Introduction
As a third-generation β-adrenergic blocker
(β-blocker), carvedilol has been widely used for treating
hypertension, myocardial infarction and heart failure (1–3).
Although carvedilol is not superior to traditional β-blockers in
blood pressure control, it still shows great benefit in the
inhibition of collagen deposition (4). A previous study demonstrated that
carvedilol’s action on blood pressure is largely mediated by
endogenous nitric oxide (NO) (5).
Considering the relationship between NO and left ventricle
remodeling (6), we hypothesized
that the effect of carvedilol on cardiac structural remodeling also
depends on endogenous NO. In the present study, it was demonstrated
that carvedilol reduces collagen volume fraction (CVF) and
perivascular collagen area (PVCA) in a rat model of unilateral
renal artery stenosis. The effect of carvedilol on cardiac
remodeling is blunted by L-NAME, a specific inhibitor of NO
synthase. The present study highlights the role of NO in the
activity of carvedilol in cardiac structural remodeling.
Materials and methods
Animals and surgery
A total of 40 male Sprague-Dawley rats were used for
narrowing the left renal artery using a modified 2-kidney 1-clip
(2K1C) method. Briefly, all rats were anesthetized with urethane [5
mg/kg, intraperitoneally (i.p)], and the left kidneys were exposed
by abdominal incision. A needle (0.25 mm, ID) was paralleled to
place on the left renal arteries, and the arteries were gently tied
together with silk. The needle was then pulled out and the blood
was allowed to fill the left kidney as soon as possible in order to
avoid irreversible damage. The abdomen was closed and the rats were
gently placed in a warm cage until waking up. The animals were
handled in strict accordance to the guidelines of the Institutional
Animal Care and Use Committee of Wuhan University (Wuhan,
China).
Blood pressure measurement and drug
administration
Systolic blood pressure (SBP) of conscious rats was
determined 4 weeks after surgery of 2K1C by the tail-cuff method.
Three measurements were made per session, and the average value was
obtained. Only the rats with SBP ≥160 mmHg (a total of 24 rats)
were used for further treatments. These rats were randomly and
equally classified into the control (Con), carvedilol-treated (Car)
or carvedilol+L-NAME-treated groups (Car+L). An additional 8 rats
were evaluated as the sham operation group (SO). These rats were
fed with vehicle (distilled water, for SO and Con) or carvedilol
(20 mg/kg/day) or carvedilol+L-NAME (20 and 25 mg/kg/day,
respectively) by gastric gavage (7,8). SBP
was continuously recorded in each rat from 1 to 8 weeks after
administration of drug or vehicle.
Determination of plasma NO levels
At 8 weeks, all the rats were sacrificed following
SBP measurement, and the blood was used to detect the NO
(NO3) levels according to a previous study (5).
Histological diagnosis
Myocardium tissues were removed from the left
ventricle (~3-mm thick) immediately following sacrifice. The
samples were fixed in 10% pre-cooled paraformaldehyde for 72 h and
embedded in paraffin for histological investigation.
Paraffin-embedded tissues were sectioned into slices (~5 μm thick)
and stained with hematoxylin and eosin (H&E). Masson’s
trichrome staining was performed to assess myocardial fibrosis.
Images were observed under an optical microscope at magnifications
of ×400 (H&E) or ×200 (Masson’s). In order to quantitatively
evaluate myocardial fibrosis, CVF and PVCA were calculated. CVF was
defined as the sum of all the connective tissue areas of the entire
section, divided by the sum of all the connective tissue and muscle
areas in five fields of the section. PVCA was calculated as the
ratio of the perivascular fibrotic area to the coronary vessel area
from five fields of the section.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Two-sample comparisons were performed using Student’s
unpaired t-test, and four-sample comparisons were performed using
one-way ANOVA (SPSS13.0). A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Four weeks after binding of the left renal artery,
SBP in the Con group was significantly increased. As expected,
carvedilol effectively decreased SBP only one week following drug
treatment. SBP in the Car group reached a relatively lower lever
over time compared with the level at the initiation of the treament
and remained stable. Although the mean value of SBP was higher in
the Car+L group, no statistical difference was detected between the
Car and Car+L groups (149±7 mmHg, n=8, week 8 vs. 155±7 mmHg, n=8,
week 8; P>0.05; Table I).
 | Table ISBP following drug administration
(mmHg). |
Table I
SBP following drug administration
(mmHg).
| Time (weeks) | SO (n=8) | Con (n=8) | Car (n=8) | Car+L (n=8) |
|---|
| 0 | 110±9 | 187±9a | 190±11a | 179±16a |
| 1 | 109±7 | 189±12a | 172±13a,b | 167±11a,b |
| 2 | 114±11 | 203±21a | 165±4a,b | 162±16a,b |
| 3 | 120±10 | 197±10a | 147±6a,b | 150±10a,b |
| 4 | 121±6 | 210±22a | 151±17a,b | 160±20a,b |
| 5 | 127±8 | 198±9a | 139±10a,b | 151±9a,b |
| 6 | 121±6 | 188±16a | 144±10a,b | 150±6a,b |
| 7 | 119±5 | 191±19a | 148±9a,b | 157±14a,b |
| 8 | 124±9 | 190±15a | 149±7a,b | 155±7a,b |
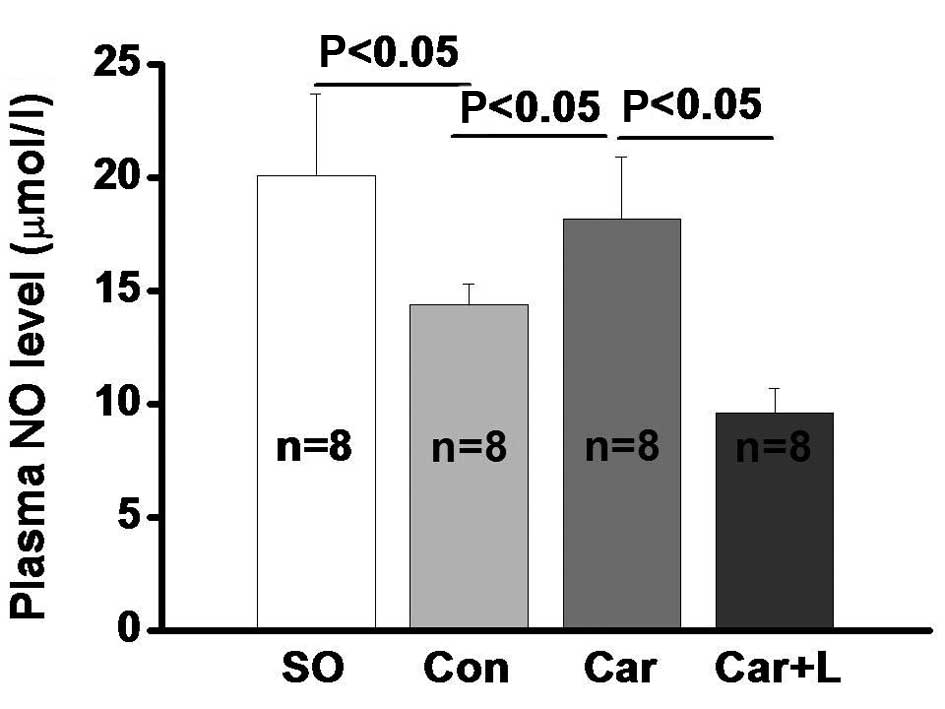
As previously reported (9), the concentration of plasma NO was
significantly reduced in rats with hypertension (20.1±3.6 μmol/l in
SO vs. 14.4±0.9 μmol/l in Con; P<0.05; Fig. 1). Carvedilol administration
reversed the reduction of NO, although this effect was blocked by
L-NAME (18.2±2.7 μmol/l in Car vs. 9.6±1.1 μmol/l in Car+L;
P<0.05).
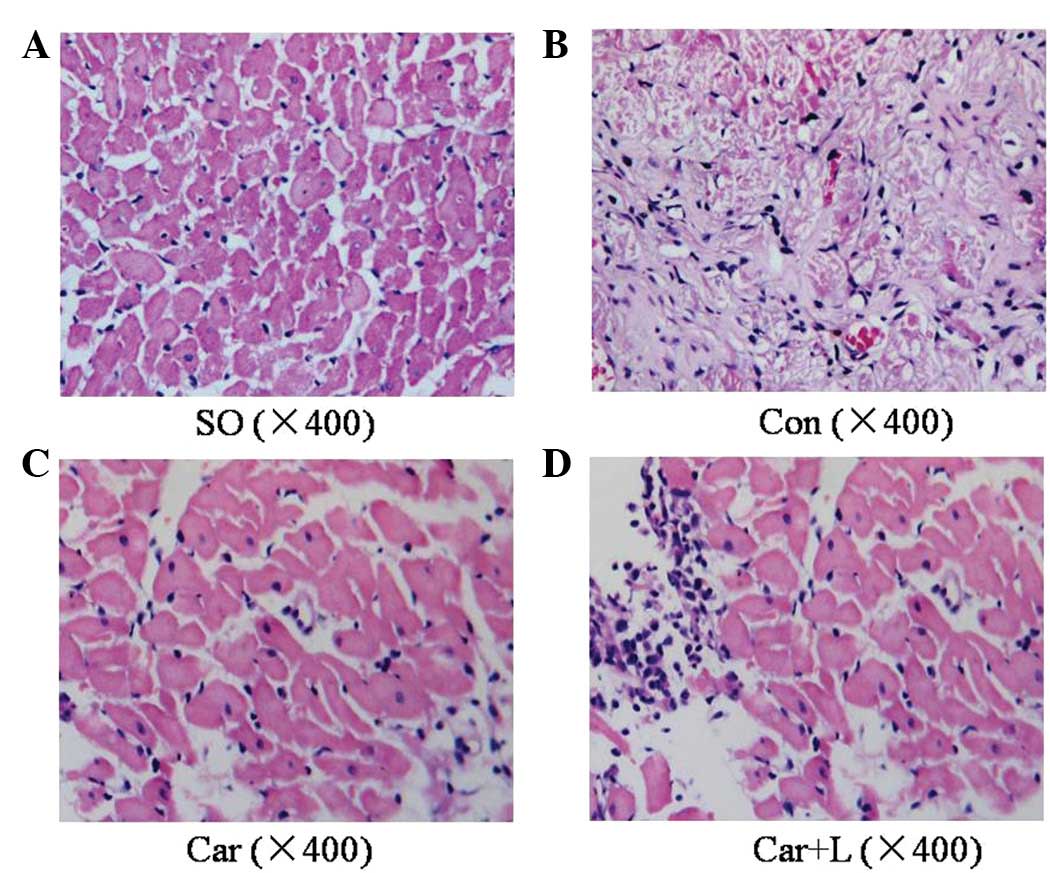
H&E and Masson’s staining showed that cardiac
fibers exhibited an irregular shape and arrangement with myocardial
fibrosis in the Con group. Moreover, the number of cardiac myocytes
was markedly reduced. Carvedilol significantly suppressed these
changes, although when combined with L-NAME, these effects were
significantly blunted (Figs. 2 and
3). To quantitatively evaluate the
influence of NO inhibition on the anti-remodeling effects of
carvedilol, CVF and PVCA were calculated. Our results showed that
carvedilol significantly decreased both CVF and PVCA in the
hypertensive heart, although L-NAME blunted the effects caused by
carvedilol (Table II).
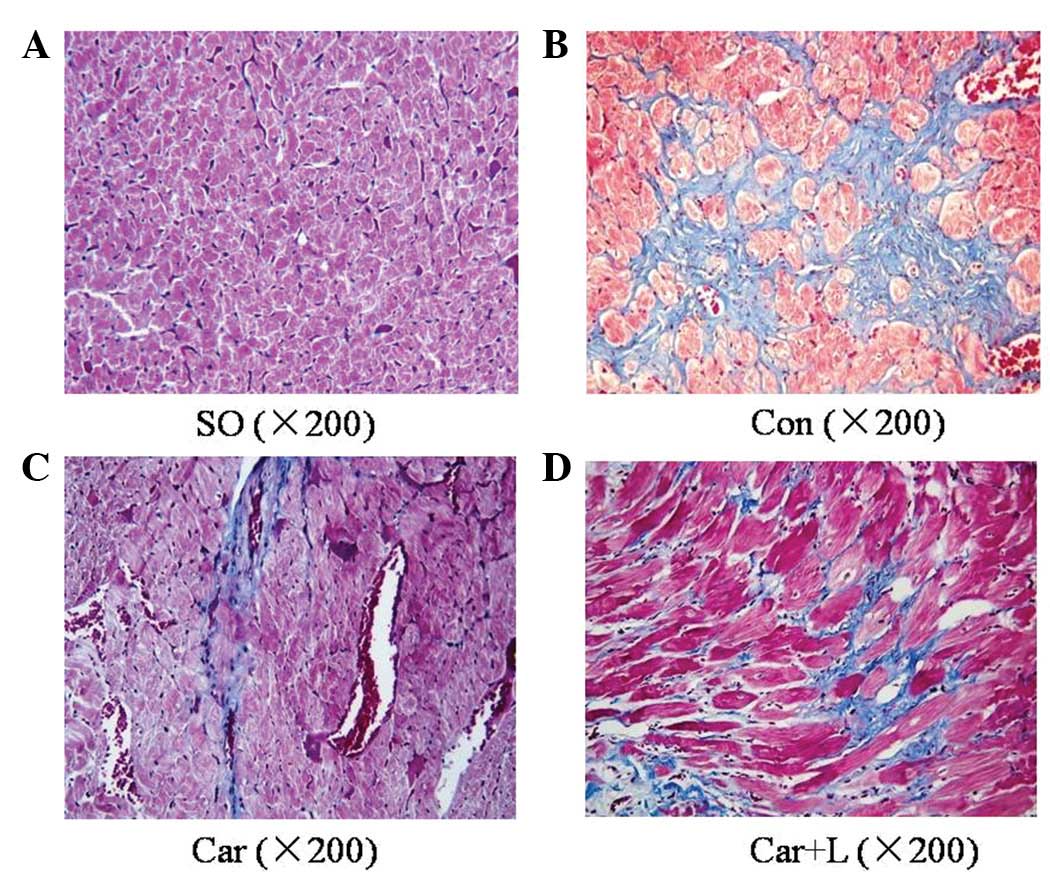 | Figure 3Masson’s trichrome staining for each
group following treatment. Typical tissue sections from the (A) SO,
(B) Con, (C) Car and (D) Car+L groups (magnification, ×200). (A)
The red area shows cardiac muscle fibers, and the muscles were
largely replaced by collagen (blue area in B). (C) Carvedilol
rescues myocytes from fibrosis, although (D) when combined with
L-NAME, this effect is attenuated. SO, sham operation; Con,
control; Car, carvedilol-treated; Car+L,
carvedilol+L-NAME-treated. |
 | Table IIComparisons of CVF and PVCA following
different treatments. |
Table II
Comparisons of CVF and PVCA following
different treatments.
| Variable | SO (n=8) | Con (n=8) | Car (n=8) | Car+L (n=8) |
|---|
| CVF (%) | 2.0±0.5 | 6.9±0.9a | 4.4±0.6a,b | 5.2±1.0a–c |
| PVCA (%) | 1.5±0.3 | 8.2±1.7a | 3.9±0.8a,b | 7.2±0.9a,c |
Discussion
Different from traditional β-blockers, the mechanism
underlying the blood pressure-lowering effect of carvedilol is more
complicated (10). As well as the
blockade of α1, β1 and β2 adrenergic receptors, Afonso et
al(5) demonstrated that
carvedilol’s action on blood pressure was largely dependent on NO
(5). However, this conclusion was
obtained from normal rats, and it remains unknown whether NO plays
an important role on carvedilol’s action under pathological
conditions. In the present study, high blood pressure was
successfully induced by narrowing the left renal artery in the Con
group, and was significantly decreased by the administration of
carvedilol. Notably, inhibition of NO generation by L-NAME (Car+L
group) appeared not to attenuate carvedilol effect on lowering
blood pressure, although the mean value of SBP remained higher in
the Car+L group compared with that in the Car group. This may be
explained by the following factors: In the study by Afonso et
al(5), carvedilol and L-NAME
were used in normal rats for a short period of time, while in the
present study, hypertension was firstly induced in rats and then
drugs were administered for 8 weeks. Carvedilol effect on blood
pressure may shift from the NO-dependent pathway to the α- and
β-adrenergic receptor-dependent pathway after exposure to long and
continuous pathological stimulations, such as high blood pressure,
angiotensin II and catecholamine.
Besides blood pressure control, carvedilol can also
bring benefit to patients with hypertension through its
antihypotrophic effects. Previous studies have demonstrated that
carvedilol prevents apoptosis and attenuates or reverses cardiac
remodeling in various animal models, such as heart failure and
ischemia/reperfusion injury (11,12).
Since carvedilol increases the production of endogenous NO and NO
prevents hypertrophy through cGMP/PKG pathway (13), we hypothesized that NO plays an
important role in carvedilol effects on anti-remodeling. Our data
showed that carvedilol reverses the reduction of serum NO level in
rats with hypertension, and significantly decreases both CVF and
PVCA. However, in the Car+L group these effects of carvedilol on
CVF and PVCA appeared to be significantly attenuated. This means
that the anti-remodeling effect of carvedilol in hypertrophy is
largely dependent on endogenous NO. On the other hand, our results
demonstrated that, as a critical anti-remodeling factor, endogenous
NO plays an important role in the process of hypertrophy. In the
future, more studies should focus on the mechanisms underlying how
carvedilol increases the generation of endogenous NO.
As a third-generation β-blocker, carvedilol has been
widely used in patients with hypertension, heart failure or
coronary heart disease, and shows great advantages besides lowering
blood pressure. In the present study, we showed that carvedilol
significantly suppresses myocardial fibrosis and decreases both CVF
and PVCA, while these effects were attenuated by L-NAME through
inhibiting the generation of endogenous NO. Our study highlights
the role of endogenous NO in carvedilol’s action on cardiac
structural remodeling. In the future, further studies will focus on
the mechanisms underlying how carvedilol increases the generation
of endogenous NO.
References
|
1
|
DiNicolantonio JJ and Hackam DG:
Carvedilol: a third-generation β-blocker should be a first-choice
β-blocker. Expert Rev Cardiovasc Ther. 10:13–25. 2012.
|
|
2
|
Fonarow GC, Lukas MA, Robertson M, Colucci
WS and Dargie HJ: Effects of carvedilol early after myocardial
infarction: analysis of the first 30 days in Carvedilol
Post-Infarct Survival Control in Left Ventricular Dysfunction
(CAPRICORN). Am Heart J. 154:637–644. 2007. View Article : Google Scholar
|
|
3
|
Bozkurt B, Bolos M, Deswal A, Ather S,
Chan W, Mann DL and Carabello B: New insights into mechanisms of
action of carvedilol treatment in chronic heart failure patients -
a matter of time for contractility. J Card Fail. 18:183–193. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wei S, Chow LT and Sanderson JE: Effect of
carvedilol in comparison with metoprolol on myocardial collagen
postinfarction. J Am Coll Cardiol. 36:276–281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Afonso RA, Patarrão RS, Macedo MP and
Carmo MM: Carvedilol’s actions are largely mediated by endogenous
nitric oxide. Rev Port Cardiol. 25:911–917. 2006.
|
|
6
|
Inaba S, Iwai M, Furuno M, Kanno H, Senba
I, Okayama H, Mogi M, Higaki J and Horiuchi M: Role of
angiotensin-converting enzyme 2 in cardiac hypertrophy induced by
nitric oxide synthase inhibition. J Hypertens. 29:2236–2245. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li B, Liao YH, Cheng X, Ge H, Guo H and
Wang M: Effects of carvedilol on cardiac cytokines expression and
remodeling in rat with acute myocardial infarction. Int J Cardiol.
111:247–255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossoni G, Manfredi B, De Gennaro Colonna
V, Berti M, Guazzi M and Berti F: Sildenafil reduces L-NAME-induced
severe hypertension and worsening of myocardial
ischaemia-reperfusion damage in the rat. Br J Pharmacol.
150:567–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Isabelle M, Simonet S, Ragonnet C,
Sansilvestri-Morel P, Clavreul N, Vayssettes-Courchay C and
Verbeuren TJ: Chronic reduction of nitric oxide level in adult
spontaneously hypertensive rats induces aortic stiffness similar to
old spontaneously hypertensive rats. J Vasc Res. 49:309–318. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dulin B and Abraham WT: Pharmacology of
carvedilol. Am J Cardiol. 93:3B–6B. 2004. View Article : Google Scholar
|
|
11
|
Bellenger NG, Rajappan K, Rahman SL,
Lahiri A, Raval U, Webster J, Murray GD, Coats AJ, Cleland JG and
Pennell DJ; CHRISTMAS Study Steering Committee and Investigators.
Effects of carvedilol on left ventricular remodelling in chronic
stable heart failure: a cardiovascular magnetic resonance study.
Heart. 90:760–764. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schulz R, Kelm M and Heusch G: Nitric
oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res.
61:402–413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindman BR and Chakinala MM: Modulating
the nitric oxide - cyclic GMP pathway in the pressure-overloaded
left ventricle and group II pulmonary hypertension. Int J Clin
Pract Suppl. 168:15–22. 2010. View Article : Google Scholar : PubMed/NCBI
|