Introduction
The BK virus (BKV) is a small, non-enveloped,
double-stranded DNA virus with a circular DNA genome of ~5,300 bp
(1). BKV is a member of the
polyomavirus family, consisting of BKV (a major cause of renal
allograft dysfunction) (2,3), the John Cunningham virus (JCV;
responsible for progressive multifocal leukoencephalopathy)
(4,5) and simian virus 40 (SV40). The
transmission of BKV occurs mainly via the oral or respiratory route
and generally happens in early childhood with only minor symptoms
of the upper respiratory tracts or it may even be asymptomatic
(6).
BKV may be indefinitely latent in the kidney,
urinary tract or lymphoid tissue, therefore this viral infection
may be reactivated by immunosuppression and cause several
complications, including meninx encephalitis, atypical retinitis,
cystitis and tubulointerstitial nephritis (7–10).
Since the first case diagnosed by Purighalla et al in 1995
(11), BKV nephropathy (BKVN) has
emerged as a significant cause of graft dysfunction (12–14).
Early detection of BKV infection within the stages of asymptomatic
viuria and viremia has been proposed as a beneficial tool for a
preventative treatment against BKVN. Urine cytology has been
implemented for screening decoy cells, however, this provides lower
positive predictive values and is highly technician-dependent
(15). The aim of the present
study was to demonstrate the impact of BKV infection on renal
function, as well as the feasibility of urine qualitative
polymerase chain reaction (PCR) assays as a screening test for BKV
infection in renal transplantation patients.
Materials and methods
Patients and clinical specimens
In total, 250 renal transplant recipients (134 male
and 116 female; patient age range, 24–78 years with a mean age of
51.86±2.1 years) were prospectively enrolled for BKV screening with
qualitative PCR at the Chung-Shan Medical University Hospital,
Taichung, Taiwan.Urine was collected as midstream samples in a
sterile container and blood samples were collected in EDTA tubes.
The urine and blood samples were stored at 4°C and assayed within 3
days of collection. The urine specimens were centrifuged at 105,000
× g for 1 h and the resultant sediment was re-suspended in 200 μl
sterilized water. DNA was extracted by the QIAamp® DNA
Blood Mini kit (Qiagen, Basel, Switzerland) and eluted with 150 μl
elution buffer. The blood samples were centrifuged at 1,900 × g for
5 min and the DNA was extracted from the plasma using the same
protocols. Serum creatinine levels were obtained by an Olympus
AU2700 (Olympus Co Ltd., Tokyo, Japan) in the Clinical Laboratory
of the Chung Shan Medical University Hospital.
Primers and qualitative PCR
The isolated DNA was amplified by PCR using primers
consentaneous for BKV and JCV (F1, 5′-GAT GGC CCC AAC CAA AAG-3′
and R1, 5′-CTA GAA CTT CTA CTC CTC C-3′). Following denaturation
for 3 min at 95°C, the PCR reaction was subjected to 30 cycles of
30 sec at 95°C, 30 sec at 40°C and 30 sec at 72°C, followed by a
final extension at 72°C for 5 min. The amplified products were
analyzed by gel electrophoresis with the anticipated size of the
amplified product being 110 and 86 bp for BKV and JCV, respectively
(Fig. 1).
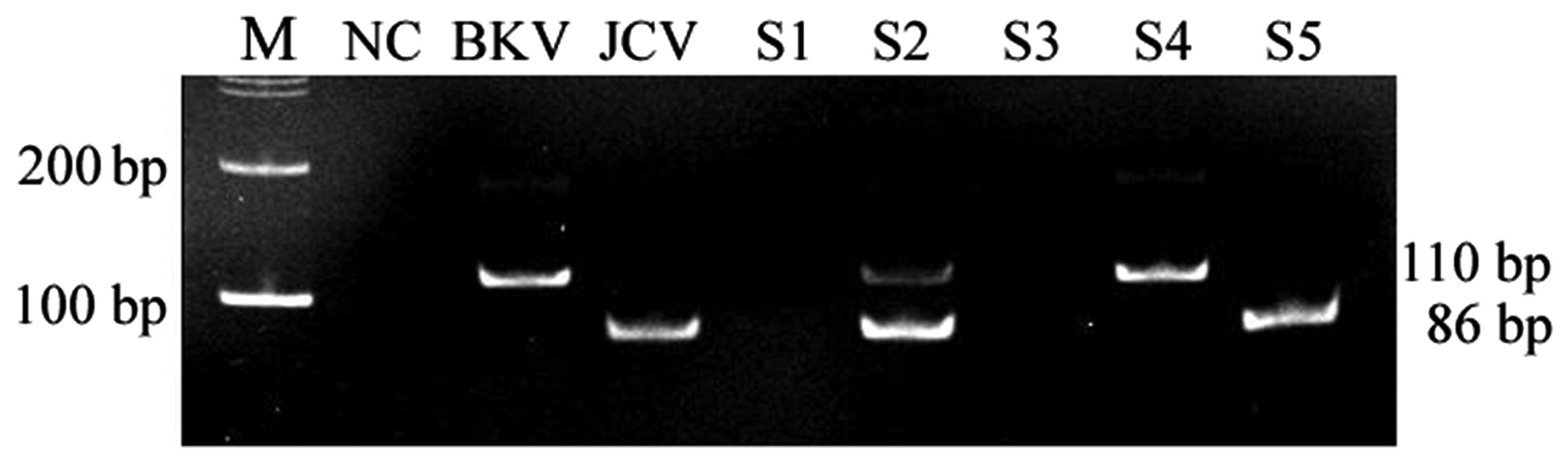 | Figure 1Gel electrophoresis of the PCR
amplified product. S1 was negative; S2 was
BKV-positive/JCV-positive; S3 was negative; S4 was BKV-positive; S5
was JCV-positive. M, 100 bp marker; NC, negative control; BKV,
positive control; JCV, positive control; S, sample; PCR, polymerase
chain reaction, BKV, BK virus, JCV, JC virus. |
Statistical analysis
A Student’s t-test was used for the statistical
analysis involving the continuous variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics and effect of BKV
on serum creatinine concentration levels
The demographic data on the renal transplant
recipients are shown in Table I.
Of the 250 patients, 134 were male and 116 were female. The patient
age ranged from 24 to 78 years (mean age, 51.86±2.1 years).
According to the results of the PCR screening, 51 (20.4%) and 96
patients (38.4%) were positive for BKV and JCV,
respectively, while 119 patients (47.6%) were negative for
the viruses together and 16 patients (6.4%) were co-infected by BKV
and JCV. Based on their infection status, the patients were
categorized into 4 groups and analyzed for the correlations between
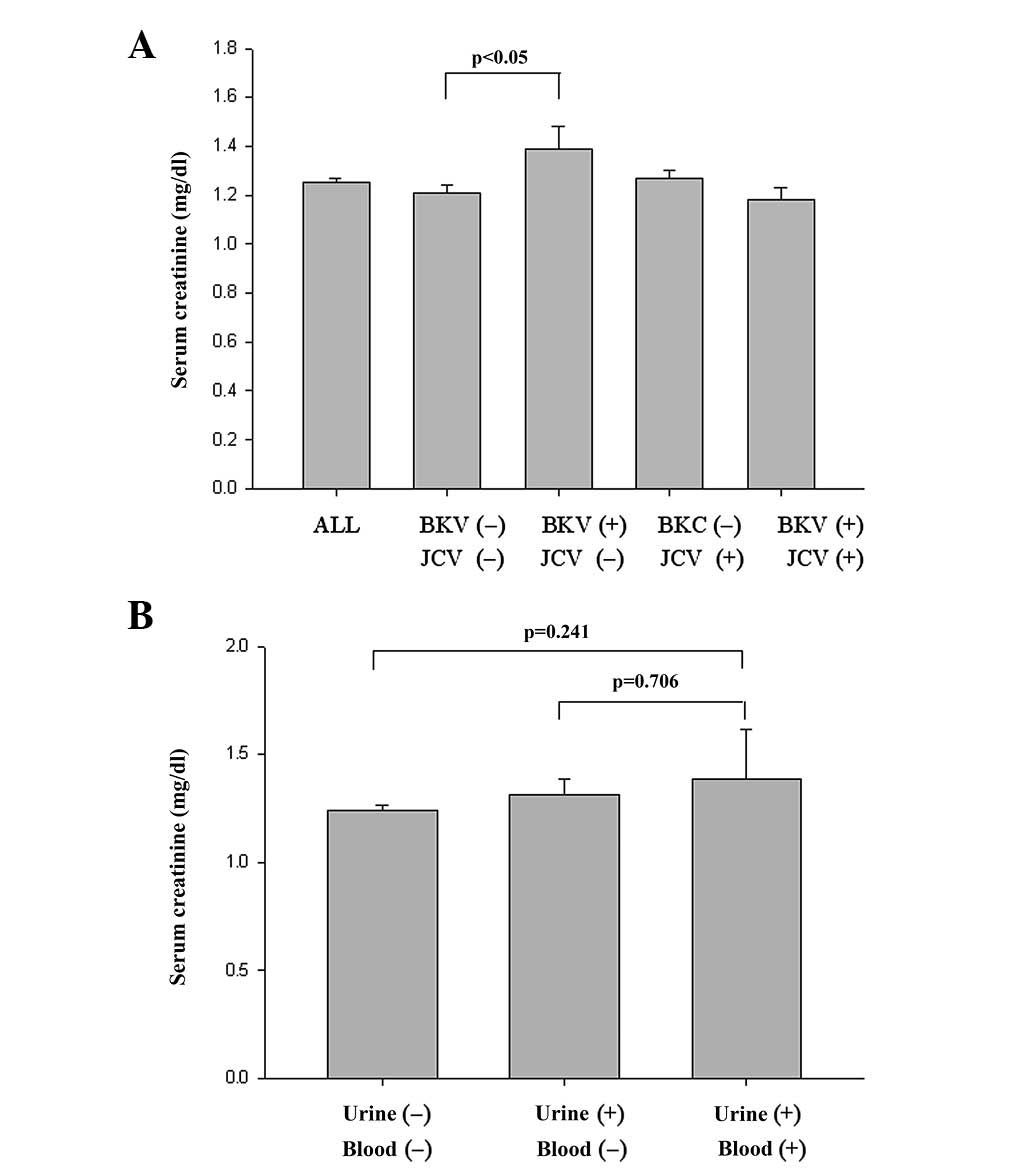
viral infection and renal function. As shown in Table I, BKV had a significant impact on
serum creatinine concentration as the mean serum creatinine
concentration of patients positive for only BKV was 1.39±0.09
mg/dl, which was significantly higher than that of patients
negative for the two viruses (1.21±0.03; P<0.05; Fig. 2A). This effect existed for each
gender. In contrast, JCV infection had no or only a slight effect
on renal function.
 | Table IDemographic data and clinical
characteristics of patients categorized based on their infection
status. |
Table I
Demographic data and clinical
characteristics of patients categorized based on their infection
status.
| | | | Gender |
|---|
| | | |
|
|---|
| | | | Male | Female |
|---|
| | | |
|
|
|---|
| Group | Number (%) | Serum creatinine
(mg/dl) | GFR (ml/min) | Number (%) | Serum creatinine
(mg/dl) | GFR (ml/min) | Number (%) | Serum creatinine
(mg/dl) | GFR (ml/min) |
|---|
| Total | 250 | 1.25±0.02 | 59.34±1.13 | 134 (53.6) | 1.37±0.03 | 63.24±1.45 | 116 (46.4) | 1.12±0.03 | 54.83±1.69 |
| I | 119 (47.6) | 1.21±0.03 | 59.21±1.72 | 54 (45.38) | 1.33±0.04 | 63.37±2.18 | 65 (54.62) | 1.11±0.04 | 55.75±5.04 |
| II | 35 (14) | 1.39±0.09 | 54.82±2.58 | 19 (54.29) | 1.5±0.12 | 56.73±3.17 | 16 (45.71) | 1.26±0.14 | 52.55±4.25 |
| III | 80 (32) | 1.27±0.03 | 61.50±1.98 | 51 (63.75) | 1.38±0.04 | 65.43±2.5 | 29 (36.25) | 1.09±0.04 | 54.6±2.85 |
| IV | 16 (6.4) | 1.18±0.05 | 59.38±4.47 | 10 (62.5) | 1.28±0.07 | 63.81±6.26 | 6 (37.5) | 1.01±0.05 | 52.01±4.96 |
Infection status of patients
With regard to further blood screening in the
patients with BKV viuria, the patients were grouped based on their
BK infection status into BKV-negative (199 patients, 79.6%), BKV
viuria (43 patients, 17.2%) and BKV viuria/viremia (8 patients,
3.2%) groups (Table II). However,
there was no significant difference in the serum creatinine
concentration between the BKV-negative (1.24±0.02 mg/dl) and BKV
viuria/viremia groups (1.39±0.23 mg/dl; P=0.241; Fig. 2B). Overall, the results suggested
that PCR monitoring of BKV with urine samples was a rapid,
non-invasive and beneficial method for the long-term care of kidney
transplant recipients. This aids in our understanding of the
significance of looking after kidney transplant patients that have
been infected by BKV.
 | Table IIDemographic data and clinical
characteristics of patients categorized based on their infection
status. |
Table II
Demographic data and clinical
characteristics of patients categorized based on their infection
status.
| | | | Gender |
|---|
| | | |
|
|---|
| | | | Male | Female |
|---|
| | | |
|
|
|---|
| Group | Number (%) | Serum creatinine
(mg/dl) | GFR (ml/min) | Number (%) | Serum creatinine
(mg/dl) | GFR (ml/min) | Number (%) | Serum creatinine
(mg/dl) | GFR (ml/min) |
|---|
| Urine (−) | | | | | | | | | |
| Blood (−) | 199 (79.6) | 1.24±0.02 | 60.14±1.73 | 105 (52.76) | 1.36±0.03 | 64.37±1.65 | 94 (47.24) | 1.1±0.03 | 55.4±1.95 |
| Urine (+) | | | | | | | | | |
| Blood (−) | 43 (17.2) | 1.32±0.07 | 56.71±2.49 | 26 (54.29) | 1.42±0.09 | 60.54±3.06 | 17 (45.71) | 1.15±0.10 | 50.95±3.91 |
| Urine (+) | | | | | | | | | |
| Blood (+) | 8 (3.2) | 1.39±0.23 | 52.89±4.69 | 3 (63.75) | 1.47±0.29 | 40.68±3.18 | 5 (36.25) | 1.34±0.35 | 58.97±4.01 |
Discussion
Although numerous studies (12,14)
in recent years have explored the reasons behind post-kidney
transplant complications, the majority extracted the patients’
blood for virus detection and extremely few performed virus
detection based on the patients’ urine. Therefore, the present
study established a viable detection method and sought to provide
valid data references for clinicians more rapidly. Subsequent to
ultracentrifugation (105,000 × g, 1 h) of the urine specimens
obtained from the 250 kidney transplant patients, DNA was extracted
for PCR analyses to assess whether polyomavirus (BKV or JVC)
infections had occurred. The results showed that the blood serum
creatinine concentrations for the kidney transplant patients
without any viral infections and for those patients with the BKV
infection only were 1.21±0.03 and 1.39±0.09 mg/dl, respectively,
thus exhibiting a significant difference (P<0.05). This result
indicated that for this group of kidney transplant patients, the
development of a BKV infection would lead to kidney damage and
decreasing kidney function. Therefore, early detection of the BKV
virus was extremely beneficial for the patients.
Generally, post-transplant diabetes mellitus (PTDM)
occurs within 3 months of transplantation (16). The results of the present study
showed that from the 250 participating kidney transplant patients,
no significant statistical difference was observed between the
blood serum creatinine concentrations and glomerular filtration
rates (GFRs) of the patients without viral infection, with BKV
infection only, with JCV infection only or with BKV and JCV
infection under the conditions of no diabetes mellitus (N-DM),
diabetes mellitus (DM) or PTDM. Although previous studies have
indicated that the occurrence of PTDM is generally related to the
use of immunosuppressants following kidney transplantation
(17,18), the patients with viral infections
and PTDM in the present study were limited and the results were not
comparable any further.
The clinical BKV nephropathy detection method
currently applied requires the patients’ blood serum creatinine
(mg/dl) concentration to reach a certain level, indicating that
conditions are already poor. The method also requires decoy cells
to be identified through urine cytology tests prior to performing
kidney biopsy confirmation, which requires a longer period of time
(19, 20). In the present study, DNA detection
was directly performed using the patients’ urine. Through
electrophoresis analysis, the results obtained using the two
centrifugation methods were observed to be identical. Besides
providing patients with earlier and more rapid test results, the
method of the present study also provides a faster reference for
clinicians to administer appropriate immunosuppressant treatments
to patients, reducing the likelihood of BKV infections damaging the
functions of the transplanted kidney.
During the present study, a noteworthy situation was
also observed. When comparing the conditions for the kidney
transplant patients infected with the two viruses, the blood serum
creatinine concentrations for the patients without viral infection
and for the patients with BKV infection only were 1.21±0.03 and
1.39±0.09 mg/dl, respectively, thus exhibiting a significant
difference (P<0.05). Following BKV infection, the blood serum
creatinine concentrations increased. However, in the patients
co-infected with BKV and JVC, the blood serum creatinine
concentration was 1.18±0.05 mg/dl and had decreased, exhibiting a
level that was an improvement on that of the patients without
infections. Whether this means that JCV infection is able to
inhibit the effects of BKV infection or not is worthy of
investigation. Currently, JCV infection is considered to only
affect the central nervous system (21,22);
thus, few studies have explored its effects on the kidneys.
Therefore, further investigations and comparisons of the
interaction and effects of JCV and BKV during coinfection are worth
pursuing.
Finally, the results of the present study have shown
that the extraction of DNA directly from the urine for PCR
detection is critical for kidney transplant patients. This method
is able to provide earlier detection of BKV viral infections, allow
prompt administration of appropriate immunosuppressant treatments
among patients and reduce the likelihood of BKV infections damaging
the functions of the transplanted kidney. Additionally, urine
cytology observations and blood BKV viral infection detection may
be combined with biopsies and immunohistochemical staining to
confirm BKV nephropathy and provide the most effective treatment
and assistance to patients who have received a kidney
transplant.
Acknowledgements
This study was supported by Chung Shan Medical
University Hospital (CSH-97-A-12), Taichung, Taiwan.
References
|
1
|
Eash S, Manley K, Gasparovic M, Querbes W
and Atwood WJ: The human polyomaviruses. Cell Mol Life Sci.
63:865–876. 2006. View Article : Google Scholar
|
|
2
|
Gardner SD, Field AM, Coleman DV and Hulme
B: New human papovavirus (B.K.) isolated from urine after renal
transplantation. Lancet. 1:1253–1257. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Padgett BL, Walker DL, ZuRhein GM,
Eckroade RJ and Dessel BH: Cultivation of papova-like virus from
human brain with progressive multifocal leucoencephalopathy.
Lancet. 1:1257–1260. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zu Rhein G and Chou SM: Particles
resembling papovaviruses in human cerebral demyelinating disease.
Science. 148:1477–1479. 1965.PubMed/NCBI
|
|
5
|
Zu Rhein GM: Polyoma-like virions in a
human demyelinating disease. Acta Neuropathol. 8:57–68.
1967.PubMed/NCBI
|
|
6
|
Goudsmit J, Wertheim-van Dillen P, van
Strien A and van der Noordaa J: The role of BK virus in acute
respiratory tract disease and the presence of BKV DNA in tonsils. J
Med Virol. 10:91–99. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moret H, Guichard M, Matheron S, et al:
Virological diagnosis of progressive multifocal
leukoencephalopathy: detection of JC virus DNA in cerebrospinal
fluid and brain tissue of AIDS patients. J Clin Microbiol.
31:3310–3313. 1993.
|
|
8
|
Sundsfjord A, Spein A, Lucht E, Flaegstad
T, Seternes OM and Traavik T: Detection of BK virus DNA in
nasopharyngeal aspirates from children with respiratory infections
but not in saliva from immunodeficient and immunocompetent adult
patients. J Clin Microbiol. 32:1390–1394. 1994.PubMed/NCBI
|
|
9
|
Chesters PM, Heritage J and McCance DJ:
Persistence of DNA sequences of BK virus and JC virus in normal
human tissues and in diseased tissues. J Infect Dis. 147:676–684.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elsner C and Dörries K: Evidence of human
polyomavirus BK and JC infection in normal brain tissue. Virology.
191:72–80. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Purighalla R, Shapiro R, McCauley J and
Randhawa P: BK virus infection in a kidney allograft diagnosed by
needle biopsy. Am J Kidney Dis. 26:671–673. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CH, Wen MC, Wang M, et al: A
regulatory region rearranged BK virus is associated with
tubulointerstitial nephritis in a rejected renal allograft. J Med
Virol. 64:82–88. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petrogiannis-Haliotis T, Sakoulas G, Kirby
J, et al: BK-related polyomavirus vasculopathy in a
renal-transplant recipient. N Engl J Med. 345:1250–1255. 2001.
View Article : Google Scholar
|
|
14
|
de Bruyn G and Limaye AP: BK
virus-associated nephropathy in kidney transplant recipients. Rev
Med Virol. 14:193–205. 2004.
|
|
15
|
Boldorini R, Brustia M, Veggiani C, et al:
Periodic assessment of urine and serum by cytology and molecular
biology as a diagnostic tool for BK virus nephropathy in renal
transplant patients. Acta Cytol. 49:235–243. 2005. View Article : Google Scholar
|
|
16
|
Kasiske BL, Snyder JJ, Gilbertson D and
Matas AJ: Diabetes mellitus after kidney transplantation in the
United States. Am J Transplant. 3:178–185. 2003. View Article : Google Scholar
|
|
17
|
Boudreaux JP, McHugh L, Canafax DM, et al:
The impact of cyclosporine and combination immunosuppression on the
incidence of posttransplant diabetes in renal allograft recipients.
Transplantation. 44:376–381. 1987. View Article : Google Scholar
|
|
18
|
Sumrani NB, Delaney V, Ding ZK, et al:
Diabetes mellitus after renal transplantation in the cyclosporine
era - an analysis of risk factors. Transplantation. 51:343–347.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cimbaluk D, Pitelka L, Kluskens L and
Gattuso P: Update on human polyomavirus BK nephropathy. Diagn
Cytopathol. 37:773–779. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hariharan S: BK virus nephritis after
renal transplantation. Kidney Int. 69:655–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Studahl M: Influenza virus and CNS
manifestations. J Clin Virol. 28:225–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khalili K, Gordon J and White MK: The
polyomavirus, JCV and its involvement in human disease. Adv Exp Med
Biol. 577:274–287. 2006. View Article : Google Scholar : PubMed/NCBI
|