Introduction
Adenoid cystic carcinoma (ACC), a relatively rare
tumor occurring mainly in the salivary glands, is a slow growing
but highly malignant tumor. In recent years, cancer treatment has
shifted to molecular-targeted therapy based on molecular
aberrations in specific neoplasms. The molecular pathology of ACC,
however, has not been fully elucidated.
Pleiomorphic adenoma gene 1 (PLAG1) and
cylindromatosis (CYLD) are genes known to affect
tumorigenesis. PLAG1 is commonly rearranged in a subset of
pleomorphic adenoma (PA) of the salivary gland by chromosomal
aberrations, resulting in gene fusion. Several fusion partners of
PLAG1, including CTNNB1, CHCHD7, LLIR
and LIFR, have been identified (1–7).
PLAG1 protein is a zinc finger protein that functions as a
DNA-binding transcription factor. Deregulated transcription of
various genes by abnormally expressed PLAG1 is hypothesized to play
a major role in the development of PA. PA is the most common
neoplasm of the salivary gland and shares specific morphological
characteristics with ACC. ACC and PA tumors are composed of
epithelial and myoepithelial cells. Ultrastructural analysis
indicates that these tumors have a similar histogenetic basis
(8). However, the role of
PLAG1 in the development of ACC remains unknown. Matsuyama
et al(9) analyzed two cases
of ACC and identified no fusion genes involving PLAG1.
CYLD is a tumor suppressor gene, the
germ-line mutation of which causes familial cylindromatosis and
Brook-Spiegler syndrome (10). The
gene encodes a cytoplasmic protein that functions as a
deubiquitinating enzyme. CYLD protein plays a role in cell
proliferation and survival by negatively regulating nuclear
factor-κB (11). There are
morphological similarities between cutaneous cylindroma and ACC,
and ACC was previously considered to be a cylindroma (12).
The present study was designed to determine the role
of the CYLD gene in ACC of the salivary gland.
Materials and methods
Materials
A total of 34 paraffin-embedded blocks of ACC of the
major and minor salivary glands were retrieved from the archival
specimens maintained at the Pathology Center of Oita University
Hospital (Oita, Japan). The study was approved by the ethic
committee of Oita University, Faculty of Medicine.
Reverse-transcription (RT)-PCR
RT-PCR analysis for the detection of PLAG1
gene fusion was performed using the method described by Matsuyama
et al(9) with minor
modifications. RNA was extracted from formalin-fixed
paraffin-embedded (FFPE) tissue using the Qiagen RNeasy FFPE kit
(Qiagen, Hilden, Germany) according to the manufacturer's
instructions. In brief, 10-μm FFPE tumor sections of each sample
were digested with proteinase K in lysis buffer. Total RNA adsorbed
to the column provided in the kit was collected in the elution
buffer. The extracted total RNA was reverse transcribed to cDNA
using the First-Strand cDNA Synthesis kit (GE Healthcare, Tokyo,
Japan).
In the present study, PLAG1-associated fusion
transcripts with catenin β 1 (CTNNB1),
coiled-coil-helix-coiled-coil-helix domain containing 7
(CHCHD7), leukemia inhibitory factor receptor α
(LIFR) and transcription elongation factor A (SII), 1
(TCEA1) were analyzed. The sequence data of the primers is
presented in Table I. The primer
sequences were those reported by Matsuyama et al(9). PCR was performed using 2.5 units Taq
DNA polymerase (AmpliTaq Gold; Perkin Elmer, Norwalk, CT, USA), 1.5
mmol/l MgCl2, PCR buffer (Perkin Elmer), 200 μmol/l each
DNP (Perkin Elmer) and 0.2 μmol forward and reverse primers. The
35-cycle PCR amplification consisted of 35 cycles of denaturation
at 94°C for 30 sec, annealing at 59°C for 30 sec and elongation at
72°C for 30 sec, followed by a final extension at 72°C for 10
min.
 | Table IPrimers for RT-PCR. |
Table I
Primers for RT-PCR.
| Primer
designation | Sequence (5′-3′) | Size of PCR product
(bp) |
|---|
| Reverse |
| PLAG1-exon
2R |
gaccgtcacagaatgaagca | |
| PLAG1-exon
3R |
gccatgcccattgactcttc | |
| Forward |
| CHCHD7-exon
1F |
gtgagccattgacgtgtttg | 124 (with exon 2R),
123 and 228 (with exon 3R) |
| CTNNB1-exon
1F |
gaggaaggtctgaggagcag | 159 (with exon 2R),
158 and 263 (with exon 3R) |
| LIFR-exon
1F |
agctcagaaagggagcctct | 105 (with exon 2R),
104 and 209 (with exon 3R) |
| TCEA1-exon
1F |
gctttgccaagaagatggac | 106 (with exon 2R),
105 and 210 (with exon 3R) |
PCR-single strand conformational
polymorphism (SSCP) direct sequencing
Tumor cells were purified from the specimen by laser
micro-dissection (LMD) using the Leica LMD system (Leica
Microsystems, Wetzlar, Germany). In brief, FFPE specimens were
sectioned at 10-μm thickness and placed on membrane slides (Leica
Microsystems). Following staining with toluidine blue, tumor cells
were selected and dissected out using a laser beam under a
microscope. Care was paid to avoid contamination by normal tissue
surrounding the ACC cells. The dissected tumor cells were digested
with proteinase K and DNA was purified using the DNeasy tissue kit
(Qiagen) according to the manufacturer's instructions. The primers
for amplification of the CYLD gene coding exons were
designed using Primer 3 (http://primer3.sourceforge.net/webif.php). Table II lists the sequence data of the
primers. PCR was performed in 25-μl sample volumes as follows: 5
min at 95°C followed by 35 cycles of 30 sec at 95°C, 30 sec at 64°C
and 30 sec at 72°C. For SSCP analysis, the PCR products were
denatured by heating in a solution of 50% formamide and 10 mM
ethylenediaminetetraacetic acid and then separated on a 12.5%
polyacrylamide gel using the Genephore system (Amersham Pharmacia,
Uppsala, Sweden). Following denaturation, single-stranded DNA
underwent 3-dimensional folding and assumed a unique conformational
state based on the base sequence. The majority of single base
changes are detected as mobility shifts (12). The gels were silver stained using a
kit (Amersham Pharmacia) to detect the mobility shifts. Mutational
analysis was performed for cases demonstrating gene aberration as
determined by SSCP. Purified PCR products from ACC and normal
tissue adjacent to the tumor were directly sequenced using the
BigDye Terminator Cycle sequencing Ready Reaction mix and ABI310
genetic analyzer (both Applied Biosystems, Foster City, CA,
USA).
 | Table IIPrimers used in PCR. |
Table II
Primers used in PCR.
| Target | Forward | Reverse | Size of PCR product
(bp) |
|---|
| Exon 4-1 |
tcttttgcggttttatgacaa |
cggtactttaaggagcttttgtg | 199 |
| Exon 4-2 |
tcaagaatgcagcgttacaga |
agaactgcatgaggttgctct | 171 |
| Exon 4-3 |
gtggggcattcaaggattc |
aggctgaacctctcctcaca | 173 |
| Exon 4-4 |
gcaacctcatgcagttctctt |
tttcttccccagatctcagc | 194 |
| Exon 4-5 |
aatagacgtgggctgtcctg |
cagacacacatgaacacaaacaa | 187 |
| Exon 5-1 |
ccccttttcctatggatcgt |
ctttccaatgcagtgtcatca | 198 |
| Exon 5-2 |
agattgtggcgtgtttgttg |
tcctggcaaaacatcacaga | 199 |
| Exon 5-3 |
tcgaacttcctcctttggaa |
gatatttaatccaaaattttcttacca | 159 |
| Exon 6-1 |
tttggaggattctttatggaaaa |
aacacacgcaaaactacaaagc | 151 |
| Exon 6-2 |
gggatggaagatttgatgga |
aaccaaacaccacctgttcc | 188 |
| Exon 7 |
ctcaaatccactgtgggtga |
accttaaagcccagcaatga | 190 |
| Exon 8 |
tttctcttctataagaatttgccttt |
ggcattatgcaaattactaaaggtt | 198 |
| Exon 9-1 |
tttttaaatgaaacttttcttgttcc |
tggattgtggttgtgagtcaa | 118 |
| Exon 9-2 |
ggatctacctcagaccctgga |
tctgatgagttagaaagaaaggatca | 173 |
| Exon 10-1 |
gagtcaatatccttgaatacatttctg |
attgggcatcttggtgagac | 194 |
| Exon 10-2 |
accgttcttcaccaccactc |
caagggtggactctcttgga | 194 |
| Exon 10-3 |
attggccacagtccactttc |
attcagtcctggtggctgac | 198 |
| Exon 10-4 |
cctgggaactcacatggtct |
gcgaaatctgcacaaaacct | 191 |
| Exon 11-1 |
ggcacggtataatgcatattga |
gctgcaatgatgcaaaccta | 168 |
| Exon 11-2 |
gcgctgtttgtgaaactgaa |
aaaacactgtcaccatcacctaa | 186 |
| Exon 12-1 |
ttttgcatcaaaatacaaaaacatt |
ctccaagccttctttttcca | 184 |
| Exon 12-2 |
ttttcagcatttggaggcta |
cctgcctcatggcactatct | 197 |
| Exon 13 |
gaaaattatcctttttcttttgcag |
aggcaaaatagcaatttgttttc | 178 |
| Exon 14-1 |
tccagcctgagtgatagagtga |
gatgcagcctccacctttt | 195 |
| Exon 14-2 |
tgtgtgccacaaaaattatgaa |
cccccaactacacagacaca | 174 |
| Exon 15 |
tgatttaaaaattttgcctgtga |
catgtctgttgaataatggcagt | 194 |
| Exon 16-1 |
ttaacattttgatttaagcatttga |
cctctgcaaatttcaggttactg | 199 |
| Exon 16-2 |
ttcccacaattcagcagttg |
aagactcccacagactttcaca | 112 |
| Exon 17-1 |
tgttttgtttgacagccatga |
tctgttatatttaattccagagaagga | 187 |
| Exon 17-2 |
attcagatgcctcgatttgg |
tgccttgggaaatactgtgtc | 199 |
| Exon 18 |
cccttccccttctcacattt |
tccattaagtgaagggaagctc | 166 |
| Exon 19-1 |
ttgaactcctgacctcgtga |
gcagagaacagcaaataactcca | 195 |
| Exon 19-2 |
cccaaagacttacccgactg |
gcagaagaaaggcgttttca | 190 |
| Exon 20-1 |
tcactggcaaaagggtttaga |
gcatcacaaagcagtcttcg | 200 |
| Exon 20-2 |
tctggaagacctgcattcct |
acagaactgccagctcgaat | 191 |
Results
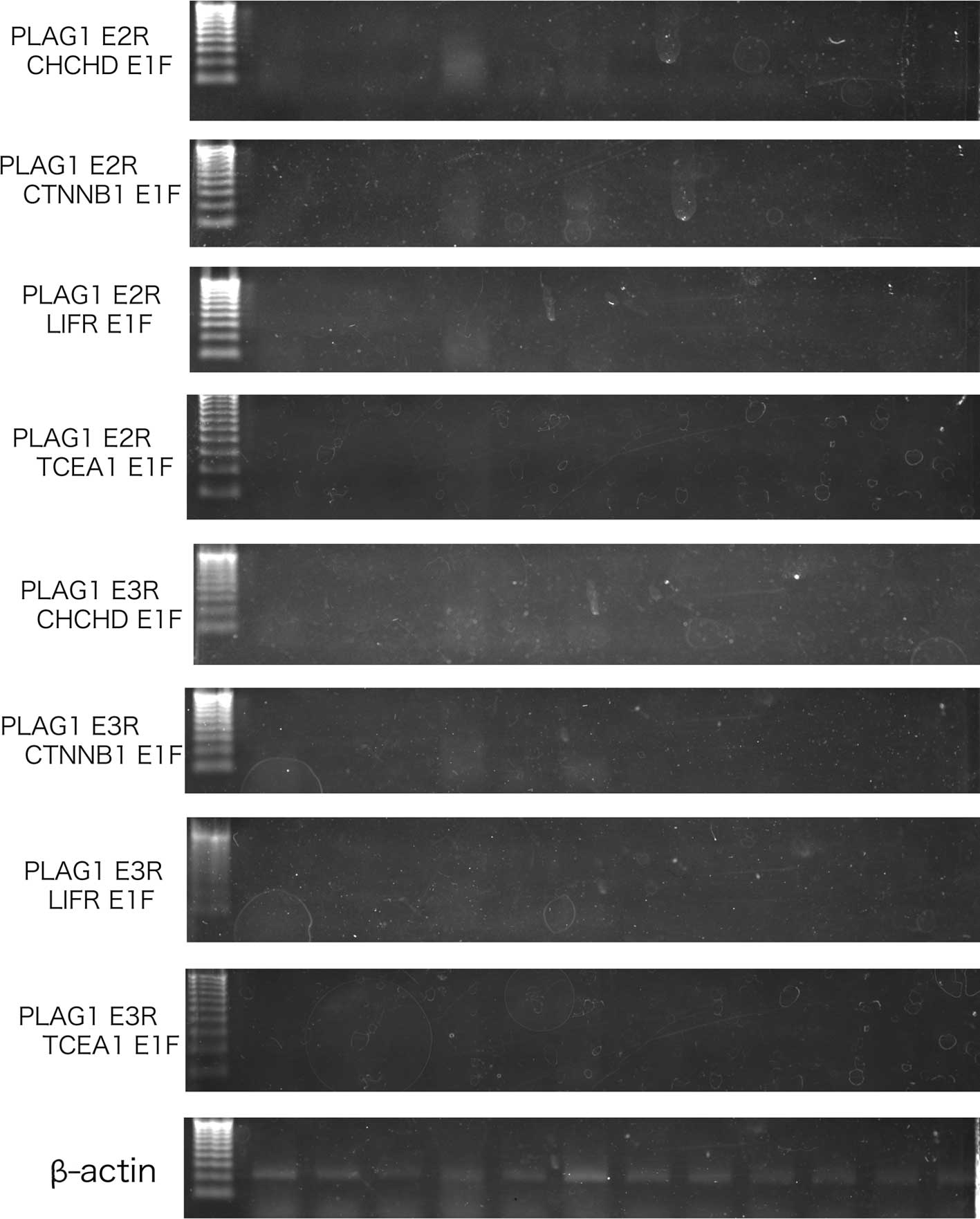
Representative RT-PCR results are presented in
Fig. 1. The β-actin product was
detected in each case. RT-PCR products for fusion genes, involving
PLAG1, were not obtained at the expected sizes.
Since 35 primer pairs were prepared, a total of
1,190 PCR analyses were performed to examine the coding region of
CYLD in the 34 cases of ACC. PCR products were obtained in
~75% of the PCR analyses. The results of PCR-SSCP analysis are
presented in Fig. 2 and aberrant
bands are indicated by arrows. These PCR products were subjected to
direct sequencing.
Fig. 3 presents
results of direct sequencing. The sample with an aberration in exon
11, identified by SSCP analysis, (Fig.
2A) was found to exhibit a silent mutation at codon 548. The
sample with an aberration in exon 16 (Fig. 2B) was also identified to have a
silent mutation, located at codon 713.
Discussion
It is well known that c-KIT, a proto-oncogene and
therapeutic target, is recurrently expressed in ACC (14,15).
A previous study reported that the chromosomal translocation
t(6;9), which is associated with
overexpression of MYB, is frequently found in ACC (16). Thus, knowledge of the molecular
pathology of ACC is increasing, however, the molecular features of
ACC remain to be elucidated. In the current study, gene-fusion
involving PLAG1 and the mutational status of CYLD
were investigated.
PLAG1, encoding a zinc finger protein, is
consistently rearranged in PAs of the salivary glands. Through
chromosomal translocation, abnormal expression of PLAG1 is
driven by a constitutionally active promoter. Overexpression of
PLAG1, acting as a transcription factor, causes deregulation of a
variety of PLAG1 target genes. The aberrant expression of these
target genes is hypothesized to be the cause of PA (17). Aberrations in PLAG1 have
been detected in neoplasms other than PA. Chromosomal rearrangement
involving PLAG1 are present in the majority of lipoblastomas
(18,19). Although the fusion partner for
PLAG1 varies, PLAG1 with a strong promoter following
chromosomal rearrangement has been identified in lipoblastoma as
well as PA (19). Thus, aberrant
expression of PLAG1 occurs in these neoplasms, acting as an
oncogene. In the present study, the gene fusions of PLAG1
and several fusion partners, specifically, CTNNB1,
CHCHD7, LIFR and TCEA1, were analyzed. These
gene fusions have been detected in PA (9). Based on the results of RT-PCR, no
gene fusion involving PLAG1 was detected in ACC. These
results are consistent with observations reported by Matsuyama
et al(9). ACC and PA have
similar histogenetic properties (8), however, the karyotypical aberrations
differ from each other (20,21).
In this study, chromosomal abnormalities of ACC were not tested,
however, gene fusion, including PLAG1, was investigated in a
relatively large number of cases. Results indicate that the
mechanism involved in the tumorigenesis of ACC is different from
that of PA.
Since cylindroma is a cutaneous neoplasm, cylindroma
and ACC do not share histogenetic characteristics, however,
myoepithelial cells participate in tumor formation in both types of
neoplasms (22). Thus, cylindroma
and ACC share morphological characteristics. CYLD, encoding
a deubiquitinating enzyme, is associated with cylindromatosis,
multiple familial trichoepithelioma and Brooke-Spiegler syndrome
(10). In addition to these
tumors, loss of CYLD expression is observed in various types of
skin cancer, including basal cell and squamous cell carcinoma
(23). Choi et al(24) identified loss of heterozygosity at
the CYLD locus in basal cell adenoma of the salivary gland.
Thus, CYLD may play a role in tumorigenesis in various
neoplasms.
In the present study, the mutational status of
CYLD was investigated in ACC. A silent mutation was detected
in only two cases, indicating that CYLD does not play a role
in ACC tumorigenesis comparable to that in Brooke-Spiegler
syndrome.
In the present study, no gene fusions of
PLAG1 or mutations of CYLD were identified,
indicating that these genes are not involved in ACC
tumorigenesis.
References
|
1
|
Asp J, Persson F, Kost-Alimova M and
Stenman G: CHCHD7-PLAG1 and TCEA1-PLAG1 gene fusions resulting from
cryptic, intrachromosomal 8q rearrangements in pleomorphic salivary
gland adenomas. Genes Chromosomes Cancer. 45:820–828. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aström AK, Voz ML, Kas K, Röijer E, Wedell
B, Mandahl N, Van de Ven W, Mark J and Stenman G: Conserved
mechanism of PLAG1 activation in salivary gland tumors with and
without chromosome 8q12 abnormalities: identification of SII as a
new fusion partner gene. Cancer Res. 59:918–923. 1999.PubMed/NCBI
|
|
3
|
Martins C, Fonseca I, Roque L, Pereira T,
Ribeiro C, Bullerdiek J and Soares J: PLAG1 gene alterations
in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic
adenoma: a combined study using chromosome banding, in situ
hybridization and immunocytochemistry. Mod Pathol. 18:1048–1055.
2005. View Article : Google Scholar
|
|
4
|
Kas K, Voz ML, Röijer E, Aström AK, Meyen
E, Stenman G and Van de Ven WJ: Promoter swapping between the genes
for a novel zinc finger protein and beta-catenin in pleiomorphic
adenomas with t(3;8)(p21;q12) translocations. Nat Genet.
15:170–174. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voz ML, Aström AK, Kas K, Mark J, Stenman
G and Van de Ven WJ: The recurrent translocation t(5;8)(p13;q12) in
pleomorphic adenomas results in upregulation of PLAG1 gene
expression under control of the LIFR promoter. Oncogene.
16:1409–1416. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bullerdiek J, Wobst G, Meyer-Bolte K,
Chilla R, Haubrich J, Thode B and Bartnitzke S: Cytogenetic
subtyping of 220 salivary gland pleomorphic adenomas: correlation
to occurrence, histological subtype and in vitro cellular behavior.
Cancer Genet Cytogenet. 65:27–31. 1993. View Article : Google Scholar
|
|
7
|
Mark J, Dahlenfors R and Wedell B: Impact
of the in vitro technique used on the cytogenetic patterns in
pleomorphic adenomas. Cancer Genet Cytogenet. 95:9–15. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orenstein JM, Dardick I and van Nostrand
AW: Ultrastructural similarities of adenoid cystic carcinoma and
pleomorphic adenoma. Histopathology. 9:623–638. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsuyama A, Hisaoka M, Nagao Y and
Hashimoto H: Aberrant PLAG1 expression in pleomorphic adenomas of
the salivary gland: a molecular genetic and immunohistochemical
study. Virchows Arch. 458:583–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bignell GR, Warren W, Seal S, et al:
Identification of the familial cylindromatosis tumour-suppressor
gene. Nat Genet. 25:160–165. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massoumi R: CYLD: a deubiquitination
enzyme with multiple roles in cancer. Future Oncol. 7:285–297.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ellis GL and Auclair PL: Adenoid cystic
carcinoma. Tumors of the Salivary Glands. Rosai J and Sobin LH:
Armed Forces Institute of Pathology; Washington D.C: pp. 203–216.
1996
|
|
13
|
Orita M, Iwahana H, Kanazawa H, Hayashi K
and Sekiya T: Detection of polymorphisms of human DNA by gel
electrophoresis as single-strand conformation polymorphisms. Proc
Natl Acad Sci USA. 86:2766–2770. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holst VA, Marshall CE, Moskaluk CA and
Frierson HF Jr: KIT protein expression and analysis of c-kit gene
mutation in adenoid cystic carcinoma. Mod Pathol. 12:956–960.
1999.PubMed/NCBI
|
|
15
|
Jeng YM, Lin CY and Hsu HC: Expression of
the c-kit protein is associated with certain subtypes of salivary
gland carcinoma. Cancer Lett. 154:107–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Persson M, Andrén Y, Mark J, Horlings HM,
Persson F and Stenman G: Recurrent fusion of MYB and NFIB
transcription factor genes in carcinomas of the breast and head and
neck. Proc Natl Acad Sci USA. 106:18740–18744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Dyck F, Declercq J, Braem CV and Van
de Ven WJ: PLAG1, the prototype of the PLAG gene family:
versatility in tumour development (review). Int J Oncol.
30:765–774. 2007.PubMed/NCBI
|
|
18
|
Astrom A, D'Amore ES, Sainati L, Panarello
C, Morerio C, Mark J and Stenman G: Evidence of involvement of the
PLAG1 gene in lipoblastomas. Int J Oncol. 16:1107–1110.
2000.PubMed/NCBI
|
|
19
|
Hibbard MK, Kozakewich HP, Dal Cin P,
Sciot R, Tan X, Xiao S and Fletcher JA: PLAG1 fusion oncogenes in
lipoblastoma. Cancer Res. 60:4869–4872. 2000.PubMed/NCBI
|
|
20
|
Nordkvist A, Mark J, Gustafsson H, Bang G
and Stenman G: Non-random chromosome rearrangements in adenoid
cystic carcinoma of the salivary glands. Genes Chromosomes Cancer.
10:115–121. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin C, Martins C, Jin Y, et al:
Characterization of chromosome aberrations in salivary gland tumors
by FISH, including multicolor COBRA-FISH. Genes Chromosomes Cancer.
30:161–167. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tellechea O, Reis JP, Ilheu O and Baptista
AP: Dermal cylindroma. An immunohistochemical study of thirteen
cases. Am J Dermatopathol. 17:260–265. 1995.PubMed/NCBI
|
|
23
|
Masoumi KC, Shaw-Hallgren G and Massoumi
R: Tumor suppressor function of CYLD in nonmelanoma skin cancer. J
Skin Cancer. 2011:6140972011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi HR, Batsakis JG, Callender DL, Prieto
VG, Luna MA and El-Naggar AK: Molecular analysis of chromosome 16q
regions in dermal analogue tumors of salivary glands: a genetic
link to dermal cylindroma? Am J Surg Pathol. 26:778–783. 2002.
View Article : Google Scholar : PubMed/NCBI
|