Introduction
Irritable bowel syndrome (IBS) is a common chronic
gastrointestinal disorder affecting 10–15% of the western
population, with a female predominance (1–3). IBS
is characterized by abdominal discomfort or pain associated with
altered bowel habits, and often bloating and abdominal distension
(1–3). The degree of symptoms varies between
patients, from tolerable to severe, with a considerable reduction
to quality of life and productivity (1–6).
Besides the increased morbidity caused by IBS, this disorder also
represents an economic burden to society in different forms,
including increased sick leave and overconsumption of healthcare
resources (6–8). However, IBS is not known to be
associated with the development of serious diseases or excess
mortality (9,10). IBS patients are sub-grouped on the
basis of differences in the predominant bowel pattern:
diarrhoea-predominant (IBS-D), constipation-predominant (IBS-C), or
a mixture of both diarrhoea and constipation (IBS-M) (1). It has been reported that
approximately one third of patients have IBS-D, one third have
IBS-C, and the remainder have IBS-M (1).
Several abnormalities in the endocrine cells of the
gastrointestinal tract have been reported in IBS patients (11–24).
These abnormalities have been suggested to be important in the
pathogenesis of IBS (1,25). The endocrine cells observed in the
proximal (duodenum) and distal (terminal ileum) small intestine are
quite different (25). Both the
duodenum and terminal ileum comprise serotonin and somatostatin
cells (25), whereas the duodenum
contains cholecystokinin (CCK), secretin and gastric inhibitory
polypeptide (GIP) cells, while the terminal ileum contains peptide
YY (PYY), pancreatic polypeptide (PP) and enteroglucagon cells
(25). The difference in endocrine
cell composition reflects the different functions of the proximal
and distal small intestine. The endocrine cells in the terminal
ileum of patients with IBS have yet to be investigated. This may be
due to the technical difficulties involved in obtaining biopsies
from the ileum of these patients, caused by a long looping colon
combined with visceral hypersensitivity in IBS patients.
Chromogranin A (CgA) is a 68 kDa protein comprising
439 amino-acid residues. CgA is co-stored and co-released with
monoamines and peptide hormones of the adrenal medulla, pituitary
gland, parathyroid, thyroid C-cells, pancreatic islets, endocrine
cells of the gastrointestinal tract and sympathetic nerves
(9,10). CgA is considered to be a general
marker for gut endocrine cells and endocrine tumours (9,10,26).
CgA cell density has been reported to be lowered in the duodenum
and colon, but not in the rectum (27,28).
This study was undertaken in order to investigate a possible
abnormality in the density of the endocrine cells, as detected by
CgA, in the ileum of IBS patients.
Materials and methods
Patients and controls
In total, 98 patients with IBS according to the Rome
III Criteria were included in this study (http://www.romecriteria.org) (29). These patients included 77 females
and 21 males with an average age of 35 years (range, 18–66 years).
In total, 35 patients had IBS-D, 31 had IBS-M and 32 had IBS-C. All
98 patients had symptoms for many years and could not associate the
onset of IBS symptoms with any event, particularly gastrointestinal
or other infections. The patients underwent complete physical
examinations and were investigated with blood tests: full blood
count, electrolytes, calcium, inflammatory markers, liver tests and
thyroid function tests. They underwent further gastroscopy with
duodenal biopsies, in order to exclude celiac disease.
In total, 27 subjects who underwent colonoscopy with
terminal ileum biopsies were used as controls. Of these, 20
subjects underwent a colonoscopy due to gastrointestinal bleeding,
where the source of bleeding was identified to be haemorrhoids
(18) or angiodysplasia (2). Seven of the subjects were examined
due to health worries caused by a relative having been diagnosed
with colon carcinoma. The control group consisted of 16 females and
11 males with an average age of 52 years (range, 20–69 years).
The study was performed in accordance with the
Declaration of Helsinki and was approved by the local Committee for
Medical Research Ethics. All the subjects gave oral and written
consent.
Colonoscopy
Colonoscopies were performed on both patients and
controls and biopsies were taken from the ileum and the right
(cecum, ascending and right part of transverse colon) and left
colon (left part of transverse, descending and sigmoid colon).
Biopsies were fixed in 4% buffered paraformaldehyde overnight,
embedded in paraffin and cut into 5-μm sections.
Histopathology and
immunohistochemistry
The sections were stained with haematoxylin and
eosin and immunostained with the avidin-biotin complex (ABC) method
using the Vectastain ABC kit and the 3,3′-diaminobenzidine (DAB)
peroxidase substrate kit (Vector Laboratories, Burlingame, CA,
USA). The primary antibody used was monoclonal mouse
anti-N-terminal purified CgA (Dako, Carpinteria, CA, USA; code no.
M869).
Computerized image analysis
Analysis was conducted using Olympus software
cell^D. When using x40 objectives, the frame (field) on the monitor
represents an area of 0.14 mm2 of the tissue. The number
of CgA immunoreactive cells and the area containing the epithelial
cells were measured in each field. Measurements were taken in 10
randomly chosen fields for each individual. The data from the
fields were tabulated and the number of cells/mm2 of the
epithelium were computed and automatically statistically analysed.
The immunostained sections of IBS patients and controls were coded
and mixed, and measurements were made without knowledge of the
identity of the sections.
Statistical analysis
Comparison between controls, IBS patients and IBS
sub-groups was performed by the non-parametric ANOVA test with
Dunnett’s multiple comparison test as a post hoc test.
Results
Colonoscopy, histopathology and
immunohistochemistry
The colons of the patients and control subjects were
macroscopically normal. The ileum was also macroscopically normal,
with the exception of one control subject and three of the
patients, where lymphoid hyperplasia was observed. Lymphoid
hyperplasia is a common finding in young individuals without any
pathological relevance.
Histopathological examination of the colon biopsies
revealed normal histology, excluding microscopic colitis.
Histopathological examination of the ileum revealed normal
histology and confirmed the finding of lymphoid hyperplasia in the
individuals mentioned above. CgA cells were mainly located in the
crypts (Fig. 1); these cells were
basket- or flask-shaped.
Computerized image analysis
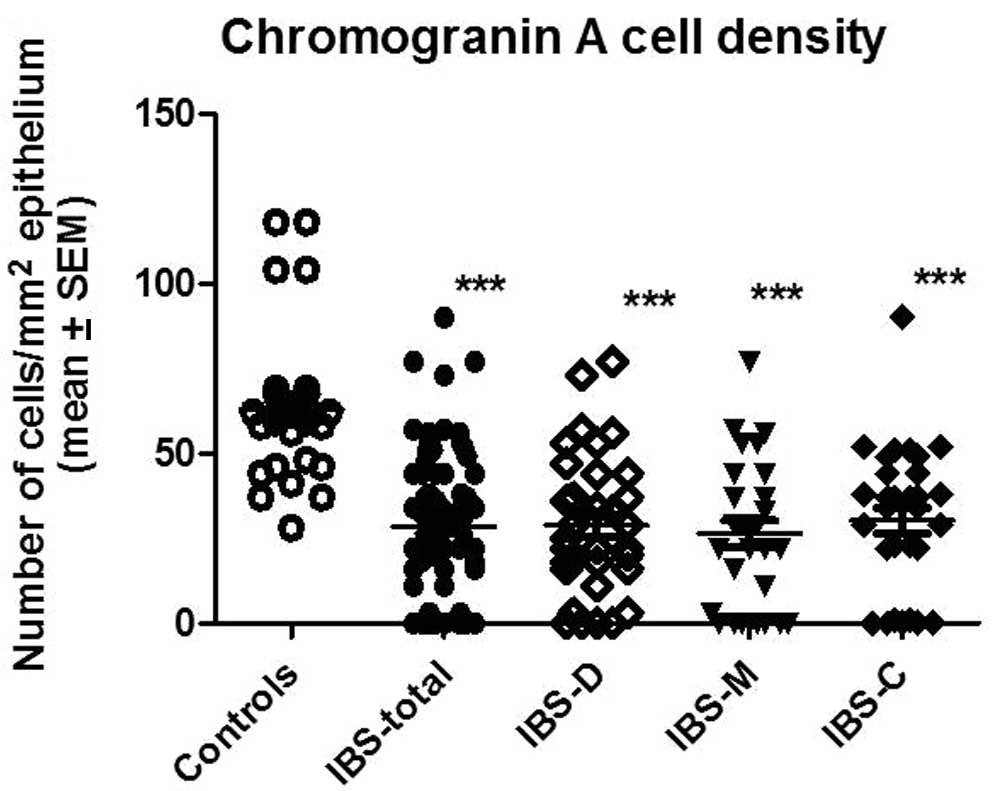
The CgA density in the controls was 63.2±4.4 (mean ±
SEM), in all IBS patients it was 28.6±2.1, in IBS-D patients it was
28.8±3.4, in IBS-M patients it was 26.5±3.9 and in IBS-C patients
it was 30.3±3.7 (Fig. 2). There
was a statistically significant difference between controls and all
IBS patients (IBS-D, IBS-M and IBS-C; P<0.0001 for all).
Discussion
The age and gender of the patients and healthy
controls used in this investigation did not match completely. The
control subjects included in the present study were slightly older
and the proportion of males to females was higher. It is not easy
to find healthy volunteers matching the age and gender of the
patients that are willing to be subjected to bowel preparation
prior to colonoscopy and colonoscopy. One must be patient and wait
for healthy subjects that undergo this examination for other
reasons. However, in previous studies, age and gender have been
found to have no effect on the density of intestinal endocrine
cells in adults (30,31). The medical history of the IBS
patients studied demonstrates that there is no association between
the onset of IBS symptoms and gastrointestinal infection and,
consequently, the patients included in this study suffer from
sporadic IBS.
This study demonstrates that CgA cell density in the
ileum of IBS patients is reduced, regardless of the subtype. It has
been reported previously that CgA cell density is also reduced in
the duodenum and colon of IBS patients (27). Thus, it appears there is endocrine
cell depletion in the small and large intestine of IBS patients.
This is notable, as IBS has been considered to be a functional
condition without detectable abnormalities. The present finding
lends support to the suggestion that CgA cell density may be used
as a biological marker for the diagnosis of IBS (1,17,27).
This would be advantageous, as currently there are no biochemical,
histopathological or radiological diagnostic tests for IBS. At
present, the diagnosis of IBS is based on symptom assessment.
The ileum contains the same types of endocrine cells
as the large intestine, serotonin, PYY, PP, somatostatin and
enteroglucagon cells. This is unsurprising as the ileum, similar to
the large intestine, contributes to the absorption of water and
electrolytes from the lumen and regulates the passage of faeces. As
mentioned previously, CgA cell density represents the total
endocrine cell content of the ileum and more studies are required
in order to determine which endocrine cell type is affected.
In conclusion, the present study demonstrates that
the total number of endocrine cells is reduced in the ileum of IBS
patients. Furthermore, it confirms that the endocrine cells are
depleted in the small and large intestine of IBS patients.
Acknowledgements
This study was supported by a grant from
Helse-Fonna.
References
|
1
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Irritable Bowel Syndrome: Diagnosis, Pathogenesis &
Treatment Options. Nova Science Publishers Inc; New York, NY:
2012
|
|
2
|
Thompson WG: A world view of IBS.
Irritable Bowel Syndrome: Diagnosis and Treatment. Camilleri M and
Spiller R: Saunders; Philadelphia and London: pp. 17–26. 2002
|
|
3
|
Drossman DA, Li Z, Andruzzi E, et al: U.S.
householder survey of functional gastrointestinal disorders.
Prevalence, sociodemography, and health impact. Dig Dis Sci.
38:1569–1580. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hungin AP, Whorwell PJ, Tack J and Mearin
F: The prevalence, patterns and impact of irritable bowel syndrome:
an international survey of 40,000 subjects. Aliment Pharmacol Ther.
17:643–650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson S, Roberts L, Roalfe A, Bridge P
and Singh S: Prevalence of irritable bowel syndrome: a community
survey. Br J Gen Pract. 54:495–502. 2004.PubMed/NCBI
|
|
6
|
Whitehead WE, Burnett CK, Cook EW III and
Taub E: Impact of irritable bowel syndrome on quality of life. Dig
Dis Sci. 41:2248–2253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Everhart JE and Renault PF: Irritable
bowel syndrome in office-based practice in the United States.
Gastroenterology. 100:998–1005. 1991.PubMed/NCBI
|
|
8
|
Harvey RF, Salih SY and Read AE: Organic
and functional disorders in 2000 gastroenterology outpatients.
Lancet. 1:632–634. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taupenot L, Harper KL and O’Connor DT: The
chromogranin-secretogranin family. N Engl J Med. 348:1134–1149.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiedenmann B and Huttner WB: Synaptophysin
and chromogranins/secretogranins – widespread constituents of
distinct types of neuroendocrine vesicales and new tools in tumor
diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 58:95–121.
1989.
|
|
11
|
El-Salhy M, Lillebø E, Reinemo A and
Salmelid L: Ghrelin in patients with irritable bowel syndrome. Int
J Mol Med. 23:703–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sjölund K, Ekman R and Wierup N:
Covariation of plasma ghrelin and motilin in irritable bowel
syndrome. Peptides. 31:1109–1112. 2010.PubMed/NCBI
|
|
13
|
Dizdar V, Spiller R, Singh G, Hanevik K,
Gilja OH, El-Salhy M and Hausken T: Relative importance of
abnormalities of CCK and 5-HT (serotonin) in Giardia-induced
post-infectious irritable bowel syndrome and functional dyspepsia.
Aliment Pharmacol Ther. 31:883–891. 2010.PubMed/NCBI
|
|
14
|
El-Salhy M, Vaali K, Dizdar V and Hausken
T: Abnormal small intestinal endocrine cells in patients with
irritable bowel syndrome. Dig Dis Sci. 55:3508–3513. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Salhy M, Gundersen D, Ostgaard H,
Lomholt-Beck B, Hatlebakk JG and Hausken T: Low densities of
serotonin and peptide YY cells in the colon of patients with
irritable bowel syndrome. Dig Dis Sci. 57:873–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El-Salhy M, Gundersen D, Hatlebakk JG and
Hausken T: Abnormal rectal endocrine cells in patients with
irritable bowel syndrome. Submitted. 2012.
|
|
17
|
El-Salhy M, Seim I, Chopin L, Gundersen D,
Hatlebakk JG and Hausken T: Irritable bowel syndrome: the role of
gut neuroendocrine peptides. Front Biosci (Elite Ed). 4:2783–2800.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coates MD, Mahoney CR, Linden DR, Sampson
JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM
and Moses PL: Molecular defects in mucosal serotonin content and
decreased serotonin reuptake transporter in ulcerative colitis and
irritable bowel syndrome. Gastroenterology. 126:1657–1664. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang SH, Dong L, Luo JY, Gong J, Li L, Lu
XL and Han SP: Decreased expression of serotonin in the jejenum and
increased numbers of mast cells in the terminal ileum in patients
with irritable bowel syndrome. World J Gastroenterol. 13:6041–6047.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JH, Rhee PL, Kim G, Lee JH, Kim YH,
Kim JJ, Rhee JC and Song SY: Enteroendocrine cell counts correlated
with visceral hypersensitivity in patients with
diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol
Motil. 18:539–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dunlop SP, Jenkins D and Spiller RC:
Distinctive clinical, psychological and histological feature of
postinfective irritable bowel syndrome. Am J Gastroenterol.
98:1578–1583. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK
and Cho SW: The alteration of enterochromaffin cell, mast cell, and
lamina propria T lymphocyte numbers in irritable bowel syndrome and
its relationship with psychological factors. J Gastroenterol
Hepatol. 23:1689–1694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spiller RC, Jenkins D, Thornley JP, Hebden
JM, Wright T, Skinner M and Neal KR: Increased rectal mucosal
enteroendocrine cells, T lymphocytes, and increased gut
permeability following acute Campylobacter enteritis and in
post-dysenteric irritable bowel syndrome. Gut. 47:804–811. 2000.
View Article : Google Scholar
|
|
24
|
Kim HS, Lim JH, Park H and Lee SI:
Increased immunoendocrine cells in intestinal mucosa of
postinfectious irritable bowel syndrome patients 3 years after
acute Shigella infection – an observation in small case control
study. Yonsei Med J. 51:45–51. 2010.PubMed/NCBI
|
|
25
|
Dunlop SP, Coleman NS, Blackshaw E,
Perkins AC, Singh G, Marsden CA and Spiller RC: Abnormalities of
5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin
Gastroenterol Hepatol. 3:349–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deftos LJ: Chromogranin A: its role in
endocrine function and as an endocrine and neuroendocrine tumor
marker. Endocr Rev. 12:181–187. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Salhy M, Lomholt-Beck B and Hausken T:
Chromogranin as a tool in the diagnosis of irritable bowel
syndrome. Scand J Gastroenterol. 45:1435–1439. 2010. View Article : Google Scholar
|
|
28
|
El-Salhy M, Mazzawi T, Gundersen D and
Hausken T: Chromogranin A cell density in the rectum of patients
with irritable bowel syndrome. Mol Med Rep. 6:1223–1225.
2012.PubMed/NCBI
|
|
29
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorder.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandström O and El-Salhy M: Aging and
endocrine cells of human duodenum. Mech Ageing Dev. 108:39–48.
1999.
|
|
31
|
Sandström O and El-Salhy M: Human rectal
endocrine cells and aging. Mech Ageing Dev. 108:219–226.
1999.PubMed/NCBI
|