Introduction
Esophageal, gastric and lung cancer are common
diseases that pose a serious global threat to health. Chemotherapy
agents such as bleomycin, mitomycin and methotrexate may cause
pulmonary toxicity, while radiotherapy may lead to radiation
pneumonitis (1,2). Lung injury seriously hampers full
implementation of treatment, limiting the potential benefits of
therapy. The early phase of lung injury is characterized by
inflammation (alveolitis), while the late phase is characterized by
the organization and deposition of collagen with remodeling
(pulmonary fibrosis) (1,2).
The characteristic clinical and histological
manifestations of acute lung injury (ALI) are initiated by a
well-described network of cytokines (3,4). The
acute phase of ALI, characterized by alveolar inflammation, is
mediated by tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1)
and transforming growth factor-β (TGF-β) (3,4). In
addition, interferon-γ (IFN-γ) is able to maintain the inflammatory
response in the lung by inducing macrophages to produce mediators,
such as TNF-α, IL-1, IL-6 and IL-8. TNF-α is an important signaling
protein that is able to initiate and continually amplify local or
systemic inflammatory responses (5).
TGF-β is a key cytokine that induces lung injury and
contributes to pulmonary fibrosis, through its actions to induce
collagen gene expression or synthesis by stimulation of fibroblast
proliferation (3,4,6,7).
Expression of the connective tissue growth factor (CTGF) gene acts
as a downstream effector of TGF-β1, and is thought to play an
important role in pulmonary fibrosis through the promotion of
extracellular matrix synthesis (8).
Numerous other signal transduction pathways have
been implicated in the pathogenesis of pulmonary fibrosis. For
example, the mitogen-activated protein kinase/extracellular
signal-related kinase (MAPK/ERK) pathway is essential for the
formation of pulmonary fibrosis (9–11).
NF-κB is a significant transcription factor that is a key mediator
of signal transduction during the acute inflammatory response and
pulmonary fibrosis (12,13).
Despite recent advances in our understanding of the
epidemiology, pathogenesis and treatment of ALI, this condition
remains a significant cause of morbidity and mortality in the
critically ill patient population (14). At present, glucocorticoids are the
most frequently used anti-inflammatory drugs for the clinical
management of ALI (9,15). However, currently there are no
approved medical anti-fibrotic therapies (16), and hence the development of
effective agents to ameliorate pulmonary fibrosis is urgently
needed.
Peiminine is the main component of
Fritillaria, and has been used for several years as a
traditional Chinese medicine for a variety of conditions including
pulmonary fibrosis. Peiminine has been reported to have effects as
a relaxant of bronchial smooth muscle and as an antitussive
(17,18). In addition, there is evidence that
alkaloids isolated from the Fritillaria bulb have
anti-inflammatory, as well as antitussive actions (17,18).
However, to date there have been no studies exploring whether or
not peiminine is able to inhibit pulmonary inflammation and
fibrosis.
The aims of this study was to determine whether or
not peiminine inhibits lung inflammation and pulmonary fibrosis in
rats. Furthermore, the effects of peiminine on lung injury were
correlated with changes in the levels of mediators implicated in
the pathogenesis of ALI.
Materials and methods
Animals
Age- and gender-matched Sprague-Dawley (SD) rats
(weight, 180–220 g) were purchased from the Experimental Animal
Center of the Nanjing Medical University, kept in a 12-h dark/light
cycle in a temperature- and humidity-controlled room, and fed a
standard laboratory diet and water. The experimental procedures
were approved by the Animal Care and Use Committee of the Nanjing
Medical University, China. Adequate measures were taken to minimize
the pain experienced by the experimental animals.
Drugs and reagents
Peiminine (purity >98%) was obtained from Gamma
Technology Development Co., Ltd. (Shenzhen China). Dexamethasone
(DXS; 0.75 mg) was purchased from Tianjin Tianyao Pharmaceutical
Co., Ltd. (Tianjin, China). Bleomycin (8 mg) was obtained from
Tianjin Taihe Pharmaceutical Co., Ltd. (Tianjin, China).
Rabbit anti-rat polyclonal antibodies against TGF-β,
CTGF, NF-κB, ERK1/2, Fas and FasL were purchased from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
IL-4 and TNF-α kits were provided by the Beijing Huaying
Biotechnology Institute (Beijing, China). The IFN-γ kit was
obtained from the Beijing Huaying Biotechnology Institute, sourced
from Adlitteram Diagnostic Laboratories, Inc. (West Palm Beach, FL,
USA).
Instruments
The microtome was purchased from Leica (Mannheim,
Germany). The optical microscope, Olympus DP71 microscope digital
camera and fully automated image acquisition system were obtained
from the Olympus Corporation (Tokyo, Japan).
The γ-911 automatic radioimmunoassay (RIA) counter
was purchased from the Science and Technology Industrial Company of
the China University, and the Stat Fax 2100 automatic microplate
reader was purchased from Awareness Technology, Inc. (Palm City,
FL, USA).
Experimental groups
Rats were randomly divided into 4 groups: the
sham-operated (n=12), the control (n=14), the DXS (n=14) and the
peiminine groups (n=10). For the latter 3 groups, intratracheal
administration of bleomycin was used to induce lung injury, to
allow comparison of the effects of peiminine and DXS. For the
sham-operated group, normal saline was applied instead of
bleomycin, as the negative control for ALI.
Development of the ALI model in rats
After allowing adjustment to the environment, the
rat was anesthetized with chloral hydrate (10%) and fixed on a
board in the supine position. For the control, DXS and peiminine
groups, bleomycin (5 mg/kg) was instilled into the trachea of the
rat using a microliter injector, based on methods described
previously in the literature (19). For the sham-operated group, normal
saline was administered instead of bleomycin. After intratracheal
instillation of bleomycin or saline, the rat was placed in a
vertical position and spun for 0.5 min to ensure that the solution
was distributed evenly within the lungs.
Administration of drugs
Rats in the sham-operated and control groups were
given 5‰ carboxymethyl cellulose sodium (CMC) solution at a dosage
of 1 ml/100 g weight; CMC was chosen as its viscosity was similar
to that of the drugs used in the other 2 groups. Rats in the DXS
group were given an equal volume of DXS solution at a dosage of
0.000405 g/kg weight. Rats in the peiminine group were administered
an equal volume of peiminine at a dosage of 0.005 g/kg weight.
Drugs were administered daily for 28 consecutive days, using
gastric gavage; it has been reported previously that 28 days are
required for the formation of lung fibrosis after administration of
bleomycin (20).
Alveolitis and pulmonary fibrosis
assay
Rats were anesthetized and sacrificed by carotid
exsanguination. The left lung was fixed with 4% paraformaldehyde in
phosphate-buffered saline (PBS) under 15–20-cm H2O
pressure. Lungs were embedded in paraffin, and 4-μm sections were
prepared. For histology, the sections were stained with hematoxylin
and eosin (H&E) and Masson’s trichrome. To assay the severity
of alveolitis and pulmonary fibrosis, the scoring method described
by Szapiel et al(21) was
used.
The grading criteria used for alveolitis were: 1
point, no alveolitis; 2 points, mild alveolitis, affecting <20%
of the total lung, showing infiltration of mononuclear cells into
the widened alveolar septa, and limited to localized regions with
involvement of nearby pleural areas; 3 points, moderate alveolitis,
affecting an area of 20–50%, with greater pleural involvement; 4
points, severe alveolitis, involving an area >50%, with
occasional monocytes in the alveolar space and bleeding caused by
consolidation.
The scoring criteria used for fibrosis were: 1
point, no fibrosis; 2 points, mild fibrosis, affecting an area
<20% of the whole lung, with fibrosis involving the pleura and
subpleural interstitium, and disorders of alveolar structure; 3
points, moderate fibrosis, involving an area of 20–50%, with
localized areas of fibrosis extending from the pleura; 4 points,
severe fibrosis, involving an area >50%, with fusion of alveolar
spaces.
Lung index assay
Rats were anesthetized and sacrificed by carotid
exsanguination, and their chest was opened to obtain the lungs. The
trachea was removed and discarded, and after drying the surface
with filter paper, the lungs were weighed. The lung index was then
calculated, based on lung weight and body weight: Lung index = lung
weight (g)/body weight (g) ×100%
Assay for inflammatory cytokines
Rats were anesthetized and sacrificed by carotid
exsanguination. The blood was collected, and centrifuged at 3,000
rpm for 10 min to obtain serum. The serum was subjected to RIA to
determine the levels of IL-4, TNF-α and IFN-γ.
Assay of cell signal transduction
pathways
The left lung was fixed with 4% paraformaldehyde in
PBS under 15–20-cm H2O pressure. The lung was embedded
in paraffin and 4-μm sections were prepared. Immunohistochemistry,
using the streptavidin-biotin complex (SABC) method, with
calculation of average optical density (IOD), was used to determine
the levels of TGF-β, CTGF, NF-κB, ERK1/2, Fas and FasL.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Statistical analyses were carried out using the SPSS 16.0
software. One-way analysis of variance (ANOVA) followed by the
Student-Newman-Keuls test were used to compare the results in the
various treatment groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
General observations
In the initial period after surgical operation, rats
in the control group (i.e., with bleomycin-induced ALI) had cold
tails and limbs, dark purple tail veins and loss of hair luster.
These symptoms gradually receded, showing improvement at 3 days,
and had almost disappeared at 7 days. Rats in the control and DXS
groups showed a reduced activity, decreased appetite and weight
loss. The weight loss was more evident in the DXS group; however,
in these two groups, body weight gradually recovered over 18–21
days. In the sham-operated and peiminine groups, no noticeable
reduction was observed in activity, appetite or weight. The
mortality rates (during the 28-day period) in the control and DXS
groups were 1/14 rats. No rats died in the other two groups during
this period.
Macroscopic observations of lung
tissue
The lung tissue of the sham-operated group was pink,
smooth and soft, with good elasticity. However, in the control
group, a significant reduction was observed in the amount of normal
lung tissue, with increasing occurrence of uneven pale foci, black
lesions and reduced elasticity. In the DXS group, the extent of the
lesions was smaller compared to the control group, although there
was still a clear difference from the sham-operated group. The
peiminine group showed no obvious changes, with lung tissue
structure resembling that of the sham-operated group.
H&E staining observed under the light
microscope
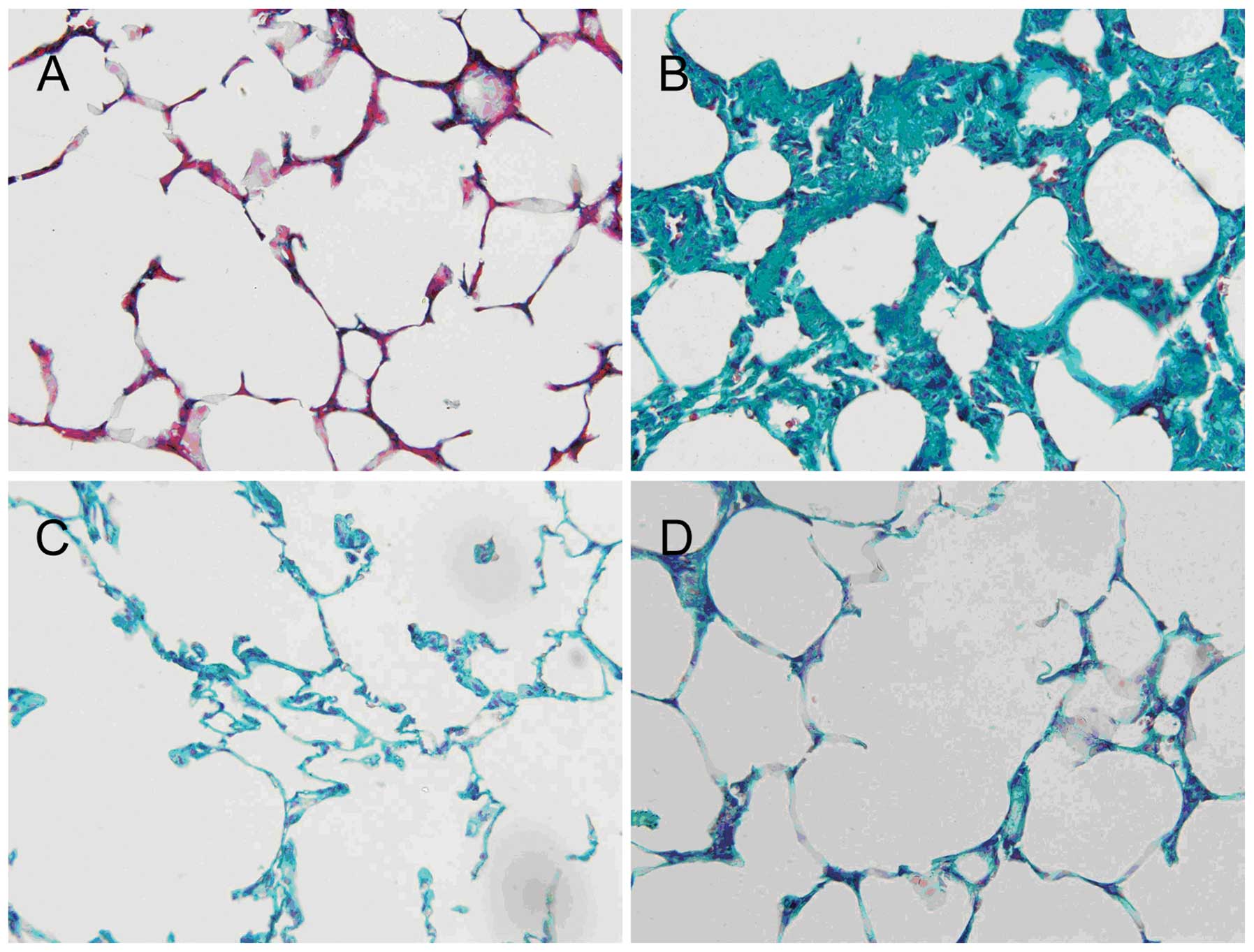
Representative examples of sections from the 4
groups are shown in Fig. 1. The
morphological characteristics of the sham-operated group were
consistent with those expected of normal lung structure. By
contrast, the control group showed widening of the alveolar septa,
interstitial edema and inflammatory cell infiltration into the
pulmonary interstitium and the alveolar spaces. Although
pathological changes were also evident in the DXS and peiminine
groups, these lesions were less severe or extensive compared to
those observed in the control group.
Masson’s trichrome staining observed
under the light microscope
Representative examples of sections from the 4
groups are shown in Fig. 2. In the
lung tissue of sham-operated rats, a relatively small amount of
collagen fibers was present. In the control group, a substantial
increase in the number of collagen fibers was evident, typical of
pulmonary fibrosis. Evidence of pulmonary fibrosis was also
observed in the DXS and peiminine groups, although to a lesser
extent compared to that observed in the control group.
Alveolitis and pulmonary fibrosis
scores
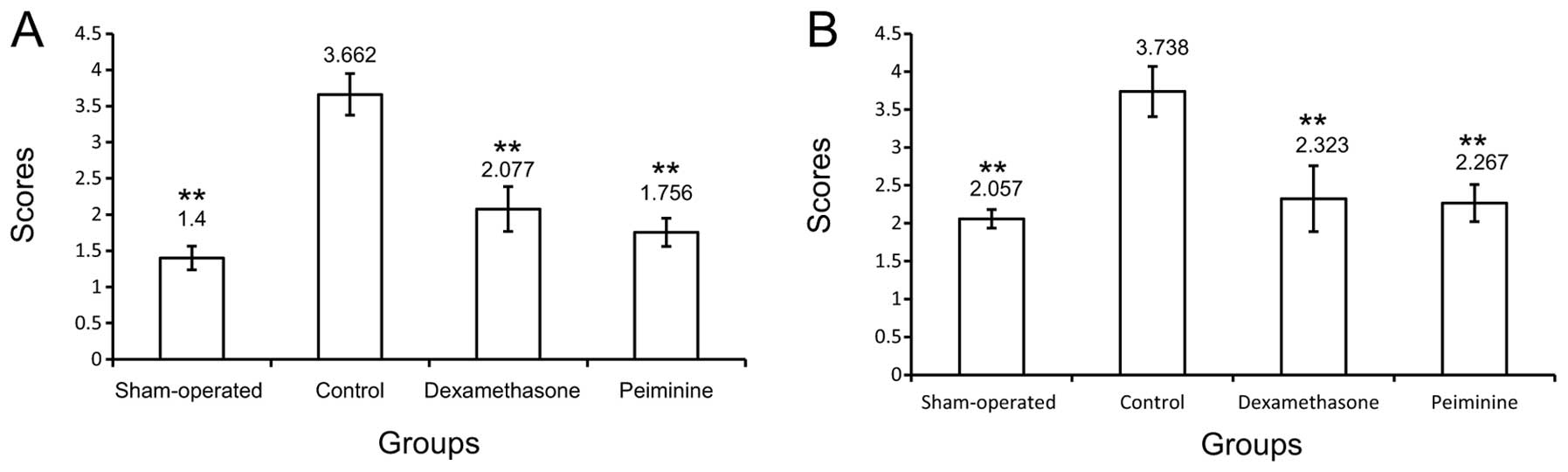
As shown in Fig. 3,
scores for alveolar inflammation and pulmonary fibrosis were
significantly higher in rats in the control group compared to rats
in the sham-operated group (P<0.01). Furthermore, the alveolitis
and pulmonary fibrosis scores in the DXS and peiminine groups were
significantly lower compared to the corresponding scores in the
control group (P<0.01).
Lung index as a measure of lung injury in
rats
Fig. 4A–C presents
data for body and lung weight as well as lung index for the 4
groups. The control group showed a significantly elevated lung
index (P<0.01) compared to the sham-operated group, which was
associated with a significant increase in lung weight (P<0.05)
and a significant decrease in body weight (P<0.01). This
increase in the lung index is indicative of bleomycin-induced lung
injury in rats of the control group. Furthermore, the peiminine
group was found to have a significantly lower lung index
(P<0.01), as well as a significantly lower lung weight
(P<0.01) compared to the control group. These results suggest
that peiminine reduced the extent of the lung injury.
Levels of inflammatory cytokines in the
blood
As shown in Fig.
4D–F, 28 days after induction of lung injury, levels of IL-4
(P<0.01) and IFN-γ (P<0.05) were significantly elevated in
the control group, compared to the sham-operated group. The levels
of TNF-α and IL-4 in the peiminine and DXS groups were not
significantly different to the corresponding values in the control
group (P>0.05). However, the levels of IFN-γ in the peiminine
and DXS groups were significantly lower compared to that of the
control group (P<0.01).
Cell signal transduction pathways
As shown in Fig. 5,
the levels of TGF-β, CTGF, NF-κB, ERK1/2, FasL and Fas were
significantly higher in the control group compared to those in the
sham-operated group (P>0.05 for Fas; P<0.01 for the
others).
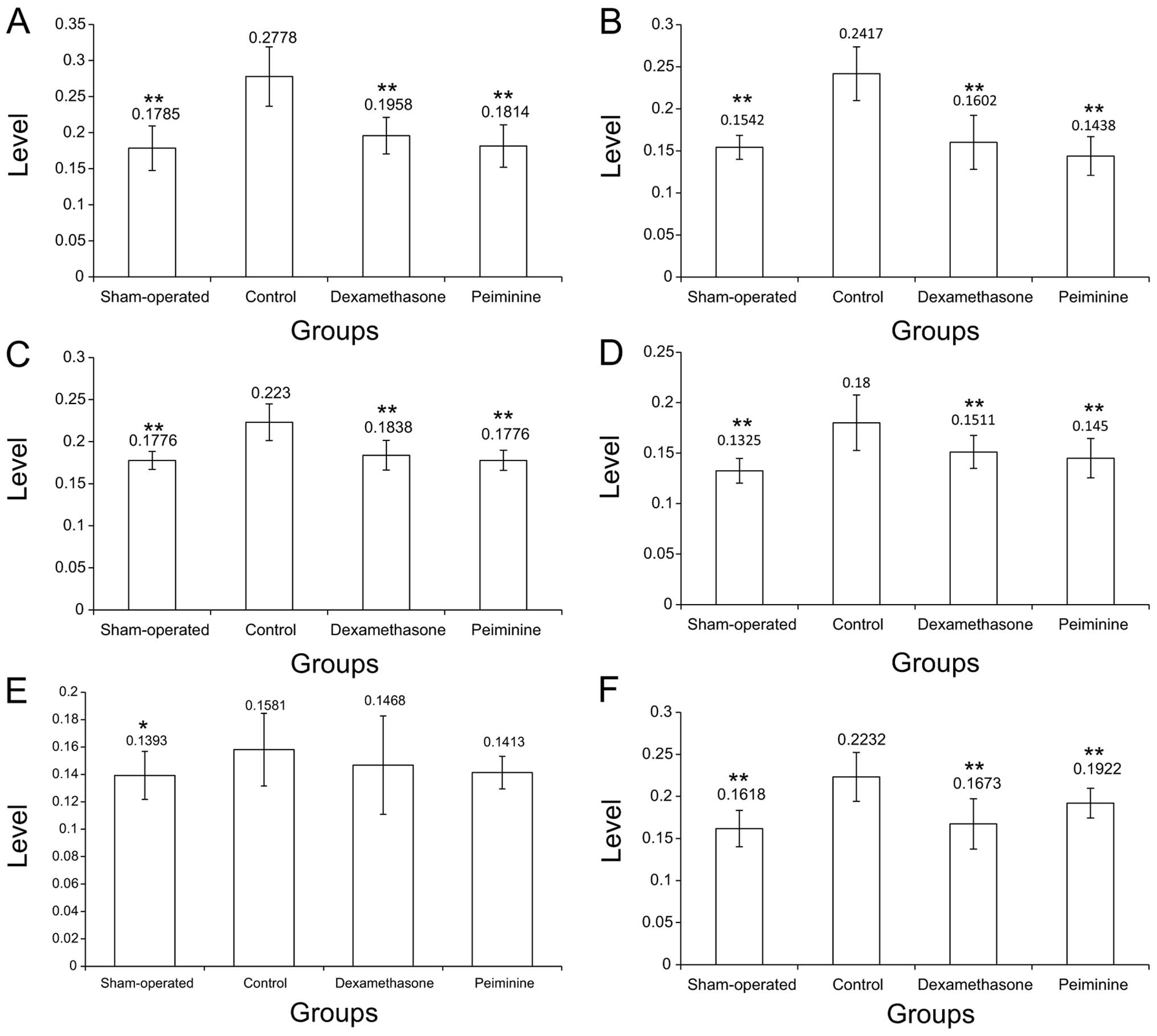 | Figure 5Levels of TGF-β, CTGF, NF-κB, ERK1/2,
Fas and FasL in lung tissue. (A) TGF-β levels in the 4 groups.
TGF-β was significantly increased (P<0.01) in the control,
compared to the sham-operated group. The dexamethasone and
peiminine groups had significantly lower TGF-β levels (P<0.01)
compared to the control group. (B) CTGF levels in the 4 groups.
CTGF was significantly increased (P<0.01) in the control,
compared to the sham-operated group. The dexamethasone and
peiminine groups had significantly lower CTGF levels (P<0.01)
compared to the control group. (C) NF-κB levels in the 4 groups.
NF-κB was significantly increased (P<0.01) in the control,
compared to the sham-operated group. The dexamethasone and
peiminine groups had significantly lower NF-κB levels (P<0.01)
compared to the control group. (D) ERK1/2 levels in the 4 groups.
ERK1/2 was significantly elevated in the control (P<0.01),
compared to the sham-operated group. The dexamethasone and
peiminine groups had significantly lower ERK1/2 levels (P<0.01)
compared to the control group. (E) Fas levels in the 4 groups. The
Fas level in the control group was significantly higher (P<0.01)
compared to that in the sham-operated group, but not significantly
different from values in the dexamethasone and peiminine groups.
(F) FasL levels in the 4 groups. FasL was significantly elevated in
the control (P<0.01), compared to the sham-operated group.
Levels in the dexamethasone and peiminine groups were significantly
lower (P<0.01) compared to the control group.
*P<0.05; **P<0.01. |
Compared to the control group, the peiminine and DXS
groups showed significantly lower levels of TGF-β, CTGF, NF-κB,
ERK1/2 and FasL (P<0.01 for all). By contrast, no statistically
significant differences were observed in these groups for Fas
(P>0.05).
Discussion
The main findings of our study are that peiminine is
as effective as DXS in reducing the degree of alveolitis and the
extent of pulmonary fibrosis (assessed using histological scoring
methods and the lung index), 28 days after bleomycin-induced lung
injury in rats. These effects of peiminine were associated with a
reduced level of serum IFN-γ, and decreased expression of TGF-β,
CTGF, ERK1/2, NF-κB and FasL in lung tissue. The beneficial actions
of peiminine may thus be due to the inhibitory effects on these
aforementioned mediators, which are known to be involved in the
pathogenesis of ALI.
Intratracheal application of bleomycin is a widely
used technique for inducing ALI in rodent animal model systems,
resulting in an initial development of pulmonary oedema that is
followed by a fibrotic interstitial reaction (20,22,23).
In the present study, histological comparison of the control
(bleomycin) and the sham-operated groups showed clear evidence of
inflammation, edema and collagen deposition in lung sections of the
control group that were not present in the sham-operated group.
Furthermore, the grading of alveolitis and pulmonary fibrosis using
established scoring systems demonstrated that bleomycin induced
these two pathological changes. In addition, levels of IFN-γ and
IL-4 in the serum were significantly increased in the control group
(compared to the sham-operated group), as were levels of TGF-β,
CTGF, ERK1/2, NF-κB and FasL in lung tissue. These data clearly
indicate that bleomycin successfully induced lung injury,
alveolitis and pulmonary fibrosis in the rats used in our study,
supporting our use of this method as a model of ALI.
Over the past decade, substantial progress has been
made in understanding the pathophysiology of lung fibrosis. The
design of successful anti-fibrotic therapies may need to focus on
mechanisms or pathways, downstream of the inflammatory process,
that mediate fibroproliferation. The identification of
intracellular signaling pathways eliciting the cellular responses
of mesenchymal cell proliferation and differentiation as well as
extracellular matrix deposition, may facilitate the development of
novel therapeutic approaches to ameliorate the global burden of
fibroproliferative diseases (9,24).
Inhibition of signal transduction proteins is now widely
acknowledged as a valid strategy to combat inflammatory disease
(23). Notably, studies have
reported that neferine, methyl palmitate, naringin, astragalin,
luteolin and paeonol have inhibitory effects on pulmonary fibrosis,
due to their actions as anti-inflammatory agents, anti-oxidants and
inhibitors of cytokines and NF-κB (22,25–30).
Our study suggests that peiminine may also have such beneficial
effects, which are comparable to those of DXS.
The process of fibrosis is promoted by early
pro-inflammatory mediators, hence blocking of these mediators may
be one approach to attenuate fibrosis. Evidence from several
clinical studies has indicated that pro-inflammatory cytokines,
notably TNF-α, IL-1 and IL-6, participate in the early development
of inflammation and play a crucial role in ALI (3,4).
TNF-α is known as a primary cytokine, since it amplifies the
inflammatory cascade to cause inflammatory injury and recruits
neutrophils into the lung (5,23).
Furthermore, IL-4 is an anti-inflammatory cytokine that is able to
inhibit the function of TNF-α and reduce inflammatory injury to
lung tissue (31). In the present
study, serum levels of TNF-α and IL-4 were not significantly
affected by peiminine and DXS, whereas the level of IFN-γ was
reduced. This would suggest that inhibitory actions of peiminine
and DXS on alveolitis and pulmonary fibrosis are not secondary to
effects on TNF-α and IL-4, but may instead be the consequence, at
least in part, of decreased secretion of IFN-γ.
Previous studies have identified a number of
chemokines, cytokines and growth factors that mediate pulmonary
fibrosis (3,4,7). Of
these, TGF-β1 is thought to be one of the key mediators that links
inflammation to fibrogenesis. CTGF is a downstream mediator of
TGF-β1 that induces connective tissue cell proliferation and
extracellular matrix deposition (8). It is therefore of note that, in our
experiments, peiminine as well as DXS caused reductions in the
tissue expression of CTGF and TGF-β. Upregulation of TGF-β1 and
CTGF are known to be critically involved in the pathogenesis of
pulmonary fibrosis (23,32). TGF-β1 is a potent pro-fibrotic
factor that plays a pivotal role in several pathological processes,
including the transition of alveolar epithelial cells to
myofibroblasts (33–35). Consistent with this hypothesis,
impaired TGF-β responsiveness appears to result in a reduction of
fibrosis (32). CTGF has been
reported to be useful in diagnosing or predicting disease
progression in certain fibrotic diseases (36), while CTGF levels in blood are
considered to reflect fibrosis in a variety of organs (37). Furthermore, inhibiting the
upregulation of CTGF can attenuate bleomycin-induced ALI and
pulmonary fibrosis (23), while
certain agents that inhibit bleomycin-induced ALI and pulmonary
fibrosis have been reported to act through inhibition of TGF-β1 and
CTGF. It is therefore reasonable to conclude that some of the
inhibitory effects of peiminine (and also DXS) on bleomycin-induced
ALI are via reduced expression of CTGF and TGF-β.
The NF-κB family of transcription factors regulates
inflammation, survival, proliferation and other biological
processes (12). There are clear
links between canonical activation of NF-κB in immune cells to the
pathogenesis of inflammatory diseases (13), and the expression of
pro-inflammatory mediators is known to be modulated by NF-κB
(26). Stimulation of the NF-κB
pathway is mediated by diverse signal transduction cascades in
response to several stress conditions, such as infection and
inflammation. We found that administration of peiminine and DXS
were associated with significant reductions in tissue NF-κB
expression. These actions may thus contribute to the protective
effects of these agents on ALI.
The MAPK-ERK signaling cascade is a major pathway
controlling cellular processes associated with fibrogenesis,
including growth, proliferation and survival. In progressive
pulmonary fibrosis associated with increased MAPK/ERK activation,
ERK has been reported to be primarily activated in the mesenchymal
cells of the fibrotic lesions (9).
Clinical findings have demonstrated an upregulation of MAPK/ERK in
human fibrotic disease (10). The
MAPK/ERK pathway is a logical target for potential fibrosis
therapy, as several fibrogenic cytokines signal through MAPK/ERK
(11), and selective inhibition of
MAPK prevents the development and attenuates the progression of
fibrosis, when administered as a rescue therapy. Our findings that
peiminine and DXS cause reduced expression of ERK1/2 in lung tissue
indicate that decreased signaling through the MAPK/ERK pathway
contributes to the anti-fibrotic effects of these drugs. In
addition, actions on FasL, which is also involved in the fibrotic
process (38), may also play a
role in the effects of peiminine and DXS.
Our study is not without limitations. First,
although we have shown that peiminine has protective effects
against bleomycin-induced ALI, it cannot be certain that such
effects would extend to other chemotherapy agents or to
radiation-induced injury. However, the bleomycin model is widely
used and validated, hence our data are likely to have applicability
to human patients, at least to a certain extent. Second, although
changes in the levels of various cytokines and mediators have been
identified following peiminine treatment in our rat model system,
the primary mediators that contribute to the beneficial actions of
peiminine are yet to be identified. In addition, it cannot be
definitively concluded that similar changes in mediator levels
would be seen in human patients. Additional studies are required to
expand upon our observations, and describe the mechanisms
underlying the actions of peiminine more precisely.
In conclusion, our findings indicate that peiminine
has beneficial effects protecting against bleomycin-induced lung
injury in rats, and that these effects are comparable to those of
DXS. Furthermore, the attenuation of pulmonary fibrosis by
peiminine is associated with a reduction in the levels of IFN-γ in
the blood, and CTGF, TGF-β, NF-κB, ERK1/2 and FasL in lung tissue.
Thus, our findings provide evidence that peiminine may have
therapeutic potential in the treatment of ALI and pulmonary
fibrosis.
Acknowledgements
This study was financially supported by the Nature
Science Foundation of the Jiangsu province of China (no.
08KJB360008) and the Chinese Postdoctoral Station of the Nanjing
Medical University (no. 201102170C).
References
|
1
|
Limper AH: Chemotherapy-induced lung
disease. Clin Chest Med. 25:53–64. 2004. View Article : Google Scholar
|
|
2
|
Graves PR, Siddiqui F, Anscher MS and
Movsas B: Radiation pulmonary toxicity: from mechanisms to
management. Semin Radiat Oncol. 20:201–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhatia M, Zemans RL and Jeyaseelan S: Role
of chemokines in the pathogenesis of acute lung injury. Am J Respir
Cell Mol Biol. 46:566–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin TR and Matute-Bello G: Experimental
models and emerging hypotheses for acute lung injury. Crit Care
Clin. 27:735–752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mukhopadhyay S, Hoidal JR and Mukherjee
TK: Role of TNFalpha in pulmonary pathophysiology. Respir Res.
7:1252006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anscher MS: Targeting the TGF-beta1
pathway to prevent normal tissue injury after cancer therapy.
Oncologist. 15:350–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson MS, Madala SK, Ramalingam TR, et
al: Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A
dependent. J Exp Med. 207:535–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blom IE, Goldschmeding R and Leask A: Gene
regulation of connective tissue growth factor: new targets for
antifibrotic therapy? Matrix Biol. 21:473–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madala SK, Schmidt S, Davidson C, Ikegami
M, Wert S and Hardie WD: MEK-ERK pathway modulation ameliorates
pulmonary fibrosis associated with epidermal growth factor receptor
activation. Am J Respir Cell Mol Biol. 46:380–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antoniou KM, Margaritopoulos GA, Soufla G,
et al: Expression analysis of Akt and MAPK signaling pathways in
lung tissue of patients with idiopathic pulmonary fibrosis (IPF). J
Recept Signal Transduct Res. 30:262–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia X, Liu B, Shi X, Ye M, Zhang F and Liu
H: Roles of the ERK, JNK/AP-1/cyclin D1-CDK4 pathway in
silica-induced cell cycle changes in human embryo lung fibroblast
cells. Cell Biol Int. 35:697–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morris GF: An alternative to lung
inflammation and fibrosis. Am J Pathol. 176:2595–2598. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson ER and Matthay MA: Acute lung
injury: epidemiology, pathogenesis, and treatment. J Aerosol Med
Pulm Drug Deliv. 23:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lamontagne F, Briel M, Guyatt GH, Cook DJ,
Bhatnagar N and Meade M: Corticosteroid therapy for acute lung
injury, acute respiratory distress syndrome, and severe pneumonia:
a meta-analysis of randomized controlled trials. J Crit Care.
25:420–435. 2010. View Article : Google Scholar
|
|
16
|
Raghu G, Collard HR, Egan JJ, et al: An
official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis:
evidence-based guidelines for diagnosis and management. Am J Respir
Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Zhu J, Wang S, et al: Antitussive,
expectorant and anti-inflammatory alkaloids from Bulbus
Fritillariae Cirrhosae. Fitoterapia. 82:1290–1294. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang D, Wang S, Chen X, et al:
Antitussive, expectorant and anti-inflammatory activities of four
alkaloids isolated from Bulbus of Fritillaria wabuensis. J
Ethnopharmacol. 139:189–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taooka Y, Maeda A, Hiyama K, Ishioka S and
Yamakido M: Effects of neutrophil elastase inhibitor on
bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care
Med. 156:260–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aono Y, Nishioka Y, Inayama M, et al:
Imatinib as a novel antifibrotic agent in bleomycin-induced
pulmonary fibrosis in mice. Am J Respir Crit Care Med.
171:1279–1285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
22
|
El-Demerdash E: Anti-inflammatory and
antifibrotic effects of methyl palmitate. Toxicol Appl Pharmacol.
254:238–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JW, Rhee CK, Kim TJ, et al: Effect of
pravastatin on bleomycin-induced acute lung injury and pulmonary
fibrosis. Clin Exp Pharmacol Physiol. 37:1055–1063. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hardie WD, Glasser SW and Hagood JS:
Emerging concepts in the pathogenesis of lung fibrosis. Am J
Pathol. 175:3–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Wang X, Chang Q, et al: Neferine,
a bisbenzylisoquinline alkaloid attenuates bleomycin-induced
pulmonary fibrosis. Eur J Pharmacol. 627:304–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Wu H, Nie YC, Chen JL, Su WW and Li
PB: Naringin attenuates acute lung injury in LPS-treated mice by
inhibiting NF-κB pathway. Int Immunopharmacol. 11:1606–1612.
2011.PubMed/NCBI
|
|
27
|
Lee HB, Kim EK, Park SJ, Bang SG, Kim TG
and Chung DW: Isolation and anti-inflammatory effect of astragalin
synthesized by enzymatic hydrolysis of tea seed extract. J Sci Food
Agric. 91:2315–2321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu PK, Wu CL, Tsai TH and Hsieh CL:
Anti-inflammatory and anticoagulative effects of paeonol on
LPS-induced acute lung injury in rats. Evid Based Complement
Alternat Med. 2012:8375132012.PubMed/NCBI
|
|
29
|
Lee JP, Li YC, Chen HY, et al: Protective
effects of luteolin against lipopolysaccharide-induced acute lung
injury involves inhibition of MEK/ERK and PI3K/Akt pathways in
neutrophils. Acta Pharmacol Sin. 31:831–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soromou LW, Chen N, Jiang L, et al:
Astragalin attenuates lipopolysaccharide-induced inflammatory
responses by down-regulating NF-κB signaling pathway. Biochem
Biophys Res Commun. 419:256–261. 2012.PubMed/NCBI
|
|
31
|
Levings MK and Schrader JW: IL-4 inhibits
the production of TNF-alpha and IL-12 by STAT6-dependent and
-independent mechanisms. J Immunol. 162:5224–5229. 1999.PubMed/NCBI
|
|
32
|
Carey WA, Taylor GD, Dean WB and Bristow
JD: Tenascin-C deficiency attenuates TGF-β-mediated fibrosis
following murine lung injury. Am J Physiol Lung Cell Mol Physiol.
299:L785–L793. 2010.
|
|
33
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
the importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ramos C, Becerril C, Montano M, et al:
FGF-1 reverts epithelial-mesenchymal transition induced by
TGF-{beta}1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell
Mol Physiol. 299:L222–L231. 2010.PubMed/NCBI
|
|
36
|
Kono M, Nakamura Y, Suda T, et al: Plasma
CCN2 (connective tissue growth factor; CTGF) is a potential
biomarker in idiopathic pulmonary fibrosis (IPF). Clin Chim Acta.
412:2211–2215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leask A, Parapuram SK, Shi-Wen X and
Abraham DJ: Connective tissue growth factor (CTGF, CCN2) gene
regulation: a potent clinical bio-marker of fibroproliferative
disease? J Cell Commun Signal. 3:89–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dosreis GA, Borges VM and Zin WA: The
central role of Fas-ligand cell signaling in inflammatory lung
diseases. J Cell Mol Med. 8:285–293. 2004. View Article : Google Scholar : PubMed/NCBI
|