Introduction
High-mobility group box protein 1 (HMGB1) is a
nuclear protein present in all mammals and conserved across
species. HMGB1 appears to have two distinct functions in cells. The
main intranuclear function of HMGB1 is to regulate transcription
and modify chromatin structure (1–3).
Extracellular HMGB1 stimulates the secretion of proinflammatory
cytokines from endothelial cells, monocytes and macrophages,
leading to inflammatory responses in target tissues (4,5).
Previous studies have demonstrated that
extracellular HMGB1 is a late cytokine mediator of delayed
endotoxin lethality (6,7). HMGB1 has been detected in the serum
of patients with sepsis, and serum HMGB1 levels are significantly
elevated in patients with poor prognoses. However, the mechanism by
which intracellular HMGB1 participates in inflammation remains
unclear.
Despite the use of antibiotics and modern intensive
care, severe sepsis has a mortality rate of ~30%, resulting in an
estimated 250,000 deaths annually in the United States (8). Microvascular injury is one of the
characteristics of sepsis-associated tissue damage, and may be
manifested by single or multiple organ failure (MOF) syndromes
(9).
The endothelium plays an important role in the
development of sepsis. The nucleus of the endothelial cell contains
large amounts of HMGB1. A number of studies have demonstrated that
the endothelium may be a critical source of HMGB1 in inflammation
(10,11). In a clinically relevant murine
model of sepsis (induced by cecal ligation and puncture),
administration of HMGB1-specific neutralizing antibodies 24 h after
the onset of sepsis rescued mice from lethal sepsis in a
dose-dependent manner (12,
13). This indicates that further
investigation of the function of HMGB1 in inflammation may be
important for the improvement of the treatment of inflammatory
diseases, particularly sepsis.
Based on the HMGB1-specific antibody results in
sepsis, we hypothesized that the downregulation of HMGB1 expression
via RNA interference (RNAi) decreases inflammatory activity.
Vectors capable of producing short hairpin RNAs (shRNAs), which are
processed to form small interfering RNAs (siRNAs), enable the
suppression of endogenous gene expression (14,15).
A previous study demonstrated the potential of chemically
synthesized antisense oligonucleotides targeting HMGB1 expression
to significantly inhibit colon cancer cell growth, migration and
invasion (16). However, the
gene-silencing effects of HMGB1 RNAi on endothelial cell function
remain unknown.
Increased expression levels of endothelial adhesion
molecules, either at the membrane level or in the plasma,
characterize the different models of sepsis (17). In vitro incubation of
endothelial cells with bacterial lipopolysaccharide (LPS) has been
shown to induce the rapid expression of intracellular adhesion
molecule 1 (ICAM1) and E-selectin mRNA (18). Moreover, there is a close
relationship between plasma levels of adhesion molecules and the
consequences of sepsis (19). In
various models of sepsis, endothelial adhesion molecules were found
to be present at elevated levels in membranes and plasma. Previous
studies have identified HOXA9 to be a key regulator of endothelial
activation (20). HOXA9 also
participates in the transcriptional activation of E-selectin in
endothelial cells (21).
In the present study, a stable, HMGB1-silencing
human umbilical vein endothelial cell (HUVEC) line was established
using RNAi technology and eukaryotic expression vectors carrying
the full-length cDNA of HMGB1 (pcDNA3.1-myc-his-HMGB1) were
constructed. The effects of subsequent HMGB1 knockdown on HOXA9,
E-selectin, ICAM1 and vascular cell adhesion molecule 1 (VCAM1)
expression were then evaluated. The potential mechanism by which
the HMGB1 gene affects endothelial cell activation was
investigated.
Materials and methods
Cells
HUVECs (CRL-2873; American Type Culture Collection,
Mannassas, VA, USA) were maintained in Dulbecco's modified Eagle's
medium (DMEM) and supplemented with 10% fetal bovine serum (FBS;
HyClone Laboratories, Inc., Logan, UT, USA), 2 mM glutamine, 100
IU/ml penicillin and 100 mg/ml streptomycin. The cells were
incubated at 37°C in a 5% CO2/95% air-humidified
atmosphere.
Western blot analysis
The cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and prepared with
radioimmunoprecipitation assay lysis buffer (50 mM Tris-HCl, 150 mM
NaCl, 1% Nonidet P-40, 0.5% deoxycholate and 0.1% sodium dodecyl
sulfate) containing a protease inhibitor mixture (Roche, Basel,
Switzerland). The samples were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA)
using a SemiDry Transfer Cell (Bio-Rad, Hercules, CA, USA).
Polyvinylidene difluoride membranes were blocked with 5% nonfat
milk and incubated with the first antibody at 4°C overnight. The
blots were then incubated with a horseradish peroxidase-conjugated
secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) for 1 h at room temperature.
Immunoreactive bands were visualized using the
SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford,
IL, USA) and LAS-3000 mini (Fuji Film, Fuji, Japan). The antibodies
against HMGB1, ICAM1, VCAM1, E-selectin and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) were obtained from Abcam (cat.
nos. ab18256, ab19569, ab2213 and ab6630; Cambridge, UK) and Santa
Cruz Biotechnology, Inc., respectively.
Plasmid construction and
transfection
The full-length HMGB1 (GenBank accession no.
NM_002128) cDNA was obtained by reverse transcription (RT)-PCR from
HUVECs and cloned into pcDNA3.1/myc-his-B (Invitrogen, Carlsbad,
CA, USA) using KpnI and EcoRI. The primers for the
full-length HMGB1 were: forward, ATGGGCAAAGGAGATCCTAAGA and
reverse, TTCATCATCATCATCTTCTTC. All the cDNAs were analyzed by DNA
sequencing to ensure that there were no mutations. HUVECs were
transfected with the constructed plasmids using Lipofectamine™ 2000
(Invitrogen), and the expression of HMGB1 was verified by western
blot analysis.
RNAi
The siRNA for a sequence targeting human HMGB1 to
the coding regions, 5′-AAGGTTGAGAGC TATTGCTGA-3′, was constructed
using the siRNA design center from GenScript (GenScript,
Piscataway, NJ, USA). A non-silencing, scrambled siRNA sequence
(5′-TTCTCCGAACGTGTCACGT-3′) was used as a control. The siRNA
sequence was inserted into the pRNA-U6.1/Neo vector (GenScript),
recombinant siRNA plasmids were transfected into HUVECs, and stable
transfectants were selected with G418.
Transfection
HUVECs in which HMGB1 was silenced were transfected
with the siRNAs targeted to HOXA9 using Lipofectamine™ 2000
according to the manufacturer's instructions. The mRNA and protein
expression levels of HMGB1 and HOXA9 were determined using
real-time PCR and western blot analysis, respectively.
Real-time PCR
Total RNA was treated with RNase-free DNase I
(Takara Bio, Tokyo, Japan) and reverse transcribed with Avian
Myeloblastosis Virus Reverse Transcriptase (Promega, Madison, WI,
USA). Real-time PCR was performed using an ABI 7500 real-time PCR
system (Applied Biosystems, Foster City, CA, USA) with an Ex Taq
RT-PCR kit (Takara Bio), according to the manufacturer's
instructions. The sequences of primers for human HMGB1, HOXA9,
ICAM1, VCAM1, E-selectin and GAPDH are shown in Table I. Amplification conditions were:
95°C for 10 sec, 40 cycles of 95°C for 5 sec, and 64°C for 34 sec.
The quantities of HMGB1, HOXA9, ICAM1, VCAM1 and E-selectin
transcripts for individual samples were normalized to GAPDH.
 | Table IPrimers for real-time PCR. |
Table I
Primers for real-time PCR.
| Target | Amplicon length
(bp) | Oligonucleotide
sequence | GenBank accession
no. |
|---|
| HMGB1 | 186 |
F:GGGGTACCATGGGCAAAGGAGATCCTAAGA | NM_002128 |
| | R:
CGGAATTCGTTTCATCATCATCATCTTCTTC | |
| HOXA9 | 152 | F:
TACGTGGACTCGTTCCTGCT | NM_152739.3 |
| | R:
CGTCGCCTTGGACTGGAAG | |
| ICAM1 | 112 | F:
ATGCCCAGACATCTGTGTCC | NM_000201 |
| | R:
GGGGTCTCTATGCCCAACAA | |
| VCAM1 | 60 | F:
CGAATGAGGGGACCACATCTA | NM_080682 |
| | R:
TGTTCGTTCCCAAAACTAACAGG | |
| E-selectin | 185 | F:
TGTGGGTCTGGGTAGGAACC | NM_000450 |
| | R:
AGCTGTGTAGCATAGGGCAAG | |
| GAPDH | 225 | F:
GAAGGTGAAGGTCGGAGTC | NM_002046 |
| | R:
CAAGCTTCCCGTTCTCAGCC | |
Statistical analysis
Data analysis was conducted using SPSS 17.0
software. Data were expressed as the means ± standard deviation
(SD). Statistical analysis between groups was performed using
Student's t-test and ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of shRNA-mediated suppression of
HMGB1 expression in HUVECs
To explore the role of HMGB1 in HUVECs, the siRNA
technique was used to knockdown HMGB1 expression. Specific siRNA
targeting HMGB1 was designed and the double-stranded siRNA was
inserted into pRNA-U6.1/Neo siRNA vector, with scrambled,
non-silencing siRNA as a control. siRNA constructs were stably
transfected into HUVECs and the silencing effect was determined
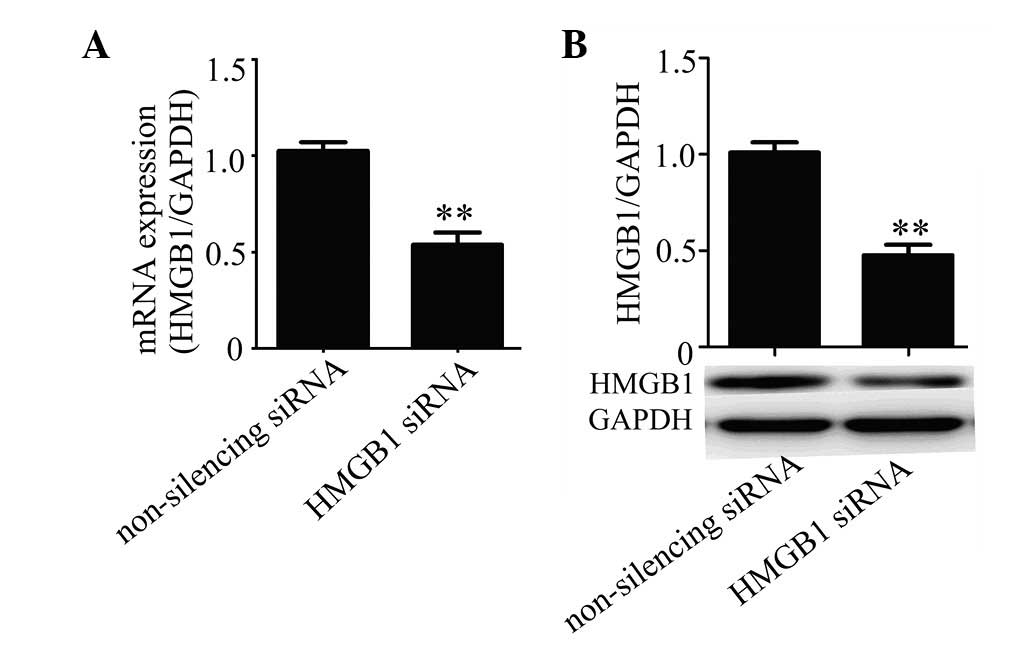
using real-time PCR and western blot analysis. The results showed
that HMGB1 siRNA was efficient in suppressing the expression of
HMGB1 in HUVECs compared with the non-silencing siRNA control at
the mRNA (Fig. 1A) and protein
(Fig. 1B) levels.
HMGB1 knockdown decreases E-selectin
expression in HUVECs
Cell adhesion molecules (CAMs) such as VCAM1, ICAM1
and E-selectin are involved in the inflammatory process (22). Inhibitors of cell adhesion
molecules may be useful therapeutic agents for vascular diseases
including atherosclerosis (23).
To explore the function of HMGB1 in inflammation, we detected the
expression of VCAM1, ICAM1 and E-selectin in HMGB1-silenced HUVECs.
The results showed that the expression levels of VCAM1, ICAM1 and
E-selectin were significantly decreased in HUVECs transfected with
HMGB1-specific siRNA compared with those in control cells, at the
mRNA (Fig. 2A) and protein
(Fig. 2B and C) levels.
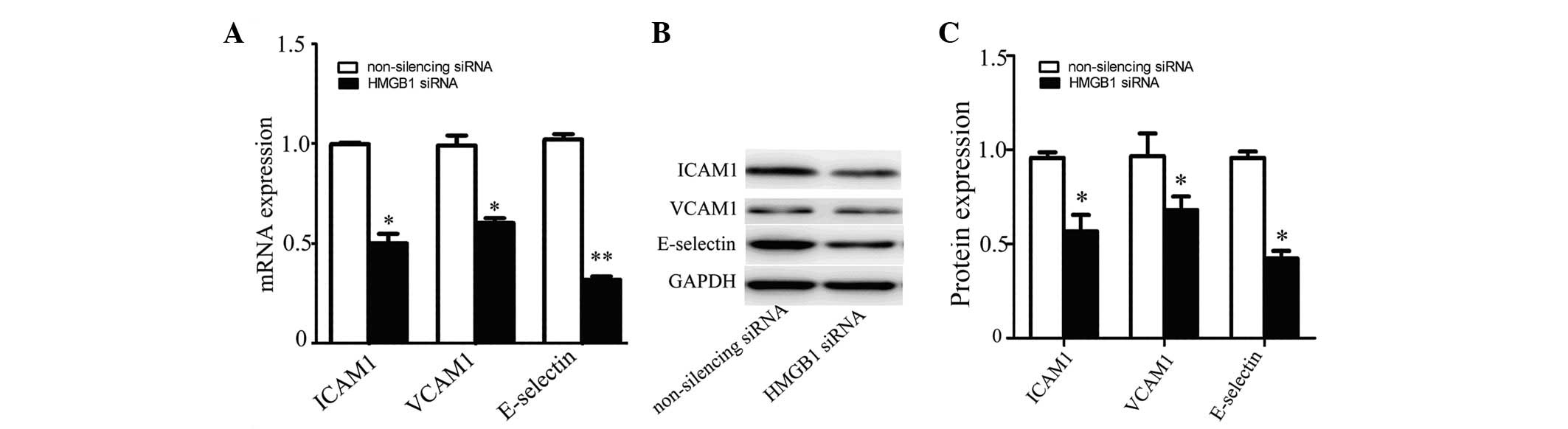 | Figure 2Expression of cell adhesion molecules
in HMGB1-silenced HUVECs. The expression of VCAM1, ICAM1 and
E-selectin in HUVECs transfected with HMGB1 siRNA or non-silencing
siRNA was detected by (A) real-time PCR and (B) western blot
analysis. VCAM1, ICAM1, E-selectin and GAPDH protein bands (C) were
measured using Image J software. Values are the means ± SD of three
independent experiments, each performed in triplicate.
*P<0.05, **P≤0.01 vs. non-silencing siRNA.
HMGB1, high-mobility group box protein 1; HUVECs, human umbilical
vein endothelial cells; VCAM1, vascular cell adhesion molecule 1;
ICAM1, intracellular adhesion molecule 1; siRNA, small interfering
RNA; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Role of HOXA9 in the regulation of
E-selectin expression
HOXA9 is a homeobox transcription factor and is
rapidly downregulated during endothelial cell activation in
response to tumor necrosis factor (TNF)-α (24). HOXA9 downregulation is considered
an essential event for endothelial cell activation. Previous
studies have demonstrated that TNF-α and LPS activate endothelial
cells by inducing the expression of leukocyte adhesion molecules
such as ICAM1, VCAM1 and E-selectin, which are not expressed by
quiescent endothelial cells (25).
Trivedi et al(21)
demonstrated that TNF-α and LPS downregulate HOXA9, which is an
essential event for the transition of endothelial cells from a
quiescent to an active state.
To explore the effects of HMGB1 silencing in HUVECs
on the expression of adhesion molecules, siRNA was used to
knockdown the expression of HOXA9 in HUVECs in which HMGB1
expression was silenced. After transfection with Lipofectamine™
2000 for 48 h, the gene-silencing effect mediated by HOXA9-specific
siRNAs in HUVECs was confirmed using RT-PCR and western blot
analysis. The expression of E-selectin was measured using real-time
PCR. The results showed that HOXA9 siRNA was efficient in
suppressing the expression of HOXA9 in HUVECs compared with the
non-silencing siRNA control, both at the mRNA (Fig. 3A) and protein (Fig. 3B) levels; HOXA9 expression
increased in HUVECs in which HMGB1 expression was silenced
(Fig. 3C). Co-transfection with
HMGB1 siRNA and HOXA9 siRNA significantly increased E-selectin
levels in HUVECs compared with those in HMGB1 siRNA-transfected and
control cells (Fig. 3D).
Effect of HMGB1 overexpression on
E-selectin expression
To further confirm the effect of HMGB1 on E-selectin
expression and to investigate the potential underlying mechanism,
the full-length HMGB1 gene was amplified by PCR according to the
sequence in GenBank and was subcloned into pcDNA-3.1-myc-his-B
vector. Then, HUVECs were transfected with
pcDNA-3.1-myc-his-B-HMGB1, using the pcDNA-3.1-myc-his-B vector as
a control. Expression of HMGB1 in HUVECs was detected by RT-PCR and
western blot analysis. The results showed expression of HMGB1 in
the HUVECs was successfully achieved with the
pcDNA-3.1-myc-his-B-HMGB1 vector at the mRNA (Fig. 4A) and protein (Fig. 4B) levels. RT-PCR showed that
overexpression of HMGB1 in HUVECs increased the expression of
E-selectin (Fig. 4C) and reduced
the expression of HOXA9 (Fig.
4D).
Discussion
Sepsis, a lethal condition following infection or
injury, is associated with a high mortality rate and afflicted
750,000 patients in the United States in 1995 (8). Pro-inflammatory cytokines lead to the
development of tissue damage, metabolic acidosis, hypotension, MOF
and even death (26). Two of these
cytokines, TNF-α and interleukin (IL)-1β, reached toxic levels in
serum from mice and human volunteers within 1 to 2 h of LPS
administration (27,28). Thus, it is not surprising that
delayed treatment therapies directed against early pro-inflammatory
mediators have been ineffective in large clinical trials (28–32).
HMGB1 has been demonstrated to be a late mediator of
lethal systemic inflammation in murine models and to be involved in
delayed endotoxin lethality and systemic inflammation. Thus, as a
pro-inflammatory cytokine, HMGB1 has been hypothesized to be an
ideal target in sepsis therapy. Previous studies have provided
evidence that therapy with anti-HMGB1 antibodies conferred
significant protection against lethality, even when the first dose
of antibody was administered 24 h after LPS administration. The
endothelium plays an important role in the development of sepsis;
the nucleus of the endothelial cell contains large amounts of
HMGB1, and a number of studies have demonstrated that the
endothelium may be a critical source of HMGB1 during inflammation
(33).
In the present study, we used HUVECs as an in
vitro model system and described the potential use of a
plasmid-based shRNA targeting HMGB1 expression in endothelial
cells. Results from the present study showed that HMGB1 is
expressed in HUVECs, which is in agreement with a previous study on
the expression of HMGB1 in endothelial cells (11). Some experimental approaches have
been reported to result in inhibition of HMGB1 function in sepsis
(29,34–37).
Of these, antibodies are difficult to administer and may produce
antigen-antibody complexes that stimulate the inflammatory response
by further activating innate immune pathways, thus promoting
further tissue damage. Antibody-based blockade strategies have
shown limited efficacy in clinical trials against sepsis (38). Future studies are required in order
to develop novel and selective antagonists for HMGB1 (39,40).
In the present study, we proposed that
downregulation of HMGB1 expression in the endothelium by RNAi may
decrease inflammatory activity. RNAi-based techniques for silencing
specific genes, either as synthetic RNA oligonucleotides or as
plasmid-encoded shRNAs, are widely used. We examined a similar
approach whereby vectors expressing three different shRNAs were
used to suppress HMGB1 expression in HUVECs.
The human HMGB1 gene is located on chromosome 13
(41), and six polymorphic loci
throughout the gene locus have recently been identified (42). The structure of HMGB1 is highly
conserved across eukaryotic species, with 99% amino acid homology
between rodents and humans (43–45).
However, despite its clinical importance, there is limited
knowledge regarding the intracellular signaling pathways activated
by HMGB1 in inflammation. To the best of our knowledge, the present
study is the first to investigate the role of potential signaling
pathways that control the function of intracellular HMGB1 in
inflammation.
Several studies have shown an increase in the
soluble form of E-selectin in healthy human volunteers receiving an
injection of LPS (46) and in
patients hospitalized for sepsis (47). Levels of E-selectin in the plasma
are positively correlated with the severity of sepsis in patients,
as evaluated by the Simplified Acute Physiology Score II or the
number of organ failures (i.e., MOF) score (48). Increased levels of adhesion
molecules in the plasma reflect an increase in the endothelial
membrane expression of the proteins and may be directly related to
activation or dysfunction of the endothelium (49). In the current study, we demonstrate
that blocking the expression of HMGB1 reduces E-selectin mRNA
expression. This is in agreement with the finding that HMGB1, and
the B-box of HMGB1 (a DNA-binding element), activate HUVECs to
upregulate adhesion molecules such as ICAM1, VCAM1 and E-selectin
in a dose-dependent manner (50).
HOXA9, besides binding to DNA, may associate with the crucial
architectural factor high-mobility group AT-hook 1 [HMGI(Y)], which
has been suggested to be a general cofactor in HOX-mediated
transcriptional activation (51).
Furthermore, high HOXA9 expression regulates endothelial cell
activation by inhibiting the activity of nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB). In other
words, downregulation of HOXA9 may be a prerequisite for
endothelial cell activation (52,53).
In conclusion, endothelial cells with reduced HMGB1
expression resulted in elevated HOXA9 expression, and consequently
decreased E-selectin expression. Additionally, the inhibition of
HOXA9 expression was found to lead to elevated E-selectin
expression. Therefore, it was confirmed that intranuclear HMGB1
regulates E-selectin expression via HOXA9. Further studies are
required to delineate the details of HOXA9 interaction and
transcriptional control of E-selectin expression.
Acknowledgements
This work was supported by the National Natural
Science Foundation of China (No. 30901438) and Shenyang science and
technology plan projects (No. 130309).
Abbreviations:
|
FBS
|
fetal bovine serum
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
HMGB1
|
high-mobility group box protein 1
|
|
HMGI(Y)
|
high-mobility group AT-hook 1
|
|
HOXA9
|
homeobox A9
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
ICAM1
|
intracellular adhesion molecule 1
|
|
IL
|
interleukin
|
|
LPS
|
lipopolysaccharide
|
|
MOF
|
multiple organ failure
|
|
NF-κB
|
nuclear factor κ-light-chain-enhancer
of activated B cells
|
|
RNAi
|
RNA interference
|
|
siRNA
|
small interfering RNA
|
|
shRNA
|
short hairpin RNA
|
|
TNF
|
tumor necrosis factor
|
|
VCAM1
|
vascular cell adhesion molecule 1
|
References
|
1
|
Skoko D, Wong B, Johnson RC and Marko JF:
Micromechanical analysis of the binding of DNA-bending proteins
HMGB1, NHP6A, and HU reveals their ability to form highly stable
DNA-protein complexes. Biochemistry. 43:13867–13874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pallier C, Scaffidi P, Chopineau-Proust S,
et al: Association of chromatin proteins high mobility group box
(HMGB) 1 and HMGB2 with mitotic chromosomes. Mol Biol Cell.
14:3414–3426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan F, Gu L, Guo S, Wang C and Li GM:
Evidence for involvement of HMGB1 protein in human DNA mismatch
repair. J Biol Chem. 279:20935–20940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andersson U, Wang H, Palmblad K, et al:
High mobility group 1 protein (HMG-1) stimulates proinflammatory
cytokine synthesis in human monocytes. J Exp Med. 192:565–570.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiuza C, Bustin M, Talwar S, et al:
Inflammation-promoting activity of HMGB1 on human microvascular
endothelial cells. Blood. 101:2652–2660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Bloom O, Zhang M, et al: HMG-1 as
a late mediator of endotoxin lethality in mice. Science.
285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Yang H and Tracey KJ:
Extracellular role of HMGB1 in inflammation and sepsis. J Intern
Med. 255:320–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lehr HA, Bittinger F and Kirkpatrick CJ:
Microcirculatory dysfunction in sepsis: a pathogenetic basis for
therapy? J Pathol. 190:373–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bae JS and Rezaie AR: Activated protein C
inhibits high mobility group box 1 signaling in endothelial cells.
Blood. 118:3952–3959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mullins GE, Sunden-Cullberg J, Johansson
AS, et al: Activation of human umbilical vein endothelial cells
leads to relocation and release of high-mobility group box
chromosomal protein 1. Scand J Immunol. 60:566–573. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang H, Ochani M, Li J, et al: Reversing
established sepsis with antagonists of endogenous high-mobility
group box 1. Proc Natl Acad Sci USA. 101:296–301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin S, Wang H, Yuan R, et al: Role of
HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med.
203:1637–1642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paul CP, Good PD, Winer I and Engelke DR:
Effective expression of small interfering RNA in human cells. Nat
Biotechnol. 20:505–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paddison PJ, Caudy AA, Bernstein E, Hannon
GJ and Conklin DS: Short hairpin RNAs (shRNAs) induce
sequence-specific silencing in mammalian cells. Genes Dev.
16:948–958. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuniyasu H, Chihara Y and Kondo H:
Differential effects between amphoterin and advanced glycation end
products on colon cancer cells. Int J Cancer. 104:722–727. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shapiro NI, Yano K, Sorasaki M, Fischer C,
Shih SC and Aird WC: Skin biopsies demonstrate site-specific
endothelial activation in mouse models of sepsis. J Vasc Res.
46:495–502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nooteboom A, van der Linden CJ and
Hendriks T: Modulation of adhesion molecule expression on
endothelial cells after induction by lipopolysaccharide-stimulated
whole blood. Scand J Immunol. 59:440–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sessler CN, Windsor AC, Schwartz M, et al:
Circulating ICAM-1 is increased in septic shock. Am J Respir Crit
Care Med. 151:1420–1427. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bandyopadhyay S, Ashraf MZ, Daher P, Howe
PH and DiCorleto PE: HOXA9 participates in the transcriptional
activation of E-selectin in endothelial cells. Mol Cell Biol.
27:4207–4216. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trivedi CM, Patel RC and Patel CV:
Differential regulation of HOXA9 expression by nuclear factor kappa
B (NF-kappaB) and HOXA9. Gene. 408:187–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weissberg PL and Bennett MR:
Atherosclerosis - an inflammatory disease. N Engl J Med.
340:1928–1929. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen JW, Chen YH, Lin FY, Chen YL and Lin
SJ: Ginkgo biloba extract inhibits tumor necrosis
factor-alpha-induced reactive oxygen species generation,
transcription factor activation, and cell adhesion molecule
expression in human aortic endothelial cells. Arterioscler Thromb
Vasc Biol. 23:1559–1566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel CV, Sharangpani R, Bandyopadhyay S
and DiCorleto PE: Endothelial cells express a novel, tumor necrosis
factor-alpha-regulated variant of HOXA9. J Biol Chem.
274:1415–1422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross R: The pathogenesis of
atherosclerosis: a perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tracey KJ, Beutler B, Lowry SF, et al:
Shock and tissue injury induced by recombinant human cachectin.
Science. 234:470–474. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hesse DG, Tracey KJ, Fong Y, et al:
Cytokine appearance in human endotoxemia and primate bacteremia.
Surg Gynecol Obstet. 166:147–153. 1988.PubMed/NCBI
|
|
28
|
Beutler BA, Milsark IW and Cerami A:
Cachectin/tumor necrosis factor: production, distribution, and
metabolic fate in vivo. J Immunol. 135:3972–3977. 1985.PubMed/NCBI
|
|
29
|
Fink MP: Another negative clinical trial
of a new agent for the treatment of sepsis: rethinking the process
of developing adjuvant treatments for serious infections. Crit Care
Med. 23:989–991. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fisher CJ Jr, Dhainaut JF, Opal SM, et al:
Phase III rhIL-1ra Sepsis Syndrome Study Group: Recombinant human
interleukin 1 receptor antagonist in the treatment of patients with
sepsis syndrome. Results from a randomized, double-blind,
placebo-controlled trial. JAMA. 271:1836–1843. 1994. View Article : Google Scholar
|
|
31
|
Eskandari MK, Bolgos G, Miller C, Nguyen
DT, DeForge LE and Remick DG: Anti-tumor necrosis factor antibody
therapy fails to prevent lethality after cecal ligation and
puncture or endotoxemia. J Immunol. 148:2724–2730. 1992.
|
|
32
|
Remick D, Manohar P, Bolgos G, Rodriguez
J, Moldawer L and Wollenberg G: Blockade of tumor necrosis factor
reduces lipopolysaccharide lethality, but not the lethality of
cecal ligation and puncture. Shock. 4:89–95. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C
and Guo RX: HMGB1 activates nuclear factor-κB signaling by RAGE and
increases the production of TNF-α in human umbilical vein
endothelial cells. Immunobiology. 215:956–962. 2010.
|
|
34
|
Unoshima M: Therapeutic effect of
anti-HMGB1 antibody and anti-RAGE antibody on SIRS/sepsis. Nihon
Rinsho. 62:2323–2329. 2004.(In Japanese).
|
|
35
|
Parrish W and Ulloa L: High-mobility group
box-1 isoforms as potential therapeutic targets in sepsis. Methods
Mol Biol. 361:145–162. 2007.PubMed/NCBI
|
|
36
|
Eriksson M: Should high mobility group
box-1 protein (HMGB1) be measured in patients with severe sepsis
and septic shock? If so, when, where, and how? Crit Care Med.
33:682–683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ulloa L, Ochani M, Yang H, et al: Ethyl
pyruvate prevents lethality in mice with established lethal sepsis
and systemic inflammation. Proc Natl Acad Sci USA. 99:12351–12356.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo RF and Ward PA: Role of C5a in
inflammatory responses. Annu Rev Immunol. 23:821–852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mantell LL, Parrish WR and Ulloa L: HMGB-1
as a therapeutic target for infectious and inflammatory disorders.
Shock. 25:4–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ulloa L and Messmer D: High-mobility group
box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev.
17:189–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferrari S, Finelli P, Rocchi M and Bianchi
ME: The active gene that encodes human high mobility group 1
protein (HMG1) contains introns and maps to chromosome 13.
Genomics. 35:367–371. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kornblit B, Munthe-Fog L, Petersen SL,
Madsen HO, Vindelov L and Garred P: The genetic variation of the
human HMGB1 gene. Tissue Antigens. 70:151–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bustin M and Reeves R: High-mobility-group
chromosomal proteins: architectural components that facilitate
chromatin function. Prog Nucleic Acid Res Mol Biol. 54:35–100.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ferrari S, Ronfani L, Calogero S and
Bianchi ME: The mouse gene coding for high mobility group 1 protein
(HMG1). J Biol Chem. 269:28803–28808. 1994.PubMed/NCBI
|
|
45
|
Hardman CH, Broadhurst RW, Raine AR,
Grasser KD, Thomas JO and Laue ED: Structure of the A-domain of
HMG1 and its interaction with DNA as studied by heteronuclear
three- and four-dimensional NMR spectroscopy. Biochemistry.
34:16596–16607. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuhns DB, Alvord WG and Gallin JI:
Increased circulating cytokines, cytokine antagonists, and
E-selectin after intravenous administration of endotoxin in humans.
J Infect Dis. 171:145–152. 1995. View Article : Google Scholar
|
|
47
|
Boldt J, Muller M, Kuhn D, Linke LC and
Hempelmann G: Circulating adhesion molecules in the critically ill:
a comparison between trauma and sepsis patients. Intensive Care
Med. 22:122–128. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kayal S, Jais JP, Aguini N, Chaudiere J
and Labrousse J: Elevated circulating E-selectin, intercellular
adhesion molecule 1, and von Willebrand factor in patients with
severe infection. Am J Respir Crit Care Med. 157:776–784. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Harlan JM and Winn RK:
Leukocyte-endothelial interactions: clinical trials of
anti-adhesion therapy. Crit Care Med. 30:S214–S219. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Treutiger CJ, Mullins GE, Johansson AS, et
al: High mobility group 1 B-box mediates activation of human
endothelium. J Intern Med. 254:375–385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zappavigna V, Falciola L, Helmer-Citterich
M, Mavilio F and Bianchi ME: HMG1 interacts with HOX proteins and
enhances their DNA binding and transcriptional activation. EMBO J.
15:4981–4991. 1996.PubMed/NCBI
|
|
52
|
Mark M, Rijli FM and Chambon P: Homeobox
genes in embryogenesis and pathogenesis. Pediatr Res. 42:421–429.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Trivedi CM, Patel RC and Patel CV:
Homeobox gene HOXA9 inhibits nuclear factor-kappa B dependent
activation of endothelium. Atherosclerosis. 195:50–60. 2007.
View Article : Google Scholar : PubMed/NCBI
|