.5) in a skin reconstitution model of
nude mice. Another study showed that BMSCs induced by co-culture
with sweat gland cells participate in the formation of sweat glands
and other skin appendages (6).
Whether or not ASCs have the capability similar to BMSCs to
participate in hair follicle morphogenesis has yet to be
explored.
It has recently been recognized that ASCs are a
heterozygous cell population containing multiple cell types,
including vascular endothelial cells, pre-adipocytes, smooth muscle
cells, hematological lineage cells, hematopoietic stem cells and
mesenchymal stem cells (MSCs) (7).
Therefore, to examine the ability of ASCs to participate in hair
morphogenesis it would be ideal to use MSCs purified from ASCs.
Currently, adipose-derived MSCs (ADMSCs) are considered to reside
in a perivascular location and CD34 may be one of the specific
markers for MSC identification and enrichment from adipose tissue
(8,9).
In this study, cells freshly isolated from mouse
adipose tissue (stromal vascular fraction, SVF) were sorted using
flow cytometry to give rise to three subgroups: i) CD34+
(lineage−:CD45−:CD31−:CD34+)
cells; ii) CD34− (lineage−:
CD45−:CD31−:CD34−) cells; and iii)
unsorted SVF cells. Three subpopulations were then tested for their
potential to participate in hair follicle formation using a
previously reported hair morphogenesis model (5).
Materials and methods
Animals
Female C57BL/6 mice with confirmed pregnancy and
4–6-week-old male athymic nude mice were purchased from the Slac
Laboratory Animal Center (Shanghai, China). In addition,
C57BL/6-green fluorescent protein (GFP) transgenic mice were
purchased from the Model Animal Research Center of Nanjing
University (China). An Institutional Review Committee of the
Shanghai Jiao Tong University School of Medicine approved all
animal study protocols.
Cell isolation and flow cytometry
sorting
As previously described (8), the inguinal adipose tissues were
harvested from 6–8-week-old GFP-transgenic mice and then digested
at 37°C with 0.075% collagenase (NB4, Serva, Coger, Paris, France)
in phosphate-buffered saline (PBS) containing 2% bovine serum
albumin (BSA) for 60–90 min. After filtration through a 40-μm nylon
filter mesh (BD Falcon, Franklin Lakes, NJ, USA) and
centrifugation, the freshly isolated SVF cell portion was
resuspended in PBS containing 1% fetal bovine serum (FBS) for
antibody labeling.
For cell sorting, isolated SVF cells were stained
with phycoerythrin (PE)-conjugated rat anti-mouse CD34, PE-Cy5
conjugated rat anti-mouse CD45 and CD31 (1:100 in PBS containing 1%
FBS, BD Biosciences, San Jose, CA, USA) and biotin-conjugated rat
anti-mouse hematological lineage cocktail (1:100 in PBS containing
1% FBS, BD Biosciences) followed by a PE-Cy5 conjugated
streptavidin secondary antibody (1:1,000 in PBS containing 1% FBS,
BD Biosciences). The cells were washed twice and resuspended in PBS
containing 1% FBS followed by fluorescence-activated cell sorting
(FACS, Beckman Coulter, Miami, FL, USA) and analyzed on a FACSort
with CellQuest Pro v5.2.1 software (BD Biosciences). Sorted cells
were collected as the subpopulations: i) CD34+
(lineage−:CD45−:CD31−:CD34+)
cells; ii) CD34−
(lineage:CD45−:CD31−:CD34−) cells;
and iii) unsorted SVF cells. These three groups of cells were used
for subsequent experiments.
Isolation of mouse fetal epidermal and
dermal cells
Briefly, fetal mouse dorsal skins were harvested
from C57BL/6 mouse embryos of gestational day 17.5 (E17.5), and
then incubated in 0.2% dispase (Roche Diagnostics, Indianapolis,
IN, USA) dissolved in Dulbecco’s modified Eagle’s medium (DMEM,
Gibco BRL, Gaithersburg, MD, USA) containing 10% FBS overnight at
4°C. Subsequently, the epidermis was mechanically separated from
the dermis using forceps. Then, the epidermis and the dermis were
digested with 0.2% collagenase (Sigma, St. Louis, MO, USA) in PBS
for 1 h at 37°C. After digestion, the two dissociated cell types
were washed in DMEM containing 10% FBS and then collected and
counted before grafting.
Cell grafting
The hair morphogenesis model was performed as
previously described (5). Male
athymic nude mice were anaesthetized by intraperitoneal injection
of pentobarbital sodium (1.3 mg/kg body weight). Briefly,
1×106 of the CD34+ (lineage−:
CD45−:CD31−:CD34+) cells,
CD34−
(lineage−:CD45−:CD31−:
CD34−) cells and unsorted SVF cells were mixed with
1×106 fetal mouse epidermal cells and 1×106
fetal dermal cells respectively, and then injected subcutaneously
by inserting the pipette tip into the dorsal skin (n=3). After
recovery from anesthesia, mice were caged individually and then
sacrificed at 3 weeks post-cell transplantation and tissue
specimens were harvested and subjected to various studies.
Histological analysis and
immunofluorescent staining
For the immunofluorescent analysis, freshly
harvested dorsal skin, including implanted tissue, were fixed for 4
h at room temperature in PBS containing 4% paraformaldehyde and 10%
glutaraldehyde, and then switched to PBS containing 30%
glutaraldehyde overnight at 4°C. The fixed tissues were embedded
quickly into embedding medium (OCT). The tissues were subsequently
frozen and sectioned at the thickness of 10 μm. Sections were first
permeabilized in PBS supplemented with 0.25% Triton X-100 for 60
min followed by blocking with 10% goat serum in PBS at room
temperature. The sections were then stained with rabbit anti-mouse
CD31 (1:50, in PBS containing 1% BSA, Abcam, Cambridge, UK), rabbit
anti-mouse CK15 (1:200, in PBS containing 1% BSA, Abcam) overnight
at 4°C, followed by the incubation with the secondary antibodies
Alexa Fluor 488 or Alexa Fluor 555-conjugated goat anti-rabbit IgG
(1:1,000, in PBS containing 1% BSA, Invitrogen, Eugene, OR, USA)
for 30 min at 37°C. Fluorescent staining of lipids was performed
using LipidTox (1:200 in PBS with 1% BSA, Invitrogen) as described
previously (3). Sections were
finally counterstained with DAPI (1:1,000 in PBS with 1% BSA,
Sigma). For histological analysis, the sections were stained with
hematoxylin and eosin (H&E). To analyze the participation rate,
section slides were observed under a fluorescent microscope to
determine the participation of GFP-labeled cells in the formed
tissues of each examined slide.
Quantitative and statistical
analyses
The positive and negative slide numbers derived from
the groups of hair follicle, blood vessel and fat tissue were
counted. Both positive and negative numbers derived from 3 examined
specimens of each group were respectively combined, and a
Chi-square analysis of the data was performed. A P-value of
<0.05 was considered to indicate a statistically significant
result (Table I).
 | Table IQuantitative analysis of cell
participation rate in forming different tissues. |
Table I
Quantitative analysis of cell
participation rate in forming different tissues.
| CD34+
cells | CD34−
cells | SVF |
|---|
|
|
|
|
|---|
| Examined
specimens | HF | BV | AT | HF | BV | AT | HF | BV | AT |
|---|
| 1 | 27/106 | 45/106 | 44/106 | 0/50 | 0/50 | 12/50 | 0/115 | 41/115 | 99/115 |
| 2 | 30/90 | 40/90 | 39/90 | 0/31 | 0/31 | 4/31 | 0/95 | 30/95 | 84/95 |
| 3 | 28/128 | 59/128 | 53/128 | 0/40 | 0/40 | 11/40 | 0/120 | 36/120 | 103/120 |
| Participation rate
(%) | 26.89±5.86 | 44.33±1.82 | 43.39±1.90 | 0.00±0.00 | 0.00±0.00 | 21.47±7.62 | 0.00±0.00 | 32.41±2.92 | 86.78±1.43 |
Results
Sorting of CD34+ cells
Adipose-derived SVF cells were sorted based on
antibody cocktail staining. Generally, 6.14±2.95% of
CD34+ cells could be isolated from the gated SVF
portion. Further functional characterization revealed that
CD34+ cells possessed more potent multi-differentiation
potential, stronger proliferative capability and an increased
colony forming ability (data not shown) than the cells of the other
two groups, indicating that CD34+ cells may represent
the mesenchymal stem cells enriched from adipose tissue
(ADMSCs).
Participation of CD34+ cells
in hair follicle morphogenesis
As previously described (5) and outlined in Fig. 1A, CD34+,
CD34− and unsorted cells were mixed with mouse fetal
keratinocytes and dermal fibroblasts, respectively, and implanted
subcutaneously into the back of nude mice. After 3 weeks of in
vivo implantation, larger tissue blocks were observed in the
CD34+ cell group compared with the CD34− and
SVF cell groups. Furthermore, more hair follicles were formed in
the CD34+ cell group than in the other two groups,
whereas an evident fat mass was observed in the SVF cell group
(Fig. 1B). These findings suggest
that CD34+ cells may further enhance hair follicle
morphogenesis compared with CD34− cells and unsorted SVF
cells.
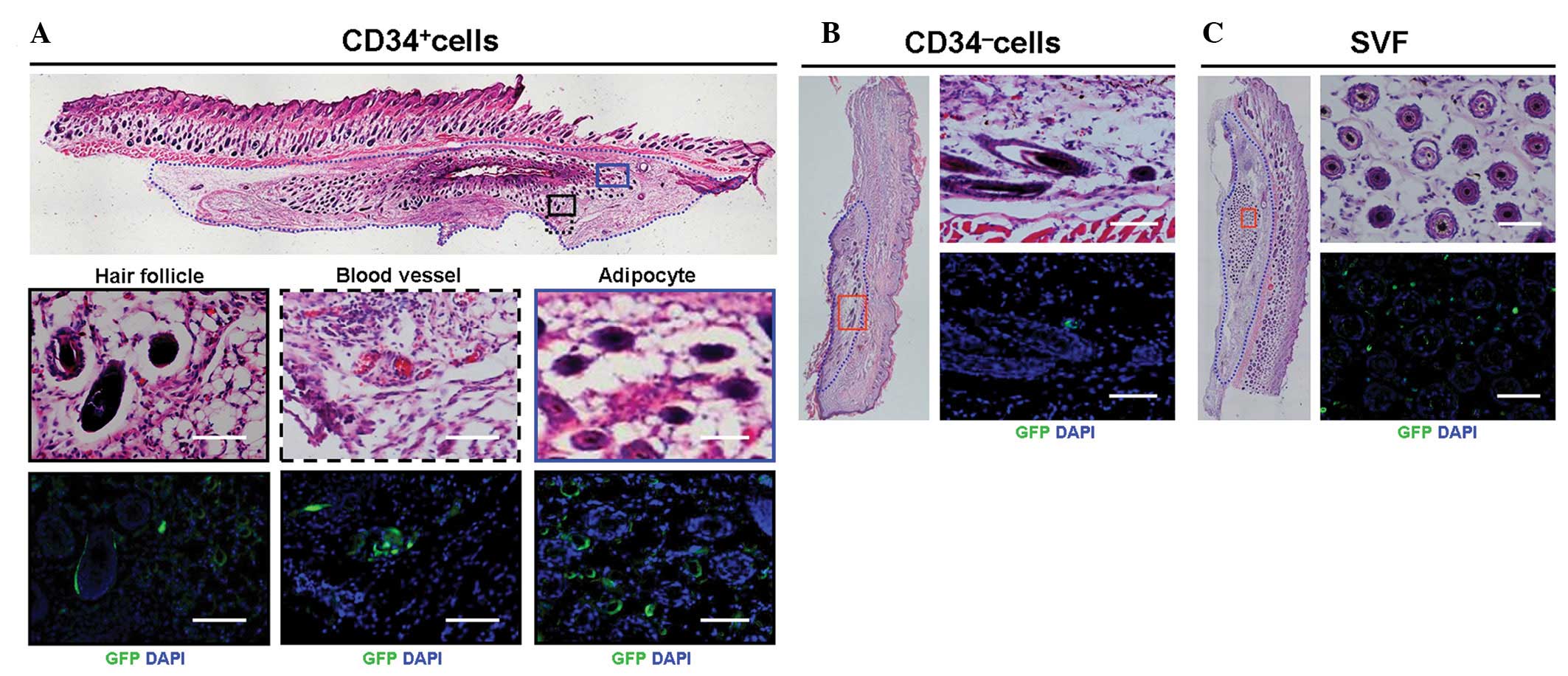
As shown in Fig. 2,
when analyzed with H&E staining, CD34+ cells were
able to participate in forming hair follicle, blood vessel and fat
tissue, as GFP-labeled cells were located at the respective tissues
(Fig. 2A), but not in other parts
of skin tissue. By contrast, no participation of hair follicle
morphogenesis was observed in both CD34− cell and
unsorted cell groups (Fig. 2B and
C), indicating that there is a difference in differentiation
potential among the three groups of cells.
Characterization of differentiated
CD34+ cells in developed skin tissue
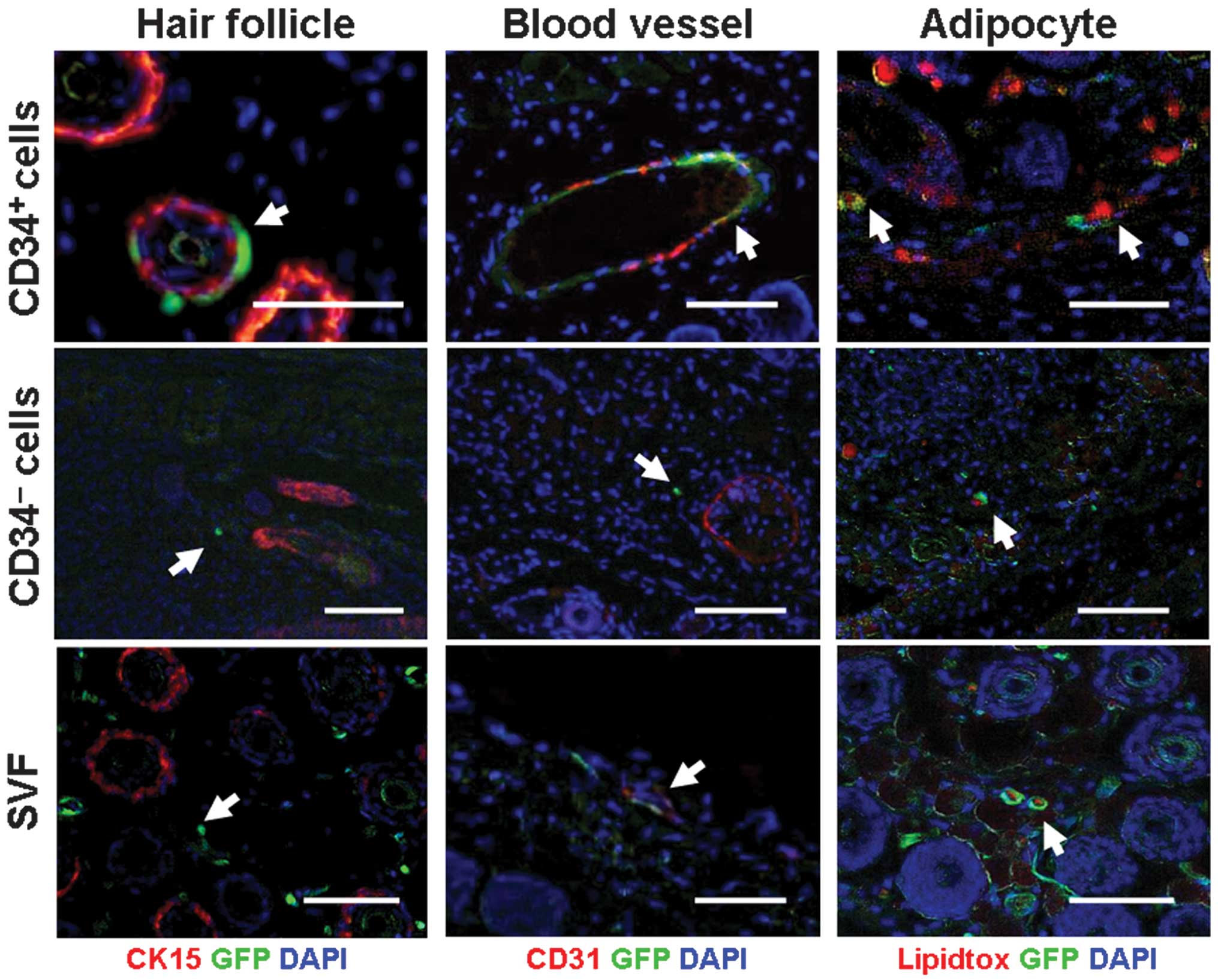
To further confirm the histological findings,
immunofluorescent staining was employed to characterize the formed
tissue. Consistent with the histological findings, co-localization
of GFP cells and other fluorescent staining indicated that
CD34+ cells were capable of participating in the
formation of multiple tissues in developed skin (Fig. 3, top panel). Firstly, GFP-positive
cells were often observed surrounding the outer root sheath of hair
follicles. However, GFP cells (green) were actually located at the
immediate outlayer of the CK15-positive cell layer (a marker for
the outer root sheath of the hair follicle, red), demonstrating no
co-localization to CK15 (Fig. 3,
top left). This finding suggests that CD34+ cells may be
integrated into the formed dermal sheath (DS). In addition,
co-localization of GFP cells (green) and CD31 cells (red)
demonstrated the participation of CD34+ cells in forming
blood vessels (Fig. 3, top
middle). Additionally, co-localization of GFP (green) in the
cytoplasm with intracellular lipid drops (red) demonstrated the
participation of CD34+ cells in forming fat tissue
(Fig. 3, top right).
In contrast to CD34+ cells, no
involvement of CD34− cells and unsorted SVF cells in
hair follicle morphogenesis (Fig.
3, middle left and bottom left) was observed. Although their
involvement in fat tissue formation was observable (Fig. 3, middle right and bottom right),
involvement in blood vessel formation was only observed in unsorted
SVF cells (Fig. 3, middle bottom),
but not in CD34− cells (Fig. 3, middle central).
Quantitative analysis of cell
participation rate
As shown in Table
I, the participation rates for the hair follicle, blood vessel
and fat tissue groups were 26.89±5.86, 44.33±1.82 and 43.39±1.90%
for the CD34+ group, 0.00±0.00, 0.00±0.00 and
21.47±7.62% for the CD34+ group and 0.00±0.00,
32.41±2.92, and 86.78±1.43% for the SVF group, respectively.
Statistical analysis revealed a significant difference in the
participation rates among each of the three groups (P<0.05).
Discussion
Adult stem cells are derived from various tissues of
the body and are able to differentiate and form different types of
tissue. Therefore, adult stem cells, such as BMSCs, ASCs, umbilical
cord (UC)-MSCs and placenta MSCs are widely explored as potential
therapeutic cell sources for tissue and organ regeneration
(10–12).
The best-studied adult stem cells are BMSCs and
their therapeutic potential has already been shown by reported
clinical trials with favorable outcomes (13,14).
However, use of BMSCs is limited as harvesting them in large
quantities may be harmful to the human body due to loss of
hematopoietic stem cells (15). By
contrast, ASCs possess several advantages over BMSCs as a promising
cell source for tissue regeneration and clinical therapy, including
easy access to the source, no harm to the donor and the
availability of multiple cell types (16,17).
Nevertheless, the heterogeneity of the ASC
population is also a disadvantage for its application in
regenerative medicine (7), since
the multiple differentiation potential of ASCs is inhibited by the
interference of some factors released from different cell types in
the pooled population. For example, the difficulty of regenerating
homogeneous cartilage-like tissue by pooled adipose-derived cells
(18) is likely due to the
functional interference to ADMSCs by other cell types. Therefore,
purification and relative enrichment with a specific marker would
be a reasonable approach for the functional evaluation of
ADMSCs.
A study by Rodeheffer et al(8) showed that ADMSCs may be enriched by a
series of surface markers including CD29, Sca-1 and CD34. Tang
et al(9) showed that ADMSCs
may be the vascular pericytes residing in the adipose stromal
vascular fraction. Several other studies also demonstrated similar
results. Lin et al(19) and
Traktuev et al(20)
illustrated that ASCs reside at in a perivascular location and CD34
is abundantly expressed in all blood vessels in human adipose
tissue. All this evidence suggests that CD34 may be able to serve
as a relatively specific marker to enrich ADMSCs. Our study showed
that mouse primary CD34+ ASCs sorted from SVF portion
cells are more potent in cell proliferation, clone formation and
multi-differentiation compared with CD34− and unsorted
SVF cells. In addition, the CD34+ cell population also
largely overlaps with the CD29+ and Sca-l+
cell populations (data not shown). These results indicate that CD34
may serve as a relatively specific marker for enriching ADMSCs.
Hair follicles consist of concentric epithelial
sheaths (hair shaft, inner root sheath, outer root sheath)
surrounded by DS that is connected to a dermal papilla (DP) at the
base of the follicle (21). In
fact, numerous studies have shown that the hair follicle’s
regenerative cycle, induced by follicular stem cells, is closely
associated with adipose tissue and adipogenic lineage cells. For
example, the thickness of the intradermal adipocyte layer in the
hair follicle active cycle (anagen) increases significantly
compared with the thickness of that in the resting phase of the
hair cycle (22,23). Furthermore, reduced intradermal
adipose tissue in transgenic mice models result in abnormalities in
skin structure and function such as hair loss, epidermal
hyperplasia and abnormal sebaceous gland function (4,24,25).
In particular, Festa et al(3) recently reported that adipose lineage
cells, including mature adipocyte and preadipocytes
(Lineage−:
CD29+:Sca-1+:CD34+:CD24+),
have been defined as skin niche cells that positively regulate hair
follicle stem cell activity.
More importantly, hair follicle morphogenesis,
involving multiple tissue and cell types, has been employed as a
model to test the differentiation potential of adult MSCs. A study
by Kataoka et al(5)
confirmed that BMSCs were capable of differentiating into various
cell types, including epidermal keratinocytes, sebaceous gland
cells, follicular epithelial cells, dendritic cells and endothelial
cells, using this model. In addition, skin-derived precursors
(SKPs) can clonally differentiate into dermal cell types and induce
hair follicle morphogenesis (26).
Yoo et al(27) showed that
UC-MSCs, another type of adult stem cell, is able to differentiate
and form DP-like tissues and induce new hair follicles.
In this study, we examined the multi-potency of
mouse adipose-derived MSCs purified by CD34 using a reported hair
follicle-reconstitution model. The results demonstrated that only
CD34+ cells were able to participate in hair follicle
morphogenesis by contributing to the DS formation. Quantitative
analysis revealed a participation rate of ~27% in DS structures in
the CD34+ group, whereas a 0% participation rate was
found in the other two groups with a statistically significant
difference among the three groups (P<0.05). A number of studies
suggest that DS plays a key role in hair induction. For example,
Yamao et al(28) reported
that DS formation is critical to the normal development of hairs
with hair shafts. In addition, previous studies demonstrating that
DS cells and DP cells can be transformed into each other (29), and that lower DS cells can
regenerate the DP (30). Moreover,
the study by Biernaskie et al(26) also highlighted that SKPs are
capable of differentiating into interfollicular dermal cell types
in vivo, including DS and DP cells.
This study also revealed that CD34+ cells
may be involved in blood vessel formation by differentiating into
endothelial cells, besides the differentiation to adipocytes. By
contrast, CD34− cells were unable to participate in hair
follicle morphogenesis and blood vessel formation. Unsorted SVF may
participate in blood vessel formation due to their contained
endothelial cells in the pool. However, quantitative analysis
revealed there was a significant difference in the participation
rate between CD34+ and SVF groups (P<0.01). These
results, in addition to previous findings (8,9,19,20),
indicate that CD34 may serve as a valid marker to enrich
ADMSCs.
When compared with BMSCs that participate in the
morphogenesis of multiple skin appendages, CD34-enriched adipose
cells appear less potent than BMSCs in multiple differentiations,
particularly in skin cell differentiation and tissue formation.
This finding also agrees with other findings that the
differentiation potential varies among different types of adult
MSCs, and BMSCs appear to possess better stemness compared with
other adult-type MSCs (15,18).
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30872694, 31170937). The authors also
appreciate the technical support from Dr Jingjun Chen, Dr Wanrao
Xia, Mr. Demin Ying, Ms. Lijuan Zong and Ms. Juanjuan Wu.
References
|
1
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gronthos S, Franklin DM, Leddy HA, Robey
PG, Storms RW and Gimble JM: Surface protein characterization of
human adipose tissue-derived stromal cells. J Cell Physiol.
189:54–63. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Festa E, Fretz J, Berry R, et al:
Adipocyte lineage cells contribute to the skin stem cell niche to
drive hair cycling. Cell. 146:761–771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen HC, Smith SJ, Tow B, Elias PM and
Farese RV Jr: Leptin modulates the effects of acyl
CoA:diacylglycerol acyltransferase deficiency on murine fur and
sebaceous glands. J Clin Invest. 109:175–181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kataoka K, Medina RJ, Kageyama T, et al:
Participation of adult mouse bone marrow cells in reconstitution of
skin. Am J Pathol. 163:1227–1231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Fu X, Ouyang Y, Cai C, Wang J and
Sun T: Adult bone-marrow-derived mesenchymal stem cells contribute
to wound healing of skin appendages. Cell Tissue Res. 326:725–736.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshimura K, Shigeura T, Matsumoto D, et
al: Characterization of freshly isolated and cultured cells derived
from the fatty and fluid portions of liposuction aspirates. J Cell
Physiol. 208:64–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodeheffer MS, Birsoy K and Friedman JM:
Identification of white adipocyte progenitor cells in vivo. Cell.
135:240–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang W, Zeve D, Suh JM, et al: White fat
progenitor cells reside in the adipose vasculature. Science.
322:583–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuci S, Kuci Z, Latifi-Pupovci H, et al:
Adult stem cells as an alternative source of multipotential
(pluripotential) cells in regenerative medicine. Curr Stem Cell Res
Ther. 4:107–117. 2009.PubMed/NCBI
|
|
12
|
Kakinuma S, Tanaka Y, Chinzei R, et al:
Human umbilical cord blood as a source of transplantable hepatic
progenitor cells. Stem Cells. 21:217–227. 2003.PubMed/NCBI
|
|
13
|
Wakitani S, Imoto K, Yamamoto T, Saito M,
Murata N and Yoneda M: Human autologous culture expanded bone
marrow mesenchymal cell transplantation for repair of cartilage
defects in osteoarthritic knees. Osteoarthritis Cartilage.
10:199–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo W, Wang H, Zou S, et al: Bone marrow
stromal cells produce long-term pain relief in rat models of
persistent pain. Stem Cells. 29:1294–1303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wagner W, Wein F, Seckinger A, et al:
Comparative characteristics of mesenchymal stem cells from human
bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol.
33:1402–1416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aust L, Devlin B, Foster SJ, et al: Yield
of human adipose-derived adult stem cells from liposuction
aspirates. Cytotherapy. 6:7–14. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varma MJ, Breuls RG, Schouten TE, et al:
Phenotypical and functional characterization of freshly isolated
adipose tissue-derived stem cells. Stem Cells Dev. 16:91–104. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Afizah H, Yang Z, Hui JH, Ouyang HW and
Lee EH: A comparison between the chondrogenic potential of human
bone marrow stem cells (BMSCs) and adipose-derived stem cells
(ADSCs) taken from the same donors. Tissue Eng. 13:659–666. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin G, Garcia M, Ning H, et al: Defining
stem and progenitor cells within adipose tissue. Stem Cells Dev.
17:1053–1063. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Traktuev DO, Merfeld-Clauss S, Li J, et
al: A population of multipotent CD34-positive adipose stromal cells
share pericyte and mesenchymal surface markers, reside in a
periendothelial location, and stabilize endothelial networks. Circ
Res. 102:77–85. 2008. View Article : Google Scholar
|
|
21
|
Hardy MH: The secret life of the hair
follicle. Trends Genet. 8:55–61. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chase HB, Montagna W and Malone JD:
Changes in the skin in relation to the hair growth cycle. Anat Rec.
116:75–81. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansen LS, Coggle JE, Wells J and Charles
MW: The influence of the hair cycle on the thickness of mouse skin.
Anat Rec. 210:569–573. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jong MC, Gijbels MJ, Dahlmans VE, et al:
Hyperlipidemia and cutaneous abnormalities in transgenic mice
overexpressing human apolipoprotein C1. J Clin Invest. 101:145–152.
1998. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stone SJ, Myers HM, Watkins SM, et al:
Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J
Biol Chem. 279:11767–11776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biernaskie J, Paris M, Morozova O, et al:
SKPs derive from hair follicle precursors and exhibit properties of
adult dermal stem cells. Cell Stem Cell. 5:610–623. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo BY, Shin YH, Yoon HH, Seo YK, Song KY
and Park JK: Application of mesenchymal stem cells derived from
bone marrow and umbilical cord in human hair multiplication. J
Dermatol Sci. 60:74–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamao M, Inamatsu M, Ogawa Y, et al:
Contact between dermal papilla cells and dermal sheath cells
enhances the ability of DPCs to induce hair growth. J Invest
Dermatol. 130:2707–2718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tobin DJ, Gunin A, Magerl M, Handijski B
and Paus R: Plasticity and cytokinetic dynamics of the hair
follicle mesenchyme: implications for hair growth control. J Invest
Dermatol. 120:895–904. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McElwee KJ, Kissling S, Wenzel E, Huth A
and Hoffmann R: Cultured peribulbar dermal sheath cells can induce
hair follicle development and contribute to the dermal sheath and
dermal papilla. J Invest Dermatol. 121:1267–1275. 2003. View Article : Google Scholar : PubMed/NCBI
|