Introduction
Myocardial infarction (MI) leads to cardiomyocyte
loss and scar formation. Damaged myocytes in infarcted tissues
undergo progressive replacement by cardiac fibroblasts (CFBs) to
form scar tissue that causes a remodeling of the left ventricle
(LV). Consequently, cell-based therapeutic strategies for
myocardial repair have recently attracted considerable interest,
emerging as a promising cellular therapy for ischemic heart disease
(IHD). Recently, a growing body of evidence has shown that
mesenchymal stem cell (MSC) transplantation decreases fibrosis in
the post-infarcted heart (1).
As a major cellular component of the heart, CFBs are
directly involved in collagen synthesis of the infarct zone. One
novel strategy to reduce scar formation after MI is pivotal to
inhibit CFB proliferation and collagen synthesis. It has been shown
that mesenchymal stem cell conditioned medium (MSC-CM)
significantly attenuates CFB proliferation and collagen synthesis
(2).
Bone marrow-derived MSCs are multipotent progenitor
cells. They are easy to obtain and expand readily. They are also
self-renewing and capable of repairing infarcted myocardium.
Integrin-linked kinase (ILK) is a critical component of the cardiac
mechanical stretch sensor. It not only plays an important role in
maintaining myocardial contractility and function, it is also
important in anti-apoptosis and angiogenesis. Recent studies have
shown that in rat models, ILK-MSC delivery decreased the fibrotic
heart area after MI (3). However,
the potential mechanism of ILK-MSCs after MI is not yet fully
elucidated.
Therefore, we hypothesized that ILK-MSCs attenuate
CFB proliferation and collagen synthesis through paracrine effects.
To test this hypothesis, we investigated the effects of ILK-MSC-CM
on CFB proliferation and gene expression of cytokines. We implanted
ILK-MSC-CM into infarcted hearts and analyzed the cardiac
morphological and functional changes in the implanted and injured
hearts.
Materials and methods
Ethics
This study was approved by the Ethics Committee of
Nanjing Drum Tower Hospital, Nanjing, China (DTH ERBA
66.01/026D/2010).
Preparation and adenoviral transduction
of MSCs
MSCs were isolated from the bone marrow of
Sprague-Dawley rats and were expanded according to previously
reported protocols (4). The cells
were suspended in Dulbecco’s modified Eagle medium-low glucose
(DMEM-LG; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine
serum (FBS; Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin.
Fourth-passage cells were transduced with a recombinant adenoviral
vector harboring human wild-type ILKcDNA and humanized recombinant
green fluorescent protein (hrGFP). Transduction efficiency was
assessed by fluorescence-activated cell sorting (FACS) analysis
(Becton-Dickinson, Franklin Lakes, NJ, USA), western blotting,
real-time PCR and immunofluorescence.
Preparation of CFBs
CFBs were prepared and cultured as previously
described with minimum modification (5,6).
Treatment of CFBs with conditioned
medium
Conditioned medium was collected from a 72-h culture
of fourth-passage MSCs and ILK-modified MSCs (ILK-MSCs) and
normalized by cultured cell numbers. After removing the cell
debris, the supernatant was transferred into dedicated
ultrafiltration tubes (Amicon Ultra-PL 5; Millipore, Billerica, MA,
USA), concentrated and desalted according to the manufacturer’s
protocol.
CFBs were incubated in MSC-CM or ILK-MSC-CM for 72
h. The control group was incubated in DMEM-high glucose (DMEM-HG)
containing 5% FBS for the same period of time.
RT-PCR
Total RNA was extracted from cultured cells using
RNAprep pure cell kit (Tiangen, Beijing, China). RNA quality was
assessed by the ratio of absorbance at 260 and 280 nm. One
microgram of total RNA was used for first strand cDNA synthesis by
Reverse Transcriptase from Invitrogen. Quantitative real-time PCR
was carried out using the ABI Prism 7000 sequence detection system
(Applied Biosystems) with SYBR green (Applied Biosystems). All
samples were plated in 48-well plates with each well containing 1
μl cDNA for a total reaction volume of 10 μl. Reactions were
carried out at 95°C for 10 min, followed by 40 cycles of 95°C for 5
sec and 60°C for 34 sec. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) mRNA amplified from the same samples served as an internal
control. Primers were designed by using Primer 3 software. Primer
sequences were: ILK, forward: 5′-GATTGTGCCTATCCTTGAGAAGAT-3′ and
reverse: 5′-TAAACTTTATTGTGACAGGCGG-3′; CTGF, forward:
5′-GGCTGGAGAAGCAGAGTCGT-3′ and reverse:
5′-GATGCACTTTTTGCCCTTCTTAA-3′; α-SMA, forward:
5′-TTCGTTACTACTGCTGAGCGTGAGA-3′ and reverse:
5′-AAGGATGGCTGGAACAGGGTC-3′; Col1a1, forward:
5′-ATCAGCCCAAACCCCAAGGAGA-3′ and reverse:
5′-CGCAGGAAGGTCAGCTGGATAG-3′; Col3a1, forward:
5′-TGATGGGATCCAATGAGGGAGA-3′ and reverse:
5′-GAGTCTCATGGCCTTGCGTGTTT-3′; MMP-2, forward:
5′-GATCTGCAAGCAAGACATTGTCTT-3′ and reverse:
5′-GCCAAATAAACCGATCCTTGAA-3′; MMP-9, forward:
5′-CGCAAGCCTCTAGAGACCAC-3′ and reverse: 5′-TGGGGGATCCGTGTTTATTA-3′;
TIMP-1, forward: 5′-CTGCAACTCGGACCTGGTTATAAGG-3′ and reverse:
5′-AACGGCCCGCGATGAGAAACTCC-3′; TIMP-2, forward:
5′-ATTCCGGGAATGACATCTATGGCAAC-3′ and reverse:
5′-CTGGTACCTGTGGTTTAGGCTCTTC-3′; GAPDH, forward:
5′-GGGGTGAGGCCGGTGCTGAGTA-3′ and reverse:
5′-CATTGGGGGTAGGAACACGGAAGG-3′. Each experiment was repeated three
times.
Western blotting
To identify ILK protein expression, western blotting
was performed with rabbit antibodies against ILK. MSCs infected
with Ad-ILK-hrGFP were cultured with complete medium for 72 h.
After a brief wash with PBS, cells were lysed in 0.1% Triton-X 100,
10 mM TRIS pH 8, 1 mM EDTA, and supplemented with a protease and
phosphatase inhibitor cocktail. Proteins were separated by 10%
TRIS-HCL gel electrophoresis (Bio-Rad), resolved onto 0.2 μm
nitrocellulose membranes, and blocked in nonfat milk in
TRIS-buffered saline supplemented with 0.1% Tween-20.
Immunoreactivity against ILK or GAPDH was visualized with
appropriate horseradish-peroxidase-conjugated antibodies and ECL
plus Western Blotting Detection Reagents. Densitometric analysis
was performed with Abode Photoshop. The ILK signals were normalized
to signals of GAPDH. Experiments were performed in triplicate and
repeated three times.
5-ethynyl-2′-deoxyuridine (EdU)
staining
EdU staining was performed using
Click-iT® EdU Imaging kit (cat no. Cc103338; Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s protocol.
Acute myocardial infarction
Ligation of the LAD artery was performed as
previously described (7). Briefly,
animals were anesthetized by intraperitoneal injection with a
combination of xylazine (10 mg/kg) and ketamine (80 mg/kg),
endotracheally intubated, and mechanically ventilated. After a left
thoracotomy through the fifth intercostal space, the heart was
exposed, the pericardial sac was cut, and the heart exteriorized
through the space. The LAD was ligated with a 6–0 silk suture
approximately midway between the left atrium and the apex of the
heart. Successful MI was identified by blanching of the left
ventricular muscle and the presence of ST elevation on
electrocardiograms.
Prior to surgery, animals were randomized into four
groups. Accordingly, a total volume of 600 μl of MSC-CM, ILK-MSC-CM
or complete medium was injected in five different sites at the
infarct border zone (BZ) 30 min after LAD ligation. Control animals
were operated at the same way, with the exception that no injection
was performed. The chest was closed, and animals were weaned from
the ventilator and allowed to recover.
Echocardiography
Echocardiographic investigation was performed three
days and 4 weeks after MI.
Histological analysis
Animals were sacrificed, and cardiovascular tissues
were collected at the end of the experiment. Paraffin-embedded
sections (from basal, mid-region and apical blocks: 3 slices/block)
were stained with hematoxylin and eosin (H&E) for morphological
examination or Masson’s trichrome staining was performed for the
assessment of interstitial fibrosis.
Statistical analysis
Statistical analysis was performed with SPSS for
Windows (version 16.0). All the data were expressed as the means ±
standard error (SE). Siginificant differences among experimental
conditions were determined by two-tailed Student’s t-tests for
two-group comparisons or ANOVA followed by Newman-Keuls test for
multiple-group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Transduction of MSCs with adenoviral
vector expressing ILK
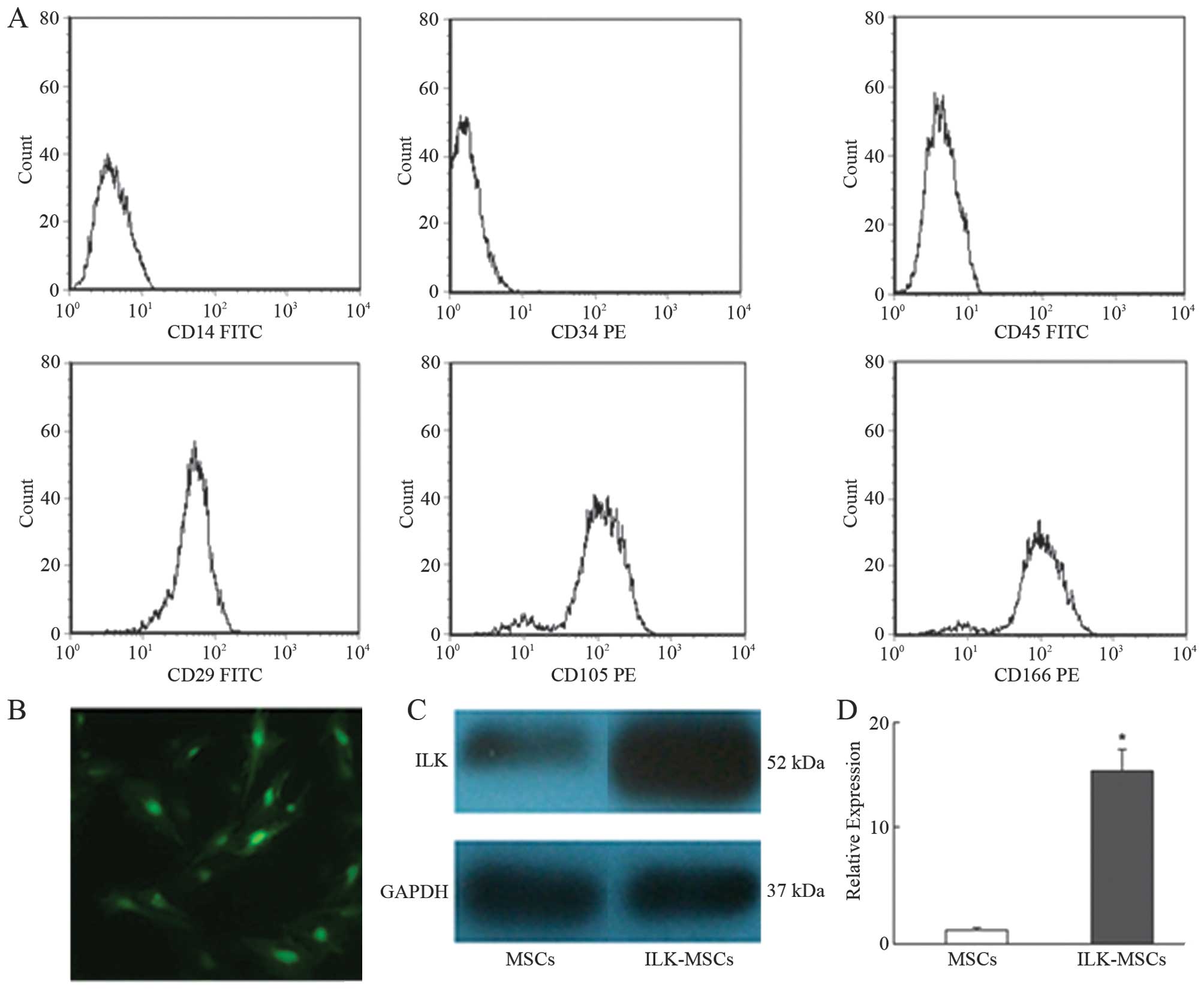
FACS analysis demonstrated that MSCs in culture
expressed low levels of CD14, CD34 and CD45, while high levels of
CD29, CD105 and CD166, in agreement with previously published data
regarding MSC cell surface markers (Fig. 1A).
The viral transduction efficacy was 85–95% in MSCs
infected with Ad-ILK-hrGFP at 200 MOI and cultured for 72 h. The
expression of hrGFP in ILK-MSCs was confirmed by photomicrograph,
qRT-PCR and western blotting (Fig.
1B-D).
Decrease of the quantity of EdU-positive
CFBs
Our results showed that the percentage of CFBs
positively stained for EdU was significantly decreased in both
MSC-CM and ILK-MSC-CM groups. The relative number of EdU-positive
cells was 41±2, 29±4 and 22±3% for the control, MSC-CM and
ILK-MSC-CM groups, respectively. The relative number of
EdU-positive cells showed a ~30 and 46% decrease in the MSC-CM and
ILK-MSC-CM group compared to the control group. In addition, the
ILK-MSC-CM group showed a ~25% decrease in the percentage of
EdU-positive cells compared to the MSC-CM group (Fig. 2A and B).
 | Figure 2Effects of ILK-MSC-CM on CFB
proliferation and gene expression of cytokines. (A) Representative
imaging showing that the ILK-MSC-CM group contained fewer
EdU-positive cells than the MSC-CM and control groups. EdU (red),
Hoechst 33342 (blue). Original magnification, ×200. (B)
Quantification of EdU-positive CFBs from 10 fields of view. Data
are represented as the number of EdU-positive cells relative to the
total number of cells. Real-time PCR for (C) Col1a1, (D) Col3a1,
(E) MMP-2, (F) MMP-9, (G) TIMP-1, (H) TIMP-2, (I) α-SMA, (J) CTGF
gene expression of CFBs after 72 h of culture in the indicated
medium. Values are the means ± SE. *P<0.05 vs. SM;
#P<0.05 vs. MSC-CM. Each group n=3. ILK,
integrin-linked kinase; MSC-CM, mesenchymal stem cell-conditioned
medium; SM, standard medium; CFB, cardiac fibroblasts. |
Effect of conditioned medium on cytokine
gene expression
The results demonstrated that gene expression of
Col1a1, Col3a1, TIMP-1, TIMP-2, α-SMA and CTGF was significantly
downregulated when CFBs were cultured in conditioned medium
compared to standard medium. However, MMP-2 and MMP-9 gene
expression in CFBs incubated with conditioned medium was
significantly upregulated. The TIMP-1, TIMP-2, α-SMA and CTGF gene
expression levels of CFBs were significantly lower in the
ILK-MSC-CM-treated compared to the MSC-CM-treated cells. ILK-MSC-CM
reduced Col3a1 to a level lower compared to MSC-CM. ILK-MSC-CM
significantly upregulated MMP-2 expression, while it downregulated
MMP-9 expression compared to MSC-CM (Fig. 2C-F and H-J).
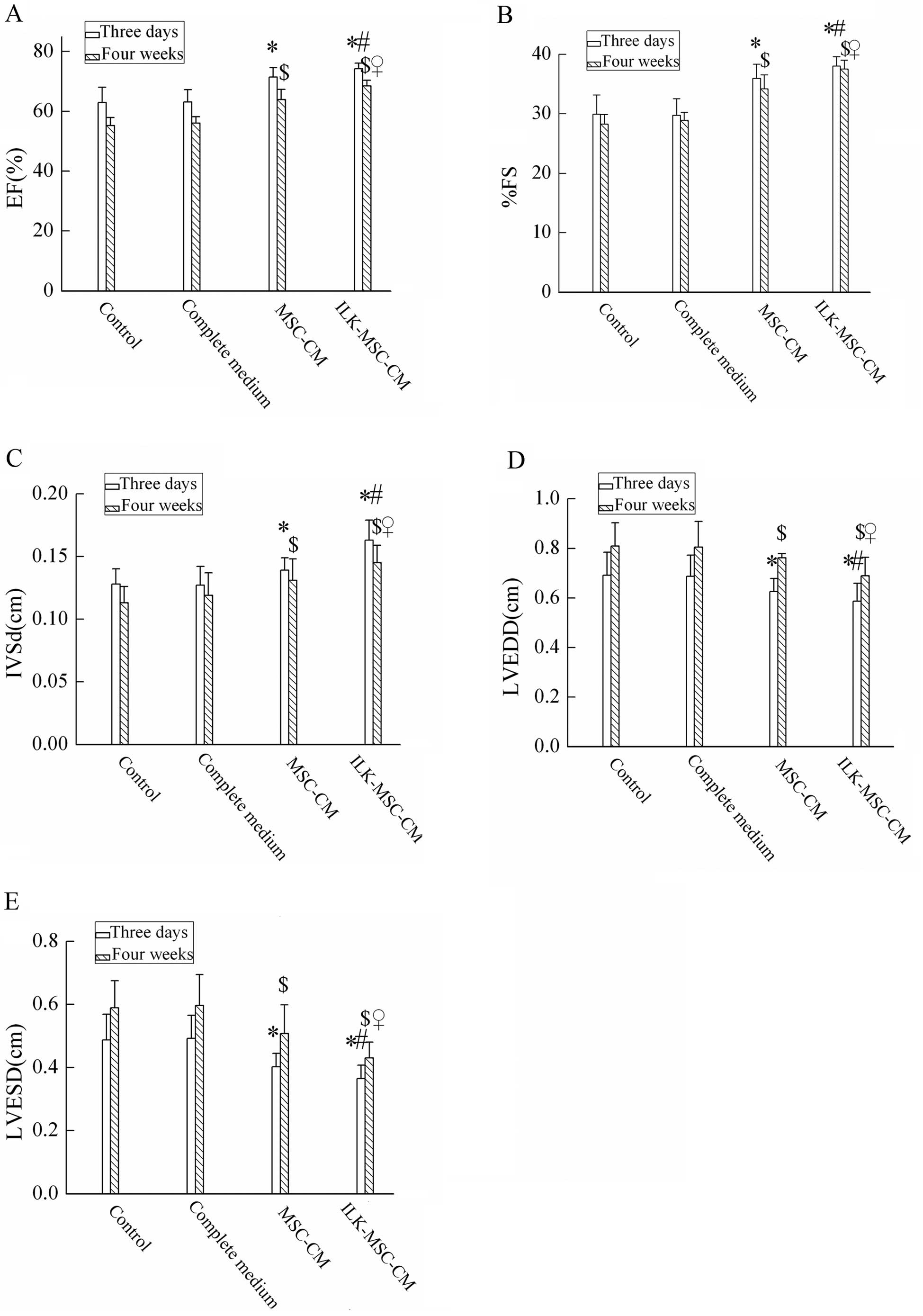
Cardiac function as detected by
echocardiography
After the ligation of the LAD artery, the MI model
detected by echocardiography was successfully established. Three
days and 4 weeks after MI, LV ejection fraction (EF), percent LV
fractional shortening (%FS), and interventricular septal thickness
in diastole (IVSd) demonstrated significant improvement in the
ILK-MSC-CM group compared to the control, complete medium and
MSC-CM groups (Fig. 3A-C). In
parallel with this, LV end-systolic dimension (LVESD) and LV
end-diastolic dimension (LVEDD) were lower in the ILK-MSC-CM group
(Fig. 3D and E). No significant
difference in LV posterior wall thickness (LVPW) was detected.
Histological analysis
The effect of ILK-MSC-CM on myocardial injury 4
weeks after infarction was evaluated by H&E staining. The mean
infarct size in the control animals was 35±3% of the LV. Injection
of complete medium had a similar injury effect, limiting the size
of the infarct to 36±4% of the LV. However, injection of MSC-CM had
a modest protective effect, limiting the size of the infarct to
30±4% of the LV. By contrast, injection of ILK-MSC-CM significantly
limited the infarct size to 22±3% of the LV. Compared to the other
three groups, this translated into a relative reduction in the
infarct size of the ILK-MSC-CM group (Fig. 4A-D).
Reduction of interstitial fibrosis in
infarct boder zone
Masson’s trichrome staining for interstitial
fibrosis in the infarct BZ in the four experimental groups is shown
in Fig. 4E. Collagen volume
fractions (CVF) were significantly lower in the ILK-MSC-CM group
compared to the other three groups. Consequently, ILK-MSC-CM
treatment significantly inhibits collagen synthesis and suppresses
collagen deposition, resulting in a reduction in ventricular
remodeling (Fig. 4F).
Discussion
In this present study, we studied the paracrine
effects of ILK-MSCs on CFBs in vitro and in vivo. The
following were demonstrated: i) MSC-CM and ILK-MSC-CM significantly
inhibited CFB proliferation, while ILK-MSC-CM further suppressed
CFB proliferation compared to MSC-CM. ii) MSC-CM and ILK-MSC-CM
distinctly inhibited type I and III collagen gene expression in
CFBs. ILK-MSC-CM further restrained type III collagen gene
expression. iii) MSC-CM and ILK-MSC-CM significantly increased
MMP-2 and MMP-9 gene expression in CFBs. iv) MSC-CM and ILK-MSC-CM
significantly decreased TIMP-1 and TIMP-2 gene expression in CFBs.
v) MSC-CM and ILK-MSC-CM decreased α-SMA and CTGF gene expression
in CFBs. ILK-MSC-CM further suppressed α-SMA and CTGF gene
expression compared to MSC-CM. (6)
More significant improvement occurred regarding the infarct area,
cardiac geometry (LVEDD, LVESD and IVSd), contractility (EF, %FS)
and pathological changes of the infarcted heart with ILK-MSC-CM
transplantation.
In the mammalian heart, normal cardiac function is
regulated by concordant and dynamic interactions of two major cell
types, cardiomyocytes and CFBs, which account for ~90% of all the
cells in the myocardium. The extracellular matrix (ECM) regulates
the structure of the heart and interactions between cellular and
non-cellular components. In the normal heart, CFBs are mainly
recognized as regulators of ECM metabolism, thereby maintaining
myocardial structure. These cells control ECM homeostasis and
produce MMPs and TIMPs to maintain a balance between the synthesis
and degradation of ECM components. CFBs also play a pivotal role in
the remodeling that occurs in the heart in response to pathological
changes, such as MI, heart failure and hypertension.
CFBs have the ability to synthesize and secrete
collagen type I and III and form a three-dimensional network around
bundles or laminae of myocytes (8). Inhibition of the collagen network
formation results in abnormally shaped hearts or rupture of the
ventricular wall. Under circumstances that accompany myocardial
remodeling, normally quiescent CFBs undergo phenotypic modulation
to myofibroblasts. In the remodeled heart, myofibroblasts become
highly proliferative and invasive, actively remodeling the cardiac
interstitium by increasing MMP secretion and collagen turnover
(9–11). CFBs, by themselves, secrete
increased amounts of growth factors and cytokines, which are able
to activate MMPs and regulate TIMPs, thereby contributing to
remodeling. In long-term heart remodeling, cumulative changes lead
to a net accumulation of collagen, cardiac fibrosis and loss of
cardiac function. Therefore, previous studies have shown that
myocardial scar tissue formation eventually leads to congestive
heart failure and malignant arrhythmias (2). In this respect, reduction of fibrosis
improves the ventricular compliance to a certain extent and delays
the process of heart failure.
Bone marrow-derived MSCs are self-renewing,
multipotent, and can differentiate into several distinct cell
types, including new cardiomyocytes. MSC transplantation has been
shown to improve cardiac function and decrease fibrosis in the
heart and other organs such as the lung, liver and kidney. Previous
studies have demonstrated that paracrine effects may contribute to
the improvement of cardiac function and scar loss following
transplantation of MSCs (12–14).
For instance, MSC-CM has been shown to significantly attenuate the
proliferation of CFBs compared to CFB-conditioned medium (2). However, the underlying mechanisms of
MSC therapy remain controversial.
ILK is a 59-kDa ankyrin-repeat containing
serine/threonine protein kinase that interacts with the cytoplasmic
domain of α1 and α3 integrins and regulates integrin-dependent
functions (15–17). ILK functions as a scaffold in
forming multiprotein complexes connecting integrins to the actin
cytoskeleton and signaling pathways. ILK activity is stimulated by
adhesion to the ECM and by growth factors in a PI3K-dependent
manner. ILK regulates multiple signaling pathways and has been
implicated in the regulation of numerous cellular processes
including proliferation, migration, cell spreading, invasion,
differentiation, transformation and survival in various cell types.
Recent evidence have shown that ILK-MSC transplantation further
improves cardiac function and decreases fibrosis in the infarcted
myocardium when compared with myocardium that has been transplanted
with MSCs alone (18). However,
there is still a lack of related experiments on whether ILK-MSCs
play an important role through paracrine mechanisms to improve
ventricular remodeling.
Many of the functional effects of CFBs are mediated
through differentiation of CFBs to myofibroblasts, cells that
express contractile proteins, including α-SMA, a myofibroblast
marker. Differentiating fibroblasts express α-SMA, indicating
acquisition of a secretory, myofibroblast phenotype, a transition
that correlates with increased secretion of profibrotic molecules
such as collagen and fibronectin (19,20).
The present study demonstrated that ILK-MSC-CM downregulates α-SMA
expression and suppresses the differentiation of CFBs to
myofibroblasts.
CTGF is a 38-kDa protein that contains 38 conserved
cysteine residues and a heparine binding domain (21). Recently, it has been reported that
CTGF promotes proliferation and ECM production in connective tissue
(22,23). This study demonstrated that
ILK-MSC-CM inhibits CTGF expression, which is beneficial to ECM
degradation.
MMP-2 and MMP-9 are two members of the MMP family, a
group of zinc-dependent endopeptidases known to hydrolyze many
components of the ECM (24). MMP-2
and MMP-9 play a fundamental role in ECM remodeling due to their
ability to initiate and continue degradation of fibrillar
collagen.
Belonging to a group of four endogenous proteins,
TIMPs firmly regulate MMP activity (25,26).
TIMPs bind to the active site of MMPs at a stoichiometric 1:1 molar
ratio with differential affinities, thereby blocking access to
their extracellular substrates. Several studies have shown that
TIMP-2 has a maximum affinity for MMP-2, whereas TIMP-1 forms a
specific complex with pro-MMP-9 (27–29).
In normal cardiac tissue, MMPs and TIMPs are co-expressed and
tightly regulated to maintain integrity of the cardiac interstitium
(30). Consequently, the MMP/TIMP
system is a crucial contributor to ECM turnover and heart failure
progression.
In the present study, it was found that the
MMP-9/TIMP-1 ratio was not differentiated in MSC and ILK-MSC
groups, whereas the MMP-2/TIMP-2 ratio significantly increased in
ILK-MSC compared to the MSC group. Elevation of the MMP-2/TIMP-2
ratio was conducive to the degradation of collagen. Furthermore, it
was favorable to angiogenesis and heart function improvement
accompanied by escalation of MMP-2 and MMP-9.
In conclusion, the data presented here demonstrate
that ILK-MSCs exert paracrine anti-fibrotic effects at least in
part through inhibition of CFB proliferation and downregulation of
collagen synthesis. The overexpression of ILK is able to regulate
the partial paracrine effects of MSCs. These features of ILK-MSCs
could be beneficial for the treatment of heart failure in which
fibrotic changes are involved.
Acknowledgements
The authors would like to thank Professor Zheng Xing
from the University of Minnesota for expert technical assistance,
and Congzhu Shi from the University of Pennsylvania for helpful
comments and suggestions.
References
|
1
|
Jaquet K, Krause KT, Denschel J, et al:
Reduction of myocardial scar size after implantation of mesenchymal
stem cells in rats: what is the mechanism? Stem Cells Dev.
14:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohnishi S, Sumiyoshi H, Kitamura S and
Nagaya N: Mesenchymal stem cells attenuate cardiac fibroblast
proliferation and collagen synthesis through paracrine actions.
FEBS Lett. 581:3961–3966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song SW, Chang W, Song BW, et al:
Integrin-linked kinase is required in hypoxic mesenchymal stem
cells for strengthening cell adhesion to ischemic myocardium. Stem
Cells. 27:1358–1365. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwase T, Nagaya N, Fujii T, et al:
Comparison of angiogenic potency between mesenchymal stem cells and
mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc
Res. 66:543–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen K, Chen J, Li D, Zhang X and Mehta
JL: Angiotensin II regulation of collagen type I expression in
cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone.
Hypertension. 44:655–661. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frangogiannis NG, Dewald O, Xia Y, et al:
Critical role of monocyte chemoattractant protein-1/cc chemokine
ligand 2 in the pathogenesis of ischemic cardiomyopathy.
Circulation. 115:584–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Ma N, Ong LL, et al: Bcl-2
engineered MSCs inhibited apoptosis and improved heart function.
Stem Cells. 25:2118–2127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldsmith EC, Hoffman A, Morales MO, et
al: Organization of fibroblasts in the heart. Dev Dyn. 230:787–794.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber KT: Fibrosis in hypertensive heart
disease: focus on cardiac fibroblasts. J Hypertens. 22:47–50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown RD, Ambler SK, Mitchell MD and Long
CS: The cardiac fibroblast: therapeutic target in myocardial
remodeling and failure. Annu Rev Pharmacol Toxicol. 45:657–687.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camelliti P, Borg TK and Kohl P:
Structural and functional characterisation of cardiac fibroblasts.
Cardiovasc Res. 65:40–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai W, Hale SL and Kloner RA: Role of a
paracrine action of mesenchymal stem cells in the improvement of
left ventricular function after coronary artery occlusion in rats.
Regen Med. 2:63–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Zhang S, Zhang Y, Yu B, Xu Y and
Guan Z: Paracrine action mediate the antifibrotic effect of
transplanted mesenchymal stem cells in a rat model of global heart
failure. Mol Biol Rep. 36:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu RX, Chen X, Chen JH, Han Y and Han BM:
Mesenchymal stem cells promote cardiomyocyte hypertrophy in vitro
through hypoxia-induced paracrine mechanisms. Clin Exp Pharmacol
Physiol. 36:176–180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Attwell S, Mills J, Troussard A, Wu C and
Dedhar S: Integration of cell attachment, cytoskeletal
localization, and signaling by integrin-linked kinase (ILK),
CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell.
14:4813–4825. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan C, Mui A and Dedhar S: Integrin-linked
kinase regulates inducible nitric oxide synthase and
cyclooxygenase-2 expression in an NF-kappa B-dependent manner. J
Biol Chem. 277:3109–3116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Troussard AA, McDonald PC, Wederell ED, et
al: Preferential dependence of breast cancer cells versus normal
cells on integrin-linked kinase for protein kinase B/Akt activation
and cell survival. Cancer Res. 66:393–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding L, Dong L, Chen X, et al: Increased
expression of integrin-linked kinase attenuates left ventricular
remodeling and improves cardiac function after myocardial
infarction. Circulation. 120:764–773. 2009. View Article : Google Scholar
|
|
19
|
Gabbiani G: Evolution and clinical
implications of the myofibroblast concept. Cardiovasc Res.
38:545–548. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Petrov VV, Fagard RH and Lijnen PJ:
Stimulation of collagen production by transforming growth
factor-beta 1 during differentiation of cardiac fibroblasts to
myofibroblasts. Hypertension. 39:258–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen MM, Lam A, Abraham JA, Schreiner GF
and Joly AH: CTGF expression is induced by TGF-beta in cardiac
fibroblasts and cardiac myocytes: A potential role in heart
fibrosis. J Mol Cell Cardiol. 32:1805–1819. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Frazier K, Williams S, Kothapalli D,
Klapper H and Grotendorst GR: Stimulation of fibroblast cell
growth, matrix production, and granulation tissue formation by
connective tissue growth factor. J Invest Dermatol. 107:404–411.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Igarashi A, Okochi H, Bradham DM and
Grotendorst GR: Regulation of connective tissue growth factor gene
expression in human skin fibroblasts and during wound repair. Mol
Biol Cell. 4:637–645. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olson MW, Gervasi DC, Mobashery S and
Fridman R: Kinetic analysis of the binding of human matrix
metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase
(TIMP)-1 and TIMP-2. J Biol Chem. 272:29975–29983. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vanhoutte D, Schellings M, Pinto Y and
Heymans S: Relevance of matrix metalloproteinases and their
inhibitors after myocardial infarction: A temporal and spatial
window. Cardiovasc Res. 69:604–613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors of metalloproteinases: evolution, structure and
function. Biochim Biophys Acta. 1477:267–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Menon B, Singh M and Singh K: Matrix
metalloproteinases mediate beta-adrenergic receptor-stimulated
apoptosis in adult rat ventricular myocytes. Am J Physiol Cell
Physiol. 289:C168–C176. 2005. View Article : Google Scholar
|
|
28
|
Goldberg GI, Marmer BL, Grant GA, Eisen
AZ, Wilhelm S and He CS: Human 72-kilodalton type IV collagenase
forms a complex with a tissue inhibitor of metalloproteases
designated TIMP-2. Proc Natl Acad Sci USA. 86:8207–8211. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goldberg GI, Strongin A, Collier IE,
Genrich LT and Marmer BL: Interaction of 92-kDa type IV collagenase
with the tissue inhibitor of metalloproteinases prevents
dimerization, complex formation with interstitial collagenase, and
activation of the proenzyme with stromelysin. J Biol Chem.
267:4583–4591. 1992.
|
|
30
|
Brown RD, Jones GM, Laird RE, Hudson P and
Long CS: Cytokines regulate matrix metalloproteinases and migration
in cardiac fibroblasts. Biochem Biophys Res Commun. 362:200–205.
2007. View Article : Google Scholar : PubMed/NCBI
|