Introduction
Despite advances in diagnostics and intensive care
treatment, morbidity and mortality remain considerably high in
septic patients (1,2). A dysregulated systemic inflammatory
response is one of the main characteristics associated with sepsis
(3). Previous studies attempted to
inhibit inflammatory pathways via various methods, including
ciglitazone, lipid-rich nutrition and C1-esterase inhibitors.
However, none of these drugs have been shown to effectively reduce
the mortality rate (4–6). Therefore, the discovery of novel
biomarkers and therapeutic targets involved in the pathophysiology
of sepsis is in high demand by patients and caregivers.
Melatonin, synthesized by the pineal gland, is a key
molecule of the central ‘master’ clock, which controls circadian
rhythmicity (7). The plasma
melatonin level has been shown to vary according to the circadian
cycle. In healthy humans, melatonin secretion occurs almost
exclusively during night hours starting between 9:00 p.m. and 11:00
p.m., reaching peak serum levels between 1:00 a.m. and 3:00 a.m.,
and then falling to low baseline values between 7:00 a.m. and 9:00
a.m. (8). Previous studies have
shown that melatonin regulates immune function and inflammatory
response, as well as possesses indirect antioxidant activity
(9,10). In intensive care unit (ICU)
patients, the normal cyclic change between daytime and sleep during
the night is disturbed, particularly if continuous application of
ataractic and other drugs is required. A previous clinical study
has also demonstrated that circadian rhythm is disrupted in sedated
critically ill patients with severe sepsis (11). Melatonin may ameliorate the sleep
pattern of these patients and improve the survival rate (12).
A number of genes, such as Clock, Cry
1-2, Per1-2, Rev-erb and Bmal1, have been
shown to participate in the regulation of circadian rhythmicity
(13). Oscillations of circadian
genes have been identified in many peripheral tissues (14,15).
These circadian genes may be meticulously adjusted by a master
clock, which is located in the suprachiasmatic nucleus within the
brain hypothalamus. The mechanisms by which the ‘master’ clock and
the peripheral clocks interact are not completely understood.
A recent clinical trial demonstrated that endotoxin
did not affect the melatonin secretion rhythm, but suppressed the
expression of peripheral circadian genes in healthy volunteers
(16). Previous studies have also
shown that the circadian activity of the clocks is modified during
periods of systemic inflammation. Tumor necrosis factor α (TNF-α)
and interleukin 6 (IL-6), two prominent pro-inflammatory cytokines
produced during the acute phase of sepsis, have also been
implicated in the regulation of circadian activity (15,17).
It has also been shown that the normal periodicity of urinary
6-sulfatoxymelatonin α (αMT6s) excretion (a metabolic product of
melatonin) was eliminated and phase amplitudes were much lower in
the late stages of severe septic patients compared to non-septic
patients (11). Thus, it is
possible that melatonin rhythm is also altered in the early stages
of sepsis.
In the current study, we investigated the altered
circadian rhythm of melatonin and expression of two circadian genes
in peripheral blood leukocytes (PBLs) of septic patients during the
first 24 h of ICU treatment. Additionally, we assessed the release
of two pro-inflammatory cytokines, TNF-α and IL-6, in the serum of
septic and non-septic patients.
Materials and methods
Ethics statement
Written informed consent forms were signed by all
patients or, in case of unconsciousness, by their close relatives.
The study was approved by the ethical committee of the First
Affiliated Hospital of Wenzhou Medical College, Wenzhou, China.
Patients
Two groups of ICU patients were enrolled in this
study: the septic group was comprised of 11 patients who were
diagnosed with sepsis, and the control group consisted of 11
non-septic patients. All patients in the present study were
admitted to the First Affliated Hospital of Wenzhou Medical College
ICU and subjected to a similar environment, including the
light-dark cycle and the same diet. Sepsis was defined as the
systemic inflammatory response syndrome (SIRS) resulting from
infection according to the American College of Chest Physicians and
the Society of Critical Care Medicine Consensus Conference
(18). SIRS was manifested by two
or more of the following conditions as a result of infection:
temperature >38.3°C or <35.6°C; heart rate >90 beats/min;
tachypnea >20/min, or hypocapnia (PaCO2 <32 mmHg);
WBC >12,000 cells/mm3 or <4,000
cells/mm3, or >10% immature (band) forms (19). In the septic group, four of the 11
septic patients succumbed to disease during their ICU stay, whereas
seven patients recovered from sepsis (Table I). Patients with insomnia, mental
illness, those engaged in shift work prior to admission to the
hospital, or patients receiving β-blockers, melatonin and blocking
agents of melatonin were excluded from the study.
 | Table IDiagnosis of participants (n=22). |
Table I
Diagnosis of participants (n=22).
| Diagnosis | Patient no. |
|---|
| Septic group |
| Septic shock | 2 |
| Acute obstructive
suppurative cholangitis | 2 |
| Multiple trauma | 2 |
| Soft tissue
infection | 2 |
| Acute gangrenous
appendicitis | 1 |
| Intestinal
perforation | 1 |
| Acute
pyelonephritis | 1 |
| Total | 11 |
| Non-septic group |
| Cerebral
hemorrhage | 3 |
| Liver and kidney
failure | 2 |
| Acute poisoning | 1 |
| Craniocerebral
injury | 2 |
| Coronary heart
disease | 1 |
| Heat stroke | 1 |
| Status
asthmaticus | 1 |
| Total | 11 |
Study course
Peripheral blood samples were collected in evacuated
EDTA glass tubes and Paxgene tubes (Qiagen, Valencia, CA, USA) at
2:00 p.m. on the day of admission to the ICU and every 4 h
thereafter, up to 24 h, through an indwelling arterial line. Plasma
was obtained after blood samples were centrifuged at 1,400 × g for
5 min and was stored at −80°C for further analysis. The blood in
the Paxgene tubes was used for total RNA isolation.
Laboratory analyses
Total RNA was isolated from the peripheral blood
collected in the Paxgene tubes using PAXgene Blood RNA kit
(Qiagen), according to the manufacturer’s instructions. Briefly,
RNA was quantified using an Agilent 2100 Bioanalyzer (Agilent
Technologies, Palo Alto, CA, USA). cDNA was synthesized using
GoScript™ Reverse Transcription System (Promega, Beijing, China).
Real-time relative quantitative RT-PCR was performed to detect mRNA
expression levels of the target genes Cry-1 and
Per-2, which are the key genes in the regulation of
circadian clock function, as well as the reference gene β-actin,
using the 7500 Real-Time PCR System (Applied Biosystems, Foster
City, CA, USA). The PCR system contained 1X TaqMan® Gene
Expression Master Mix (Applied Biosystems), 250 nM each of the
forward and reverse primers and 0.15 μM hybridization probe in a
volume of 20 ml. The sequence of the primers and probes were as
follows: Cry-1: forward primer 5′-GCA TTT ATG CTC CAA TCT
GCA TC-3′, reverse primer 5′-AGG AGG GTT GGA TTC ATC ATC TAG-3′,
probe 5′-CAA ATA CCT TCA TTC CTT C-3′; Per-2: forward primer
5′-CCT CAG GAG CGT GAA GCA G-3′, reverse primer 5′-TCA CAA TGT GCT
CAG AGG TAA CG-3′, probe 5′-TGA AAG CCA ATG AAG AGT-3′; β-actin:
forward primer 5′-CAT TGC CGA CAG GAT GCA-3′, reverse primer 5′-CAT
CTG CTG GAA GGT GGA CAG-3′, probe 5′-AGC AAT GAT CTT GAT CTT CA-3′.
The PCR cycling parameters were set as follows: 1 cycle at 95°C for
3 min, 45 cycles at 95°C for 30 sec and 55°C for 30 sec and 1 cycle
at 95°C for 1 min, followed by a melting curve initiating at 55°C
and increasing in 0.5°C increments for 80 steps. For every sample,
target genes and reference genes were amplified simultaneously in
separate triplicate wells. Relative gene expression analysis was
performed using the 2−ΔΔCt method. Each sample was
normalized with the loading control β-actin. The Ct values were
obtained from the means of the triplicate experiments. Data are
expressed as fold change relative to time at 2:00 p.m.
Melatonin concentrations in plasma were determined
using a direct melatonin radioimmunoassay kit (Rocky Mountain
Diagnostics, Colorado Springs, CO, USA), according to the
manufacturer’s instructions.
Plasma TNF-α and IL-6 levels were measured using a
Human TNF-α Quantikine enzyme-linked immunosorbent assay (ELISA)
kit and a Human IL-6 Quantikine ELISA kit, respectively (R&D
systems, Minneapolis, MN, USA), according to the manufacturer’s
instructions.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Continuous variables are reported
as the means ± SD or the median with interquartile range (IQR).
Categorical variables were reported as frequencies and percentages.
Baseline data between septic and non-septic patients were compared
using the Student’s t-test or Chi-square test. Messenger RNA
expression levels of circadian genes are presented as the means ±
SD, and Student’s t-test was applied for the comparisons.
Comparisons between the two groups were performed with the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 22 ICU patients (11 with sepsis and 11
without sepsis) were recruited for this study. Patient
characteristics at the time of enrollment are listed in Tables I and II. Baseline data between the two groups
were comparable, with the exception of a decreased mean arterial
pressure (MAP) and a higher sepsis-related organ failure assessment
in patients with sepsis (Table
II).
 | Table IIComparison of baseline data between
septic and non-septic ICU patients. |
Table II
Comparison of baseline data between
septic and non-septic ICU patients.
| Parameter | Septic patients
(n=11) | Non-septic patients
(n=11) | P-value |
|---|
| Age, years | 59.45±19.16 | 62.55±15.24 | NS |
| Gender,
male/female | 6/5 | 2/9 | NS |
| Heart rate,
bpm | 111.91±26.42 | 99.91±28.16 | NS |
| MAP, mmHg | 71.82±24.96 | 109.27±30.96 | 0.006 |
| APACHE score | 26.09±9.57 | 21.55±5.46 | NS |
| SOFA score | 10.8±6.13 | 6.45±2.84 | 0.045 |
| ICU stay, days | 7.82±3.34 | 10.0±8.93 | NS |
| Hospital stay,
days | 30.73±33.20 | 24.82±20.05 | NS |
| Mechanical
ventilation, number of patients | 6 | 9 | NS |
| Vasoactive drug,
number of patients | 6 | 6 | NS |
| Prognosis,
improvement/mortality | 7/4 | 6/5 | NS |
Secretion of melatonin
Melatonin secretion was monitored at 4-h intervals
over 24 h in each patient. Plasma levels of melatonin in septic and
non-septic patients within the study period were 39.52 (25.81,
53.49) pg/ml (median with IQR) and 17.04 (7.06, 32.31) pg/ml,
respectively. Although melatonin concentrations tended to be higher
in the septic group, statistical significance was not observed
(P=0.055). Notably, the peak time for melatonin secretion in sepsis
patients (~6:00 p.m.) was clearly distinct from non-sepsis patients
(~2:00 a.m.). Serum melatonin levels were significantly increased
at 2 p.m. [20.97 (7.91, 80.28) pg/ml and 6:00 p.m. (21.62 (14.10,
113.95) pg/ml] in septic patients when compared with that in the
control group [0.4 (0, 10.09) pg/ml at 2:00 p.m. and 0 (0, 18.04)
pg/ml at 6:00 p.m.; P=0.03 for both time points (Fig. 1)].
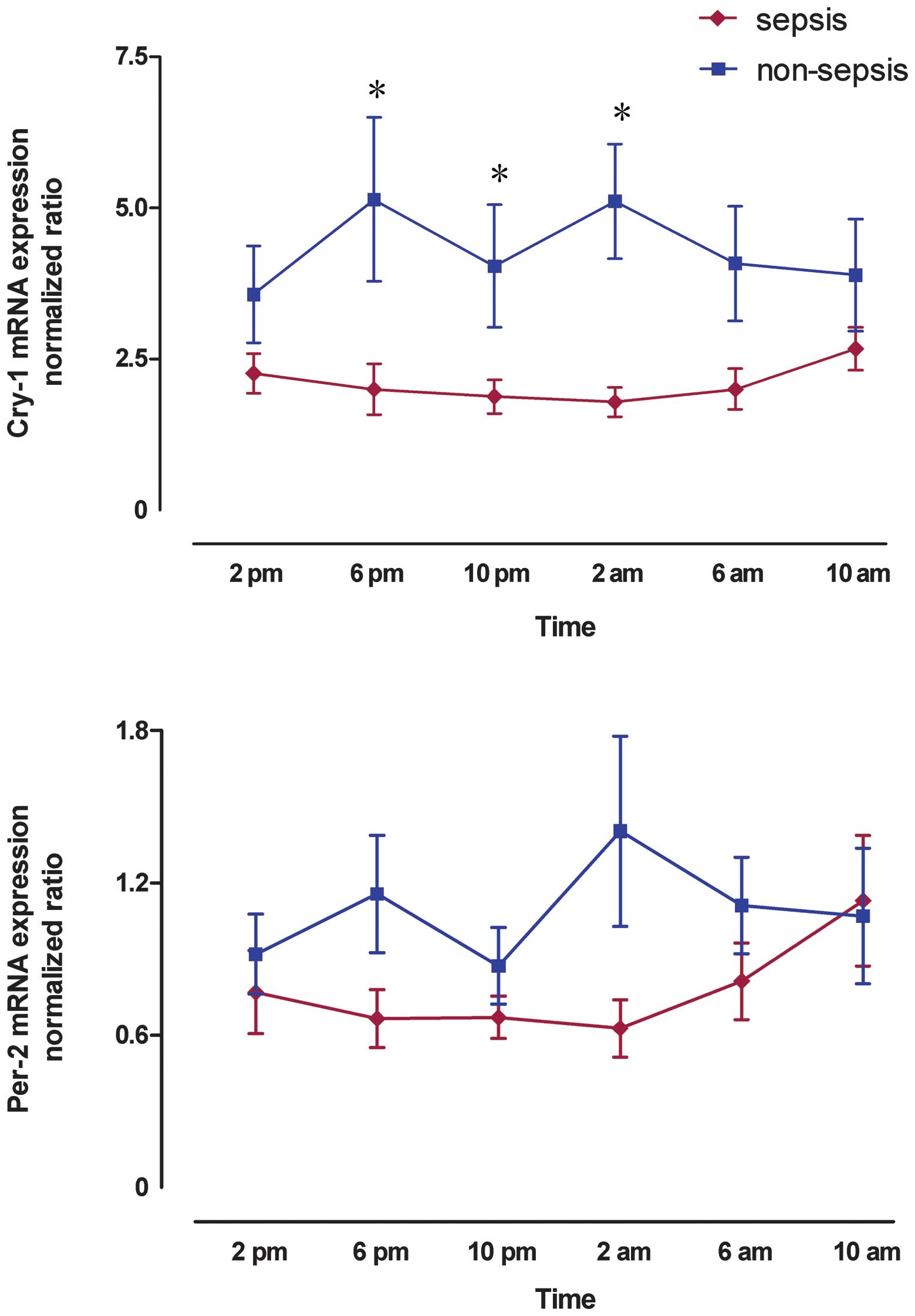
Circadian gene expression
The circadian genes Cry-1 and Per-2
were detected at the mRNA level in all the patients studied. mRNA
levels of the target genes are relative to those of the
housekeeping gene β-actin The expression of Cry-1 and
Per-2 was significantly decreased from 2:00 p.m. to 10:00
a.m. in septic patients compared with the non-septic patients
(2.10±0.32 vs. 4.30±0.66, p<0.001 for Cry-1 and 0.78±0.12
vs. 1.09±0.19, P=0.01 for Per-2; Fig. 2).
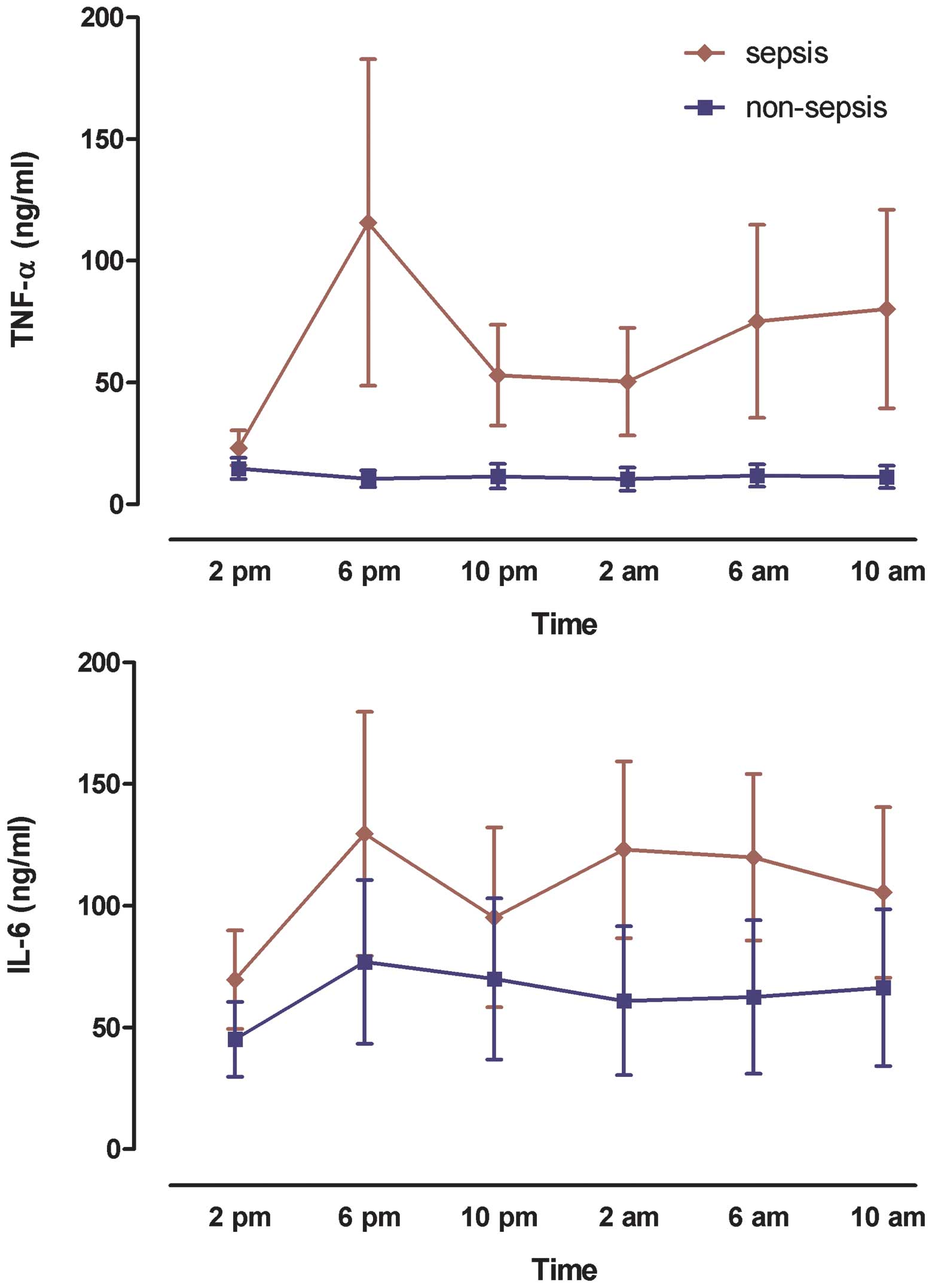
Levels of plasma pro-inflammatory
cytokines
We also monitored plasma TNF-α and IL-6
concentrations in both septic and non-septic patients at 4-h
intervals over 24 h. TNF-α and IL-6 levels were significantly
increased in septic patients [64.1 (43.6–89.126) and 41.2
(35.7–50.8) ng/ml, respectively] as compared with non-septic
patients [11.4 (10.4–12.5) and 19.1 (16–136.7) ng/ml, respectively;
P=0.04 for both]. The peak concentrations of TNF-α and IL-6
occurred at 6:00 p.m. (Fig. 3),
which was coincident with the rhythm of melatonin secretion. By
contrast, no significant peak of TNF-α or IL-6 was observed in
non-sepsis patients.
Discussion
The present study revealed altered circadian rhythm
of melatonin secretion in patients with early sepsis, featuring a
markedly shifted acrophase. The expression of clock genes
Per-2 and Cry-1 was significantly decreased in septic
patients. By contrast, plasma levels of the major pro-inflammatory
cytokines TNF-α and IL-6 increased in septic patients, reaching a
peak at 6:00 p.m., which was consistent with the altered rhythm of
melatonin secretion.
Altered sleep-wake rhythm, abnormal oxidative stress
and use of drugs may affect the secretion of melatonin. Impaired
melatonin secretion has been demonstrated in critically ill
patients, including patients with late stage sepsis (12,20).
Mundigler et al demonstrated that the secretion of melatonin
completely eradicated the natural rhythm of urinary αMT6 excretion
in septic patients who had been in the ICU for at least one week
(11). Considering the
immunomodulatory and antioxidative role of melatonin during sepsis,
abnormalities of melatonin secretion at the late stage of sepsis
may be more noticeable. However, in the current study, the
circadian rhythms of melatonin secretion also occurred in the early
stage of sepsis, although its pattern was different. This finding
is consistent with a previous study showing that the melatonin
rhythms are intact in healthy individuals challenged by endotoxin,
a model of very early-stage sepsis (16). These results suggest that
abnormalities in melatonin rhythms in early and late sepsis may be
induced by separate mechanisms.
Ten circadian clock genes have been identified in
peripheral tissues, including Per-1-3, Cry-1 and
Cry-2, Clock and Bmal1, which coordinate with
the master clock (19).
Investigations in mice with genetic alterations in circadian genes
revealed an elaborate relationship between the circadian clock and
the immune inflammatory response (21). Downregulated circadian gene
expression was reported in endotoxin-challenged rats (22) as well as endotoxin-treated healthy
volunteers (16). Consistent with
these observations, this study demonstrated that the expression of
circadian genes (Per-2 and Cry-1) were significantly
suppressed in PBLs of septic patients. Notably, this alteration
appears to be independent of melatonin rhythm. Previous studies
have demonstrated that several of the ten clock genes appeared to
follow a similar rhythm in immune cells (22,23).
Godin and Buchman proposed that a systemic inflammatory response
may cause uncoupling between day and night (24). We also showed that the suppression
of peripheral circadian genes was independent of the melatonin
rhythm, which provides some clues that there may be a severe
misalignment between the central master clock and peripheral
circadian clock genes in PBLs of septic patients.
Alteration of melatonin circadian rhythm is a result
of multiple factors (20). Certain
factors occur in serum during acute systemic inflammation and
interact with the master clock; while others, such as exposure to
certain environments, sedative drugs, endogenic catecholamines and
even specific critical illness, may also interfere with the
circadian rhythm (20). We
observed that plasma TNF-α and IL-6 levels were significantly
increased in septic patients, and peaked at 6:00 p.m., which is the
same as the shifted rhythm peak of melatonin. Melatonin exerts many
regulatory functions by modulating cellular behavior via binding to
specific receptors and intracellular targets (25,26).
It is well known that melatonin, as an anti-inflammatory molecule,
reduces oxidative tissue injuries by its antioxidant properties and
selectively inhibits the late/chronic phases of inflammatory
responses (27,28). However, the current study cannot
distinguish whether the elevated levels and altered circadian
rhythms of pro-inflammatory cytokines TNF-α and IL-6 are the result
or the cause of melatonin alternation. Further studies are required
to investigate the interaction between melatonin and
proinflammatory cytokines such as TNF-α and IL-6.
In conclusion, the present study demonstrated that
the circadian rhythm of melatonin secretion was altered in early
septic patients, with a markedly shifted peak value. The
pro-inflammatory cytokines TNF-α and IL-6 were produced in a
similar pattern as melatonin in patients with sepsis, while the
expression of circadian genes (Cry-1 and Per-2) in
PBLs was eliminated. The findings of the current study provide a
novel correlation between melatonin secretion and pro-inflammatory
cytokine release in sepsis. Considering its regulatory role in the
immune response, melatonin may be a therapeutic target for sepsis.
However, in this pilot study, it cannot be inferred from the
current data whether the elevated levels and altered circadian
rhythms of pro-inflammatory cytokines TNF-α and IL-6 are simply
caused by melatonin or if they represent a physiological
counteracting mechanism during the early stage of sepsis. Further
study is warranted to elucidate the mechanisms of altered circadian
rhythm in sepsis.
References
|
1
|
Mackenzie I and Lever A: Management of
sepsis. Br Med J. 335:929–932. 2007. View Article : Google Scholar
|
|
2
|
Dellinger RP, Levy MM, Carlet JM, Bion J
and Parker MM: Surviving Sepsis Campaign: international guidelines
for management of severe sepsis and septic shock. Crit Care Med.
36:296–327. 2008. View Article : Google Scholar
|
|
3
|
Riedemann NC, Guo RF and Ward PA: The
enigma of sepsis. J Clin Invest. 112:460–467. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chima RS, Hake PW, Piraino G, Mangeshkar P
and Denenberg A: Ciglitazone ameliorates lung inflammation by
modulating the inhibitor κB protein kinase/nuclear factor-κB
pathway after hemorrhagic shock. Crit Care Med. 36:2849–2857.
2008.PubMed/NCBI
|
|
5
|
Lubbers T, De Haan JJ, Hadfoune M, Zhang Y
and Luyer MD: Lipid-enriched enteral nutrition controls the
inflammatory response in murine Gram-negative sepsis. Crit Care
Med. 38:1996–2002. 2010.PubMed/NCBI
|
|
6
|
Dorresteijn MJ, Visser T, Cox LA, Bouw MP
and Pillay J: C1-esterase inhibitor attenuates the inflammatory
response during human endotoxemia. Crit Care Med. 38:2139–2145.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pevet P and Challet E: Melatonin: both
master clock output and internal time-giver in the circadian clocks
network. J Physiol Paris. 105:170–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brzezinski A: Melatonin in humans. N Engl
J Med. 336:186–195. 1997. View Article : Google Scholar
|
|
9
|
Escames G, Leon J, Macias M, Khaldy H and
Acuna-Castroviejo D: Melatonin counteracts
lipopolysaccharide-induced expression and activity of mitochondrial
nitric oxide synthase in rats. FASEB J. 17:932–934. 2003.
|
|
10
|
Lee YD, Kim JY, Lee KH, Kwak YJ and Lee
SK: Melatonin attenuates lipopolysaccharide-induced acute lung
inflammation in sleep-deprived mice. J Pineal Res. 46:53–57. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mundigler G, Delle-Karth G, koreny M, et
al: Impaired circadian rhythm of melatonin secretion in sedated
critically ill patients with severe sepsis. Crit Care Med.
30:536–540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bourne RS and Mills GH: Melatonin:
possible implications for the postoperative and critically ill
patient. Intensive Care Med. 32:371–379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reppert SM and Weaver DR: Coordination of
circadian timing in mammals. Nature. 418:935–941. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
James FO, Cermakian N and Boivin DB:
Circadian rhythms of melatonin, cortisol, and clock gene expression
during simulated night shift work. Sleep. 30:1427–1436.
2007.PubMed/NCBI
|
|
15
|
O’Connor MF, Motivala SJ, Valladares EM,
Olmstead R and Irwin MR: Sex differences in monocyte expression of
IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp
Physiol. 293:R145–R151. 2007.PubMed/NCBI
|
|
16
|
Haimovich B, Calvano J, Haimovich AD,
Calvano SE and Coyle SM: In vivo endotoxin synchronizes and
suppresses clock gene expression in human peripheral blood
leukocytes. Crit Care Med. 38:751–758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pollard LC, Choy EH, Gonzalez J, Khoshaba
B and Scott DL: Fatigue in rheumatoid arthritis reflects pain, not
disease activity. Rheumatology (Oxford). 45:885–889. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
American College of Chest
Physicians/Society of Critical Care Medicine consensus conference.
Definitions of sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. Crit Care Med. 20:864–874. 1992.
View Article : Google Scholar
|
|
19
|
Kusanagi H, Hida A and Satoh K: Expression
profiles of 10 circadianclock genes in human peripheral blood
mononuclear cells. Neurosci Res. 61:136–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verceles AC, Silhan L and Terrin M:
Circadian rhythm disruption in severe sepsis: the effect of ambient
light on urinary 6-sulfatoxymelatonin secretion. Intensive Care
Med. 38:804–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Malkani G and Shi X: The circadian
clock Period 2 gene regulates gamma interferon production of NK
cells in host response to lipopolysaccharide-induced endotoxic
shock. Infect Immun. 74:4750–4756. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okada K, Yano M, Doki Y, Azama T and
Iwanaga H: Injection of LPS causes transient suppression of
biological clock genes in rats. J Surg Res. 145:5–12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teboul M, Barrat-Petit MA and Li XM:
Atypical patterns of circadian clock gene expression in human
peripheral blood mononuclear cells. J Mol Med. 83:693–699. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godin PJ and Buchman TG: Uncoupling of
biological oscillators: A complementary hypothesis concerning the
pathogenesis of multiple organ dysfunction syndrome. Crit Care Med.
24:1107–1111. 1996. View Article : Google Scholar
|
|
25
|
Steinhilber D, Brungs M, Werz O,
Wiesenberg I and Danielsson C: The nuclear receptor for melatonin
repress 5-lipoxygenase gene expression in human B lymphocytes. J
Biol Chem. 270:7037–7040. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng WG, Tang ST, Tseng HP and Wu KK:
Melatonin suppresses cyclooxygenase-2 and inducible nitric oxide
synthase expression by inhibiting p52 acetylation and binding.
Blood. 108:518–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carrillo-Vico A, Lardone PJ, Naji L,
Fernandez-Santos JM and Martin-Lacave I: Beneficial pleiotropic
actions of melatonin in an experimental model of septic shock in
mice: regulation of pro-/anti-inflammatory cytokine network,
protection against oxidative damage and anti-apoptotic effects. J
Pineal Res. 39:400–408. 2005. View Article : Google Scholar
|
|
28
|
Shang Y, Xu SP, Wu Y, Jinag YX and Wu ZY:
Melatonin reduces acute lung injury in endotoxemic rats. Chin Med
J(Engl). 122:1388–1393. 2009.PubMed/NCBI
|