Introduction
In individuals who sustain a serious spinal cord
injury, <1% experience complete recovery of neurological
function and a number of these injuries result in partial or
complete paralysis (1). These
injuries reduce the quality of life of the individuals who may
become a burden to society. Therefore, therapeutic interventions
for spinal cord injury (SCI) have become one of the most intensely
studied areas in neuroscience over the last few decades. Several
cell transplantation studies have demonstrated some success in the
treatment of SCI, however, exogenous neural stem cells (NSCs),
which retain marked neurogenic activities in vitro, are
associated with low levels of differentiation when grafted into the
spinal cord (2,3).
An additional issue associated with the use of NSCs
for the treatment of SCI is that differentiated, but unpurified,
stem cell transplants from the embryo may cause tumor formation
within the central nervous system (CNS) (4). In addition, the transplantation of
xenograft cells requires the co-administration of corticosteroids
during the experimental period to prevent cell rejection, which may
complicate interpretation of the results (5,6). The
transplantation of exogenous NSCs also has ethical considerations,
since these cells are derived from embryonic tissue (7). Together, these unresolved issues have
slowed the development of exogenous NSC transplantation therapy
(8).
It is now generally accepted that NSCs reside in
specific regions of the adult mammalian CNS, including the
ependymal region of the spinal cord as well as the subventricular
zone, hippocampus and dentate gyrus of the brain (9,10).
In addition, the adult spinal cord is an abundant source of
endogenous NSCs and these cells may undergo considerable levels of
proliferation under physiological conditions, including SCI
(11). Due to the restrictive
environment of the contusive spinal cord, proliferative endogenous
NSCs mainly differentiate into astrocytes and form astrocytic
scars, which have no therapeutic effect on the re-establishment of
neural pathways (12). However, it
has been hypothesized that if this environmental restriction is
relieved by specific manipulations, then endogenous NSCs may be
able to supply new neurons, which in turn may contribute to the
reconstruction of local neuronal circuitry and facilitate the
regeneration of long-distance axonal tracts (13–15).
To date, strategies that manipulate endogenous NSCs in the context
of SCI have not been explored.
Bone morphogenetic proteins (BMPs) play critical
roles in the determination of NSC fate. Previous studies have
demonstrated that BMP-4 promotes the differentiation of NSCs into
astrocytes (16,17). BMP-4 antagonists prevent the
activation of BMP-4 receptors and thus alter the fate of NSCs
toward neurogenisis (18,19). Chordin-like protein 1 (CHRDL1),
also known as neurogenesin-1 (Ng1), ventroptin and neuralin-1, is a
BMP receptor antagonist that was originally identified in
Xenopus(20,21). CHRDL1 is predominantly secreted by
astrocytes and hypothesized to be involved in adult neurogenesis
(21). CHRDL1 has previously been
shown to induce neuronal differentiation in brain-derived NSCs in
culture (20).
The aforementioned studies indicate that CHRDL1 may
play a key role in neurogenesis in the adult hippocampus. However,
it is not yet known whether CHRDL1 also alters the fate of spinal
cord-derived NSCs from gliogenesis to neurogenesis. Thus, in the
current study, we assessed whether BMP-4 inhibition by CHRDL1
promotes the differentiation of spinal cord-derived NSCs into
neurons in vitro.
Materials and methods
Animals
hree 7-day-old Sprague-Dawley (SD) rats and one
adult SD rat (225 g) were used in this study. Animal care and use
was conducted at the Experimental Animal Center of Anhui Medical
University under a protocol approved by the Institutional Animal
Care and Use Committee.
Construction of recombinant expression
vector
One 7-day-old rat was euthanized by cervical
dislocation and the cerebrum was rapidly excised in a sterile
container on ice. The hippocampus was then separated. Total RNA was
extracted with TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA). The primers used were designed with HindIII and
XhoI sites and prepared in accordance with the published
CHRDL1 gene sequence (Gene ID: 363455) as follows: 5′
primer: 5′-CCC AAG CTT CCA GTA AAA CAC TCA GAG ACA TAT-3′; 3′
primer: 5′-CCG CTC GAG AAC AGT GGT CCT TTT CAG GTC TCT C-3′.
Primers were provided by Takara Bio, Inc. (Shiga, Japan). Reverse
transcription (RT) was performed using the RNA polymerase chain
reaction (PCR) kit (AMV, v3.0; Takara Bio, Inc.) according to the
manufacturer’s instructions. PCR experiments were performed under
the following conditions: denaturation at 94°C for 5 min;
amplification for 35 cycles of 94°C for 40 sec, 55°C for 40 sec and
70°C for 90 sec; and extension at 72°C for 5 min. Glyceraldehyde
3-phosphate dehydrogenase (GAPDH) was used as an internal
control.
The DNA fragment of the expected molecular size was
purified using the AxyPrep DNA Gel Extraction kit (AxyGen, Inc.,
Union City, CA, USA) and cloned into the pSecTag2/Hygro B vector
(Invitrogen Life Technologies) for DNA sequencing. DNA sequencing
was performed by Sangon Biotech (Shanghai, China) and the data were
compared with the GenBank database using the BLAST program. The
confirmed rat CHRDL1 gene and pSecTag2/Hygro B vector were
digested with HindIII and XhoI restriction enzymes
(Takara Bio, Inc.), respectively. The two digested products were
then incubated with T4 DNA ligase (Takara Bio, Inc.) at 16°C for 6
h to ligate the DNA. The constructed plasmid was transformed into
E. coli JM109 cells for amplification and the plasmid was
designated pSecTag2/Hygro B-CHRDL1. The plasmid was then isolated
using the AxyPrep Plasmid Miniprep kit (AxyGen, Inc.,) and
confirmed by PCR screening and restriction enzyme digestion
analysis. Three rats were used in three experimental
replicates.
Isolation, culture and identification of
NSCs from adult rat spinal cord
One adult rat was euthanized by intraperitoneal
administration of sodium pentobarbital. Under aseptic conditions,
the spinal cord was removed and immediately immersed in chilled
normal saline solution. Next, the primary cultures were prepared as
described previously (22). The
isolated cell pellet was resuspended in Dulbecco’s modified Eagle’s
medium (DMEM)/F12 (1:1) supplemented with 2% B27 (all Invitrogen
Life Technologies), 20 ng/ml epidermal growth factor (EGF), 20
ng/ml basic fibroblast growth factor (bFGF; both PeproTech, Rocky
Hill, NJ, USA), 4 mmol/l glutamine and 1% penicillin/streptomycin.
Cell suspensions were cultured in an incubator at 37°C and 5%
CO2. The media were replaced with a half-dose of fresh
medium every 2–3 days. Following cell culture for ~6 days, the
proliferation of cell clusters was observed. Cells were passaged
every 6–7 days by mechanical separation.
Following the second passage, cells were cultured
for 5 days and the observed neurospheres were plated on
poly-L-lysine coated glass coverslips placed in 6-well plates and
cultured for an additional 24 h. Immunocytochemical (ICC) analysis
was then performed to specifically detect NSCs. To induce
differentiation, the neurospheres from passage 3 were dissociated
into single cells, plated on poly-L-lysine coated glass coverslips
and grown in a medium composed of DMEM/F12, 2% B27, 10% fetal
bovine serum (FBS; Invitrogen Life Technologies) and antibiotics,
without EGF or bFGF. Media were changed every 2 days for ~6 days.
On day 7, ICC analysis was performed to detect astrocytes
and neurons.
Transfection of adult spinal cord-derived
NSCs
For the transfection experiments, cells were divided
into the following four groups: pSecTag2/Hygro B-CHRDL1
transfection alone (C), addition of BMP-4 alone (B), pSecTag2/Hygro
B-CHRDL1 transfection and addition of BMP-4 (G) and Lipofectamine
2000 (Invitrogen Life Technologies) alone as a non-transfected
control (N).
The pSecTag2/Hygro B-CHRDL1 plasmid was transfected
into NSCs using Lipofectamine 2000 according to the manufacturer’s
instructions. Prior to transfection, the neurospheres were isolated
to form single-cell suspensions by mechanical separation;
4–8×105 cells were resuspended in 500 μl growth medium
without antibiotics and seeded into each well of a 6-well plate.
For groups C and G, 4.0 μg pSecTag2/Hygro B-CHRDL1 plasmid DNA was
used. Following a 24-h incubation period, NSCs were passaged, 1:5,
into fresh growth medium containing 20 ng/ml EGF, 20 ng/ml bFGF, 2%
B27 and 1% penicillin/streptomycin. The following day, fresh growth
medium supplemented with 200 μg/ml hygromycin B was added. After
incubation for 5 days, the supernatant was collected for western
blot analysis.
NSCs were then passaged in a medium composed of
DMEM/F12, 2% B27 and 10% FBS, in the absence of EGF or bFGF. BMP-4
(10 ng/ml) was then added to the group B and G cultures. Finally, 5
days following the addition of BMP-4, a double ICC assay was
performed to assess the differentiation of NSCs.
Western blot analysis
Western blot analysis was performed to assess the
expression of CHRDL1, Smad5 and phosphorylated Smad5 proteins in
the NSCs. Briefly, 5 days following the addition of hygromycin B,
supernatant samples were collected and 5 days after addition of
BMP-4, cell extract samples were collected. Mouse anti-c-myc, goat
anti-Smad5 (both 1:500; Santa Cruz Biotechnology, Santa Cruz, CA,
USA), rabbit anti-phospho-Smad1/5 (1:200) and mouse anti-β-actin
(1:300; both Cell Signaling Technology, Beverly MA, USA) were used
as primary antibodies. Goat anti-mouse IgG antibody conjugated to
horseradish peroxidase (HRP), rabbit anti-goat and goat anti-rabbit
HRP-conjugated antibodies were used to reveal protein labeling.
Membranes were then evaluated using an electrochemiluminesence
western blot kit (Pierce Biotechnology, Inc., Rockford, IL, USA)
according to the manufacturer’s instructions.
Immunocytochemistry
Cells were grown on coverslips and fixed for 30 min
at room temperature with 4% paraformaldehyde in 0.01 mol/l PBS and
then washed three times with 0.01 mol/l PBS (pH 7.4). The cells
were then permeabilized with 0.3% Triton X-100 for 30 min at room
temperature and then washed three times with 0.01 mol/l PBS.
Non-specific interactions were blocked by incubating the cells in
5% normal goat serum in 0.01 mol/l PBS for 40 min at room
temperature. The cells were then incubated overnight at 4°C with
the following primary antibodies: mouse anti-nestin,
anti-microtubule-associated protein-2 (MAP-2; both 1:200; Santa
Cruz Biotechnology) and rabbit anti-glial fibrillary acidic protein
(GFAP; 1:200; Abcam, Cambridge, MA, USA). The following day, cells
were washed and incubated with corresponding secondary antibodies
for 1 h at room temperature: fluorescein isothiocyanate-conjugated
goat anti-mouse IgG, tetraethyl rhodamine isothiocyanate-conjugated
goat anti-rabbit IgG (both 1:100; Santa Cruz Biotechnology). When
appropriate, Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA) was
used at a concentration of 10 μg/ml for 10 min to label cell
nuclei. Fluorescent images were captured using a fluorescent
microscope system (Nikon, Tokyo, Japan).
Statistical analysis
The in vitro cell number in each of the four
experimental groups was estimated by counting 5 fields/coverslip.
Neuron purity was determined by the ratio of MAP-2-positive cells
to Hoechst 33342-positive cells. Comparisons were performed using
the χ2 test. All data were analyzed with SPSS 11.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Molecular cloning of CHRDL1 and
construction of eukaryotic vectors
RT-PCR products were analyzed by agarose gel
electrophoresis in the presence of ethidium bromide. A 1.3-kb
product band was detected of the expected molecular size (Fig. 1A) and isolated, purified and cloned
into the pSecTag2/Hygro B vector. Sequence analysis revealed that
the inserted cDNA clone was 1,275 bp and was identical to the
reported rat CHRDL1 sequence determined using the BLAST program.
The cDNA clone corresponding to the rat CHRDL1 sequence was then
sub-cloned into pSecTag2/Hygro B and confirmed by PCR screening and
double restriction enzyme analysis (Fig. 1B).
Identification of adult spinal cord
NSCs
At day 1 of culture, the majority of the cells were
observed to be floating in the medium and free-floating with
differing refractivity and the cells were three-dimensional by
morphological observation. No neurospheres were observed in the
medium. Following 3–4 days of culture, small free-floating spheres
were observed. At day 7, the cells formed suspended, spherical
clusters composed of numerous cells. The clusters were increased in
size with weakly refractive centers (Fig. 2A). When the cells were resuspended
by trituration and the suspension of single cells from the cell
clusters was passaged, cell clusters formed again, indicating that
these cell clusters may be neurospheres. Cells from the
neurospheres were successfully passaged once per week through ≥16
passages.
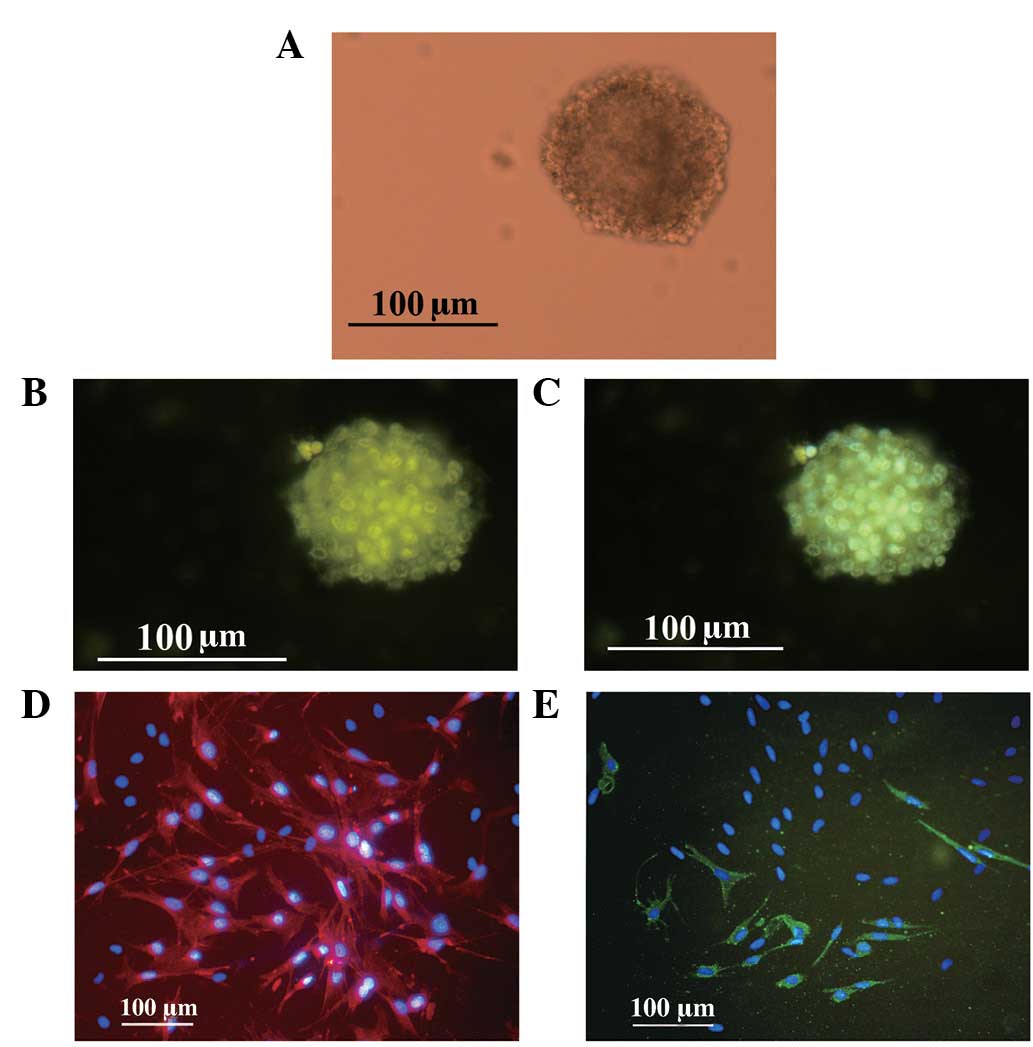 | Figure 2(A) Cultured NSCs. Cell cluster
formation, following 6 day culture of the primary cells (inverted
microscope). (B) Identification of adult NSCs in neurospheres using
an anti-nestin specific monoclonal antibody, a cell-type specific
marker of NSCs (FITC, green). (C) Merge of nestin (FITC, green) and
Hoechst 33342 (blue). (D) Identification of cell differentiation.
Astrocytes differentiated from neurospheres were GFAP positive and
identified by immunofluorescent staining (TRITC, red) and Hoechst
33342 (blue). (E) Neurons differentiated from neuronspheres were
MAP-2 positive (FITC, green) and Hoechst 33342 (blue). NSCs, neural
stem cells; FITC, fluorescein isothiocyanate; GFAP, glial
fibrillary acidic protein; TRITC, tetraethyl rhodamine
isothiocyanate; MAP-2, microtubule-associated protein-2. |
To determine the characteristics of the
free-floating neurospheres, ICC analysis was performed.
Specifically, neurospheres were examined for their expression of
nestin, an NSC marker. As demonstrated in Fig. 2B and C, the spheres exhibited
nestin positivity.
To examine the differentiation potential of adult
spinal cord-derived NSCs, newly formed neurospheres from passage 3
were dissociated into single cells and transferred into medium
containing 10% FBS. At day 6, cells were fixed and processed for
ICC staining. The phenotypes of these new cells were determined
using anti-GFAP and -MAP2 antibodies, which are specific markers
for astrocytes and neurons, respectively. Immunofluorescent
staining revealed that the majority of cells were GFAP positive
(Fig. 2D) and a number were also
MAP-2 positive (Fig. 2E). These
results indicate that these cells were NSCs and had
multidirectional differentiation potential.
Recombinant CHRDL1 protein reduces Smad5
protein levels
Western blot analysis revealed the presence of
CHRDL1 protein overexpression in the transfected NSCs. As
demonstrated in Fig. 3A,
c-myc-tagged CHRDL1 protein was detected in the supernatant. A
specific 46-kDa protein band that corresponded to the protein was
detected in groups C and G, but not in groups N and B. These
results confirmed that the CHRDL1-myc fusion protein was
successfully expressed in adult NSCs.
BMP-mediated induction of neuronal differentiation
occurs via the Smad pathways, particularly the BMP-4/Smad5
signaling pathway (23). Analysis
of cell extracts 5 days following the addition of BMP-4 revealed a
marked accumulation of phospho-Smad1/5 protein; the specific 60-kDa
protein band was detected in group B only (Fig. 3B). The absence of the specific
protein band for groups G and C demonstrated that CHRDL1 inhibited
the activation of Smad5 and that CHRDL1 does not affect the
BMP-4/Smad5 pathway independently.
Recombinant CHRDL1 protein antagonizes
BMP-4 and promotes neuronal differentiation of adult NSCs
The effect of CHRDL1 protein on adult NSCs was
determined in the presence of BMP-4. Neurospheres were dissociated
into single cell suspensions and transfected with CHRDL1 and/or
treated with BMP-4, or treated with transfection reagent. At day 5
following the addition of BMP-4, a double immunofluorescence assay
was performed (Fig. 4A-D). As
revealed in Fig. 4E, the
percentage of MAP-2-positive cells in group B (9.59%) was found to
be significantly lower than that in group N (18.92%;
χ2=5.400; P<0.05). In addition, the percentage of
MAP-2-positive cells in group G (20.65%) was observed to be
significantly higher than that in group B (9.59%;
ϕ2=7.437; P<0.05). No significant difference was
identified between groups N (18.92%) and C (22.11%; ϕ2=
0.583; P>0.05). Similar results were obtained in three
independent experiments. These results indicate that CHRDL1
expression antagonized BMP-4 and promoted the neuronal
differentiation of adult NSCs in vitro.
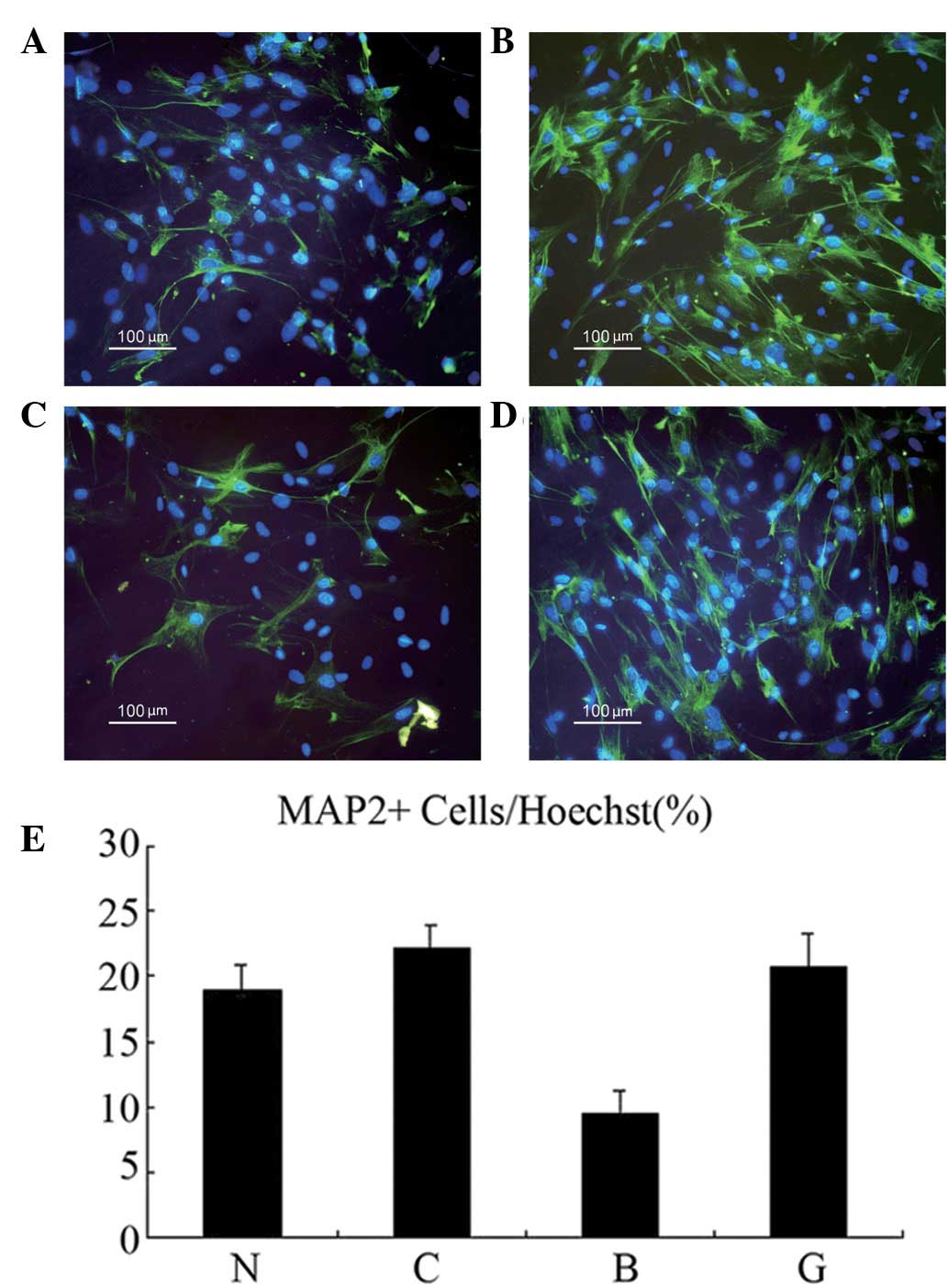 | Figure 4CHRDL1 promoted neuronal
differentiation of adult NSCs: N, control; C, transfected with
CHRDL1; B, treated with BMP-4; and G, transfected with CHRDL1 and
incubated with BMP-4. MAP-2 (FITC, green) and Hoechst 33342 (blue)
double-positive cells in groups (A) N, (B) C, (C) B and (D) G. (E)
Percentage of MAP-2-positive cells among the total number of
Hoechst 33342-positive cells. Groups: N, 18.92%; C, 22.11%; B,
9.59%; and G, 20.65%. CHRDL1, chordin-like protein 1; NSCs, neural
stem cells; MAP-2, microtubule-associated protein-2; FITC,
fluorescein isothiocyanate. |
Discussion
In the present study, CHRDL1 protein was ectopically
overexpressed in rat spinal cord-derived NSCs and considerable
levels of active CHRDL1 protein were secreted from the transfected
NSCs into the culture medium. CHRDL1 transfection of adult rat
spinal cord-derived NSCs was found to attenuate BMP-4
treatment-induced reductions in a number of the MAP-2-positive
cells. This effect appears to be mediated by the BMP-4/Smad5
pathway.
BMPs participate in diverse processes, including
neurogenesis (24,25), the formation of anterior-posterior
and dorsal-ventral axes and apoptosis (26). The BMP signaling pathway is highly
conserved and vital to a variety of organ developmental pathways.
However, the effect of BMPs on NSCs is extremely complicated as
BMPs induce NSCs to differentiate into neurons or astrocytes.
Studies have reported conflicting results concerning the effect of
BMPs on NSCs, even when assessed in the same anatomical location or
development phase (27–29). BMP-4 is expressed in the embryonic
cortex (30), indicating a
potential role in differentiation. Consistent with the hypothesis
that BMP-4 may be significant in developmental differentiation, it
has been observed to promote differentiation of spinal cord-derived
NSCs towards an astrocyte fate (31). BMP inhibitors that bind directly to
BMP and thereby prevent BMP activation, are potential factors in
the determination of neural fate commitment. Neural inducers,
including noggin, chordin and follistatin, do not have their own
receptors on target cells, instead, these molecules act by binding
to and inactivating BMP-4 (32).
Ueki et al(20) first cloned and characterized the
secretory factor, CHRDL1, and revealed that it contained an
N-terminal signal peptide, three cysteine-rich domains and shared
similarities with chordin. The C-terminal segments were composed of
amino acid sequences unique to CHRDL1 and the structural features
of CHRDL1 indicated that CHRDL1 may bind BMP-4 at BMP-binding
modules residing in cysteine-rich domains. The authors revealed
that CHRDL1 antagonizes BMP-4 and alters the fate commitment of
brain-derived NSCs from gliogenesis to neurogenesis. However,
brain- and spinal cord-derived NSCs have distinct properties and
growth requirements (33).
Therefore, in the present study, the ability of CHRDL1 to promote
the differentiation of spinal cord-derived NSCs into neurons was
determined.
In the present study, the coding region of CHRDL1
cDNA was cloned without the putative signal peptide. The coding
region of the cDNA was 1,275 bp and contained three cysteine-rich
domains that shared similarities with chordin. The gene was then
sub-cloned into the pSecTag2/Hygro B vector, an expression vector
designed for high-level expression and secretion in mammalian
hosts. The vector contains the gene encoding β-lactamase, an
N-terminal murine Ig κ chain leader sequence for protein secretion
and a C-terminal peptide containing the c-myc epitope followed by
six tandem histidine residues that are used for detection and
purification. These properties of the vector enabled us to
transfect NSCs and generate secreted, tagged CHRDL1 protein in
these cells. Restriction enzyme digestion and western blot analysis
revealed that the CHRDL1 gene had been successfully cloned
and expressed the protein in NSCs. The effects of CHRDL1 on the
proliferation and differentiation of adult NSCs derived from the
rat spinal cord in vitro were analyzed and a significant
difference was identified between groups B and N, indicating that
BMP-4 inhibits NSC differentiation into neurons. In addition, a
significant difference between groups G and B was observed,
indicating that the CHRDL1 protein promotes the differentiation of
NSCs in the presence of BMP-4. However, no statistical difference
was identified between groups G and B, which demonstrated that the
CHRDL1 protein did not promote NSC differentiation into a neuronal
fate independently. Neurosphere cultures from adult rat spinal cord
revealed that BMP-4 inhibited NSC differentiation toward a neuronal
fate. By contrast, inhibition of BMP-4 signaling by CHRDL1 markedly
increased the ratio of neurons to total cell number and this effect
was mediated by the BMP-4/Smad5 pathway.
In conclusion, the current study demonstrates that
the CHRDL1 gene induces the differentiation of endogenous
NSCs into neurons. These novel observations indicate that CHRDL1
may play a key role in adult spinal cord neurogenesis in mammals
and provide neurogenic cues for endogenous NSCs.
Acknowledgements
The authors thank Guang-Wu Li who directed the
separation of the hippocampus and Yu Chai, Dao-Jun Hu and Ren Zhao
who provided experimental assistance. The present study was funded
by the National Nature Science Foundation of China (no.
30772201).
References
|
1
|
Willerth SM and Sakiyama-Elbert SE: Cell
therapy for spinal cord regeneration. Adv Drug Deliv Rev.
60:263–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hill CE, Proschel C, Noble M,
Mayer-Proschel M, Gensel JC, Beattie MS and Bresnahan JC: Acute
transplantation of glial-restricted precursor cells into spinal
cord contusion injuries: survival, differentiation and effects on
lesion environment and axonal regeneration. Exp Neurol.
190:289–310. 2004. View Article : Google Scholar
|
|
3
|
Enzmann GU, Benton RL, Woock JP, Howard
RM, Tsoulfas P and Whittemore SR: Consequences of noggin expression
by neural stem, glial and neuronal precursor cells engrafted into
the injured spinal cord. Exp Neurol. 195:293–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roy NS, Cleren C, Singh SK, Yang L, Beal
MF and Goldman SA: Functional engraftment of human ES cell-derived
dopaminergic neurons enriched by coculture with
telomerase-immortalized midbrain astrocytes. Nat Med. 12:1259–1268.
2006. View
Article : Google Scholar
|
|
5
|
McDonald JW, Liu XZ, Qu Y, et al:
Transplanted embryonic stem cells survive, differentiate and
promote recovery in injured rat spinal cord. Nat Med. 5:1410–1412.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma H, Yu B, Kong L, Zhang Y and Shi Y:
Transplantation of neural stem cells enhances expression of
synaptic protein and promotes functional recovery in a rat model of
traumatic brain injury. Mol Med Rep. 4:849–856. 2011.PubMed/NCBI
|
|
7
|
Kulbatski I, Mothe AJ, Nomura H and Tator
CH: Endogenous and exogenous CNS derived stem/progenitor cell
approaches for neurotrauma. Curr Drug Targets. 6:111–126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galvin KA and Jones DG: Adult human neural
stem cells for cell-replacement therapies in the central nervous
system. Med J Aust. 177:316–318. 2002.PubMed/NCBI
|
|
9
|
Martens DJ, Seaberg RM and Van der Kooy D:
In vivo infusions of exogenous growth factors into the fourth
ventricle of the adult mouse brain increase the proliferation of
neural progenitors around the fourth ventricle and the central
canal of the spinal cord. Eur J Neurosci. 16:1045–1057. 2002.
View Article : Google Scholar
|
|
10
|
Sailor KA, Ming GL and Song H:
Neurogenesis as a potential therapeutic strategy for
neurodegenerative diseases. Expert Opin Biol Ther. 6:879–890. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldman SA: Directed mobilization of
endogenous neural progenitor cells: the intersection of stem cell
biology and gene therapy. Curr Opin Mol Ther. 6:466–472.
2004.PubMed/NCBI
|
|
12
|
Johansson CB, Momma S, Clarke DL, Risling
M, Lendahi U and Frisen J: Identification of a neural stem cell in
the adult mammalian central nervous system. Cell. 96:25–34. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwab ME: Repairing the injured spinal
cord. Science. 295:1029–1031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dobkin BH and Havton LA: Basic advances
and new avenues in therapy of spinal cord injury. Annu Rev Med.
55:255–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alexanian AR, Kwob WM, Pravdic D, Maiman
DJ and Fehlings MG: Survival of neurally induced mesenchymal cells
may determine degree of motor recovery in injured spinal cord rats.
Restor Neurol Neurosci. 28:761–767. 2010.PubMed/NCBI
|
|
16
|
Mabie PC, Mehler MF, Marmur R,
Papavasiliou A, Song Q and Kessler JA: Bone morphogenetic proteins
induce astroglial differentiation of oligodendroglial-astroglial
progenitor cells. J Neurosci. 17:4112–4120. 1997.PubMed/NCBI
|
|
17
|
Mehler MF, Mabie PC, Zhu G, Gokhan S and
Kessler JA: Developmental changes in progenitor cell responsiveness
to bone morphogenetic proteins differentially modulate progressive
CNS lineage fate. Dev Neurosci. 22:74–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakajima T, Ota M and Ito K:
Differentiations of autonomic neurons by BMP-independent
mechanisms. Cell Tissue Res. 332:25–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blitz IL and Cho KW: Finding partners: how
BMPs select their targets. Dev Dyn. 238:1321–1331. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ueki T, Tanaka M, Yamashita K, et al: A
novel secretory factor, Neurogenesin-1, provides neurogenic
environmental cues for neural stem cells in the adult hippocampus.
J Neurosci. 23:11732–11740. 2003.PubMed/NCBI
|
|
21
|
Sakuta H, Suzuki R, Takahashi H, et al:
Ventroptin: a BMP-4 antagonist expressed in a double-gradient
pattern in the retina. Science. 293:111–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin ZS, Zhang H, Wang W, Hua XY, Hu Y,
Zhang SQ and Li GW: Wnt-3a protein promote neuronal differentiation
of neural stem cells derived from adult mouse spinal cord. Neurol
Res. 29:847–854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao Q, Du Y, Wu W and Yip HK: Bone
morphogenetic proteins mediate cellular response and, together with
Noggin, regulate astrocyte differentiation after spinal cord
injury. Exp Neurol. 221:353–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakashima K, Takizawa T, Ochiai W, et al:
BMP2-mediated alteration in the developmental pathway of fetal
mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl
Acad Sci USA. 98:5868–5873. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yanagisawa M, Takizawa T, Ochiai W, Uemura
A, Nakashima K and Taga T: Fate alternation of neuroepithelial
cells from neurogenesis to astrocytogenesis by bone morphogenetic
proteins. Neurosci Res. 41:391–396. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mehler MF, Mabie PC, Zhang D and Kessler
JA: Bone morphogenetic proteins in the nervous system. Trends
Neurosci. 20:309–317. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Cogswell CA and LoTurco JJ: Neuronal
differentiation of precursors in the neocortical ventricular zone
is triggered by BMP. J Neurosci. 18:8853–8862. 1998.PubMed/NCBI
|
|
28
|
Mabie PC, Mehler MF and Kessle JA:
Multiple roles of bone morphogenetic protein signaling in the
regulation of cortical cell number and phenotype. J Neurosci.
19:7077–7088. 1999.PubMed/NCBI
|
|
29
|
Lim DA, Tramontin AD, Trevejo JM, Herrera
DG, Garcia-Verdugo JM and Alvarez-Buylla A: Noggin antagonizes BMP
signaling to create a niche for adult neurogenesis. Neuron.
28:713–726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shakèd M, Weissmüller K, Svoboda H,
Hortschansky P, Nishino N, Wölfl S and Tucker KL: Histone
deacetylases control neurogenesis in embryonic brain inhibition of
BMP2/4 signaling. PLoS One. 3:e26682008.PubMed/NCBI
|
|
31
|
Weible MW II and Chan-Ling T: Phenotypic
characterization of neural stem cells from human fetal spinal cord:
synergistic effect of LIF and BMP4 to generate astrocytes. Glia.
55:1156–1168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasai Y: Regulation of neural
determination by evolutionarily conserved signals: anti-BMP factors
and what next? Curr Opin Neurobiol. 11:22–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu SL, Ma ZW, Yin L, Iannotti C, Lu PH and
Xu XM: Region-specific growth properties and trophic requirements
of brain- and spinal cord-derived rat embryonic neural precursor
cells. Neuroscience. 135:851–862. 2005. View Article : Google Scholar : PubMed/NCBI
|


















