Introduction
Serious complications as a result of type 2 diabetes
mellitus (T2DM) are becoming a major health concern, among which
atherosclerosis, the leading cause of morbidity and mortality in
developed countries, is the most common disease. The most common
pathological change in atherosclerosis is the proliferation of
vascular smooth muscle cells (VSMCs), which occurs in response to
arterial injury and plays a crucial role in the atherosclerotic
process (1). Therefore, it is
necessary to elucidate the molecular mechanisms of abnormal VSMC
proliferation. It is known that insulin resistance,
hyperinsulinemia and hyperglycemia stimulate and accelerate VSMC
proliferation, hypertrophy and abnormal vascular tone in patients
with T2DM (2–4). Although certain mechanisms of
hyperglycemia- and hyperinsulinemia-induced VSMC proliferation have
been proposed to contribute to atherosclerosis, there is limited
knowledge with regard to the role of telomerase activity in VSMC
proliferation (5–7).
Telomeres are DNA-protein complexes located at the
ends of eukaryotic chromosomes, which maintain genome integrity and
stabilize the chromosome function (8). Telomeric DNA is shortened with cell
mitosis. When a critical length is reached, the telomeric ends of
chromosomes lose their protective function and induce cell
apoptosis. Telomerase activation increases telomere repeats on the
chromosome ends, maintains genome integrity and induces cellular
immortalization (9).
Studies with regard to telomerase were initially
introduced in the field of oncology (10,11).
Recently, telomerase has also been studied in proliferative
diseases, including hypertension, cardiac hypertrophy and heart
failure, and is considered to be involved in the proliferation of
abnormal cells and important in the pathogenesis of these diseases
(12–14). Animal studies have demonstrated a
relatively higher level of telomerase activation in the VSMCs of
spontaneously hypertensive rats (15). Telomerase activation may induce a
phenotypic change in VSMCs and play a role in cell remodeling,
indicating that telomerase may be important in the regulation of
tissue regeneration and cell signaling pathways in VSMC
proliferation.
Since telomerase activity is associated with cell
proliferation, aging and apoptosis and there is an imbalance
between VSMC proliferation and apoptosis in T2DM, it was
hypothesized that telomerase may also be important in VSMC
proliferation in T2DM. Therefore, the aim of the present study was
to investigate whether telomerase activity is upregulated in VSMC
proliferation in T2DM and whether the application of telomerase
inhibitors impedes VSMC proliferation in vitro. To test this
hypothesis, we examined the telomerase activity of VSMCs in a rat
model of type 2 diabetes and in cell cultures in vitro. We
also explored the effect of telomerase inhibitors on
hyperinsulinemia- and hyperglycemia-induced VSMC proliferation.
Materials and methods
Materials
Streptozotocin (STZ) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
were purchased from Sigma (St. Louis, MO, USA). Insulin
radioimmunoassay and telomerase assay kits were purchased from
Linco Research, Inc. (St Louis, MO, USA) and Roche Molecular
Biochemicals (Mannheim, Germany), respectively. Antisense
oligoribonucleotides (ASODNs; 5′-GAGCGCGGGTCATTGTGCT-3′) and sense
oligoribonucleotides (SODNs; 5′-AGCACAATGACCCGCGCTC-3′) were
designed as telomerase inhibitors according to the rat telomerase
reverse transcriptase mRNA sequence of GenBank from Beijing General
Chemical Reagent Factory (Beijing, China). Additional reagents were
purchased from Beijing General Chemical Reagent Factory.
Experimental animals
Six-week-old male Wistar rats (specific
pathogen-free quality) were purchased from the Experimental Animal
Center of Shandong University (Shandong, China) and the use of
animals was approved by the Institutional Animal Care and Use
Committee (IACUC; approval no. SCXK20100301). The rats were
randomly allocated into the normal control (NC) or diabetes
mellitus (DM) group. Rats of the NC group were fed a regular diet
(8% fat; 68% carbohydrate; 24% protein; 320 kcal/100 g), whereas
rats of the DM group were fed a high-fat diet (50.10% fat, mainly
saturated; 33.60% carbohydrate; 16.30% protein; 493 kcal/100 g).
After 8 weeks, rats of the DM group were administered a low dose of
STZ (30 mg/kg) by intraperitoneal injection, while those of the NC
group were administered the same volume of vehicle citrate buffer.
Blood glucose levels were measured 72 h after the STZ injection.
Only the rats with glucose levels >16.7 mmol/l were considered
to be a successful diabetic model (16). The diabetic model achieved was
similar to type 2 diabetes in humans. Thus, the combination of a
high-fat intake and low-dose STZ effectively induced type 2
diabetes by altering the expression of associated genes in major
metabolic tissues (17). The rats
were housed under standard laboratory conditions and had free
access to water and standard rat chow. The rats were sacrificed
four weeks after the successful establishment of the diabetic
model.
Plasma measurements
The rats were anesthetized using pentobarbital
sodium. Blood samples were then immediately collected and separated
for plasma measurements. Plasma glucose was measured using the
glucose oxidase method. Plasma insulin was measured using a
radioimmunoassay according to the manufacturer’s instructions.
Plasma triglyceride (TG), cholesterol (TC) and free fatty acid
(FFA) levels were measured using colorimetric assays.
Collection of VSMCs
The thoracic aorta was immediately dissected and
enzymatically digested at 37°C for ~3.5 h using a 0.25% trypsin
solution. Following digestion, tissue fragments were explanted in a
35-mm culture dish. Contaminated fibroblasts were separated from
the VSMCs due to their differing adhesion abilities (18).
Determination of telomerase activity
Telomerase activity in VSMCs was examined using the
telomeric repeat amplification protocol enzyme-linked immunosorbent
assay (TRAP-ELISA), according to the manufacturer’s instructions
(19). Briefly, the same amount of
protein from each sample was incubated with a biotin-labeled
telomerase substrate primer at 25°C for 15 min and primer extension
was carried out using polymerase chain reaction (PCR; 30 cycles of
94°C for 30 sec, 60°C for 30 sec and 72°C for 90 sec). The
PCR-amplified products were hybridized for 120 min and incubated
with a peroxidase-labeled anti-digoxigenin antibody for 30 min.
Following the addition of peroxidase substrate, the optical density
(OD) of each sample was measured at 450 nm with a reference
wavelength of 690 nm using an enzyme immunoassay microplate reader.
The OD values were reported as A450–A690 nm. Samples were
considered positive when the OD values were ≥0.2.
VSMC proliferation assay
VSMC proliferation was measured using the MTT assay
(20). Briefly, VSMCs were
harvested by trypsinization and plated in a 96-well plate at a
density of 1×105 cells/ml. VSMCs were then grown in 100
μl of medium at 37°C for 24 h, followed by incubation with 20 μl
MTT for 4 h. Then, 150 μl dimethyl sulfoxide (DMSO) was added to
each well and the absorbance was measured at a wavelength of 490 nm
using a microplate reader.
In vitro experiment
VSMCs from six-week-old normal Wistar rats were
cultured using the tissue explants technique and were identified
using an immunofluorescence staining method (21). The 3–5 generations of cells used in
all experiments were allocated into four groups: the normal
control, high glucose and insulin (HGI), HGI + low concentration of
ASODN or SODN and HGI + high concentration of ASODN or SODN groups.
After the cells were serum starved for 24 h, they were exposed to
normal glucose (5.5 mM) or high glucose (25 mM) and insulin (100
nM) in the presence and absence of ASODN or SODN for 24 h and then
subjected to an assessment of telomerase activity and cell
proliferation using the methods mentioned previously. In certain
experiments, 19.5 mM of mannitol was used to control variations in
osmotic pressure.
Statistical analysis
Data were subjected to statistical analysis using
the SPSS 13.0 statistical package. The data were expressed as the
mean ± standard deviation (SD). The statistical tests used were
Student’s t-test or one way ANOVA. The number of inter-group
differences were detected using the Student-Newman-Keuls (SNK)
method. The association between two variables was analyzed using
Pearson’s correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Blood parameters of rats in the studied
groups
In the DM group, a significant increase was observed
in the levels of fasting glucose (15.82±3.21 vs. 5.88±0.73 mmol/l,
P<0.01), insulin (18.99±3.68 vs. 11.24±0.68 μIU/ml, P<0.01),
FFAs (934.35±93.39 vs. 301.63±78.29 μmol/l, P<0.01) and TGs
(1.89±0.33 vs. 0.80±0.32 mmol/l, P<0.01; Table I).
 | Table ISerum levels of fasting glucose,
insulin, FFAs and TG in the studied groups (n=10). |
Table I
Serum levels of fasting glucose,
insulin, FFAs and TG in the studied groups (n=10).
| Group | Glucose (mmol/l) | Insulin (μIU/ml) | FFAs (μmol/l) | TGs (mmol/l) |
|---|
| NC | 5.88±0.73 | 11.24±0.68 | 301.63±78.29 | 0.80±0.32 |
| DM | 15.82±3.21a | 18.99±3.68a | 934.35±93.39a | 1.89±0.33a |
Telomerase activity and proliferation of
VSMCs
The expression of telomerase activity and the degree
of VSMC proliferation were significantly higher in the DM compared
with the normal control group (P<0.01; Fig. 1). To determine whether the changes
in telomerase activity and proliferation of VSMCs were induced by
HGI, we investigated the effect of HGI treatment on VSMCs in
vitro. The results showed that telomerase activation and the
proliferation of VSMCs were higher when induced by HGI. Since the
addition of 19.5 mM of mannitol caused no significant changes, we
were able to determine that the glucose effect was not a result of
hyperosmolarity. After the intervention of ASODN, telomerase
activity decreased in a concentration-dependent manner (P<0.05).
However, the same concentration of SODN did not significantly
inhibit telomerase activity (Table
II).
 | Table IITelomerase activity of VSMCs in the
in vitro groups. |
Table II
Telomerase activity of VSMCs in the
in vitro groups.
| Group | 24 h | 72 h |
|---|
| NC | 0.105±0.055 | 0.125±0.033 |
| HGI | 0.599±0.079a | 0.756±0.053a |
| SODN (5 μm) | 0.572±0.023a | 0.731±0.083a |
| ASODN (5 μm) | 0.425±0.086a,b | 0.535±0.053a,b |
| SODN (10 μm) | 0.562±0.026a | 0.709±0.094a |
| ASODN (10 μm) | 0.365±0.060a,b | 0.406±0.069a,b |
| SODN (20 μm) | 0.559±0.035a | 0.692±0.066a |
| ASODN (20 μm) | 0.174±0.039a,b | 0.185±0.046a,b |
| Negative control | 0.105±0.023 | |
| Positive control | 2.256±0.858a | |
Various concentrations of
oligoribonucleotides induce VSMC proliferation
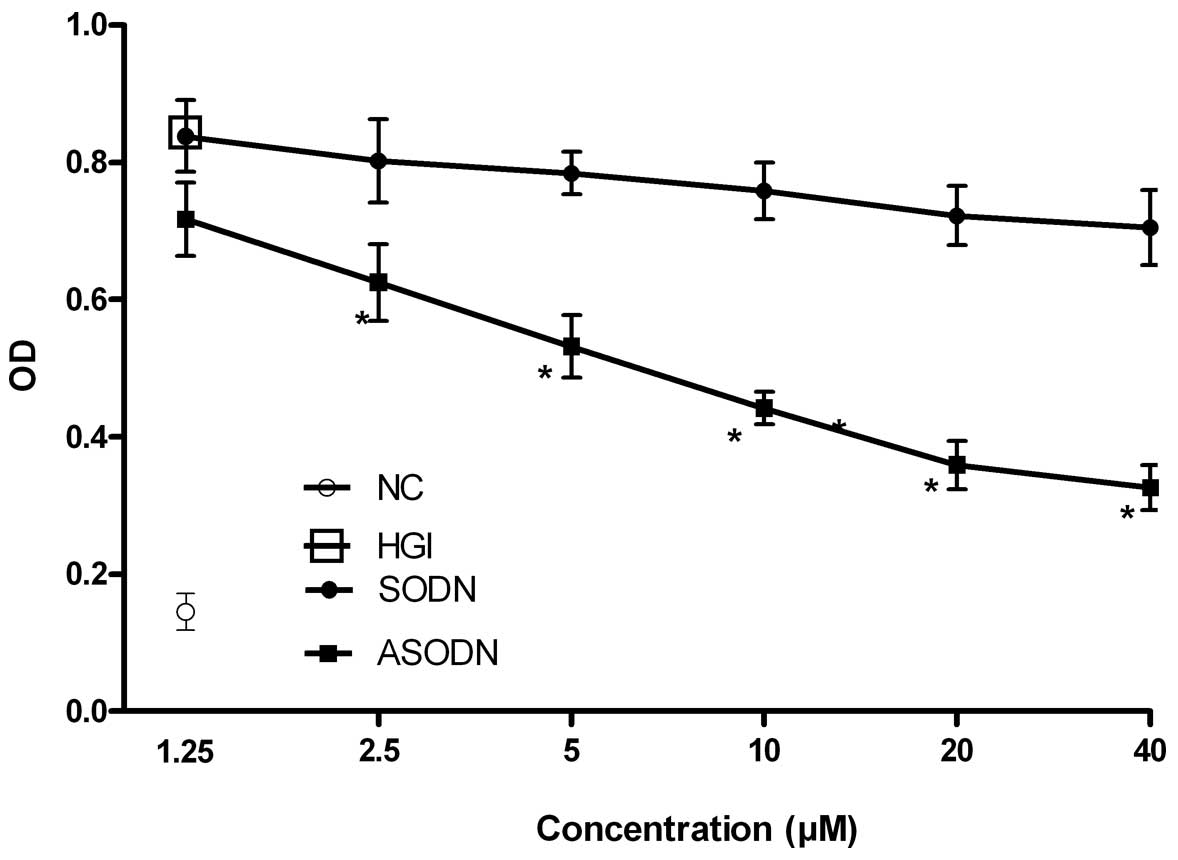
With regard to the in vitro experiments, HGI
significantly promoted VSMC proliferation. In order to examine the
role of telomerase activity in VSMC proliferation, a telomerase
inhibitor (ASODN) was used to inhibit the proliferation of VSMCs
in vitro. The results showed that ASODN significantly
inhibited the proliferation of VSMCs in a concentration-dependent
manner (P<0.05; Fig. 2).
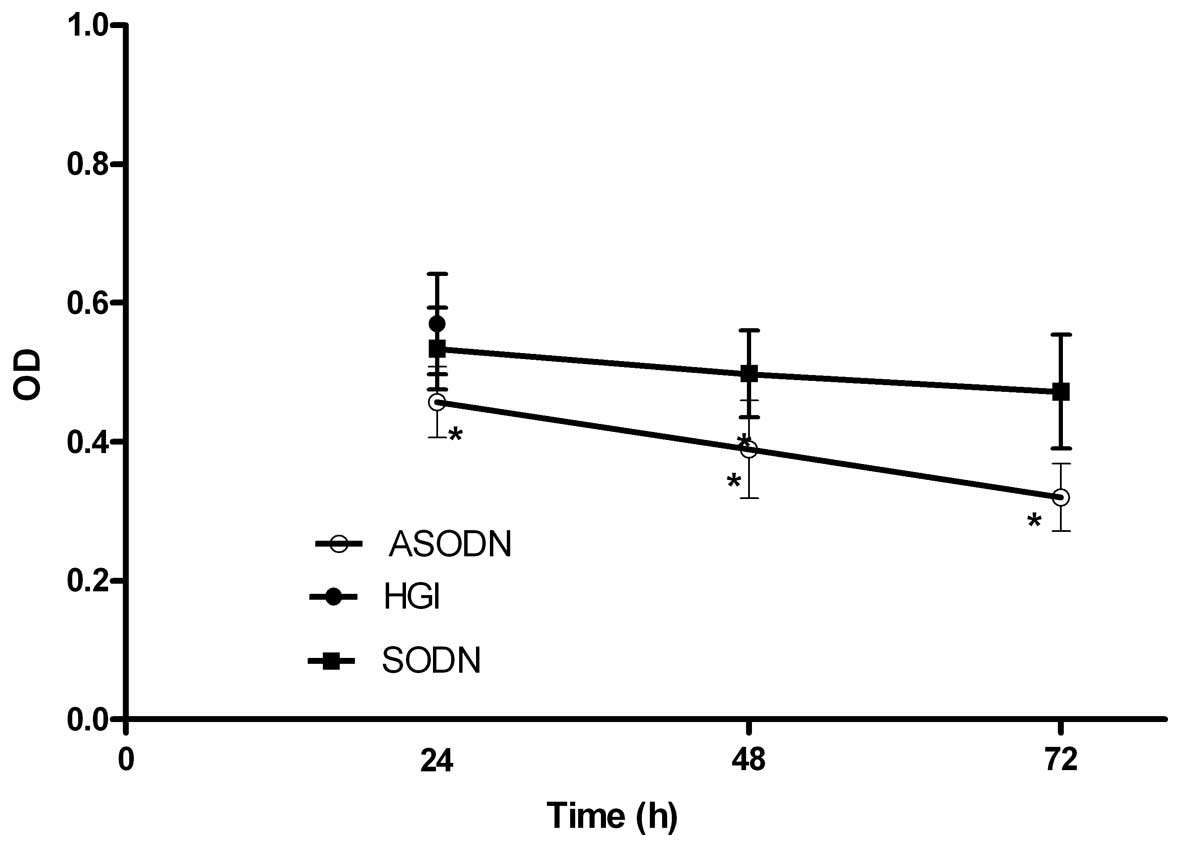
Additionally, the treatment of VSMCs induced by HGI with 20 μM
ASODN significantly inhibited the proliferation of VSMCs. At 24, 48
and 72 h after treatment, the inhibition rates were 19.8, 31.8 and
43.9%, respectively, indicating a time-dependent inhibition of VSMC
proliferation. SODN also inhibited the proliferation of VSMCs.
However, SODN caused no significant effect on VSMC proliferation
compared with ASODN (Fig. 3).
Discussion
In the present study, evidence that telomerase
activity and the proliferation of VSMCs are increased in the rat
model of T2DM and that HGI causes increased telomerase activation
and proliferation of VSMCs in vitro is provided. It was
further demonstrated that VSMC proliferation was significantly
reduced by the intervention of telomerase inhibitors (ASODNs) in
vitro.
T2DM is characterized by target-tissue resistance to
insulin, which often causes hyperglycemia and hyperinsulinemia. In
the present study, STZ-induced T2DM rats showed a significant
increase in fasting plasma glucose and insulin levels compared with
normal rats, indicating a successful model of T2DM. This diabetic
model was similar to T2DM in humans, as the high-fat diet initiated
a state of insulin resistance and then the treatment with a low
dose of STZ established a relative insulin deficiency (22). It is known that hyperglycemia and
hyperinsulinemia are associated with atherosclerosis in T2DM and
this is mainly caused by the abnormal proliferation of VSMCs. Under
physiological conditions, there is a balance between the
proliferation and apoptosis of VSMCs. When this balance is
disturbed by hyperglycemia and hyperinsulinemia, VSMC proliferation
is increased and promotes the development of atherosclerosis
(23).
Telomerase activity is known to be closely
associated with the cell proliferation and apoptosis of VSMCs.
Telomerase is wrapped in the cell nucleus and is released to repair
chromosomal DNA when cell division or DNA damage has occurred.
Thus, telomerase activity is inhibited in normal cells and is
activated in tumor or proliferating cells in normal tissues.
Telomerase is a marker of cell proliferation at the molecular level
and telomerase activity is expressed at various levels in tissues
with hyperplasia (24).
Additionally, previous studies have demonstrated that telomerase
activation may induce phenotypic changes in VSMCs by being involved
in cell remodeling processes and that it is important in the
signaling pathway of VSMC proliferation (15,25).
There are different levels of telomerase upregulation in the
abnormal proliferation of VSMCs. In the present study, telomerase
activity and the degree of VSMC proliferation were significantly
higher in diabetic compared with control rats. This finding
indicates that factors, including lipids, viral infection and
hypoxia, lead to VSMC proliferation, which initiates the
development of atherosclerotic plaques. To determine whether
hyperglycemia and hyperinsulinemia induce upregulated telomerase
activity, the telomerase activity in HGI-induced VSMC proliferation
was determined. The results showed that the expression of
telomerase activity was higher in HGI-induced VSMCs compared with
the normal control group, suggesting that telomerase activity is
upregulated in hyperglycemia- and hyperinsulinemia-induced VSMC
proliferation in T2DM.
Telomerase is a regulating enzyme. The telomerase
reverse transcriptase (TERT) catalytic subunit is the key component
that regulates telomerase activity. The TERT expression level is
closely associated with telomerase activity. Thus, ASODNs, the
synthetic short-chain nucleic acids which bind to and degrade TERT
mRNA, have been considered ideal for the inhibition of telomerase
activity (26). In the present
study, ASODN significantly inhibited VSMC proliferation in a dose-
and time-dependent manner. Although SODN demonstrated an inhibitory
effect, no significant difference was identified when compared with
the HGI group, indicating that this inhibitory effect is caused by
a specific base sequence.
In conclusion, the in vivo and in
vitro experiments in this study have shown that hyperglycemia
and hyperinsulinemia stimulated the telomerase activity and
proliferation of VSMCs, while the inhibition of telomerase activity
reduced the proliferation of VSMCs, indicating that telomerase may
be involved in the pathological process of diabetic vascular
disease. The specific molecular mechanisms require further
investigation to be fully elucidated. Telomerase inhibition may
provide a unique approach for the treatment of vascular
complications in T2DM.
References
|
1
|
Bruemmer D and Law RE: Thiazolidinedione
regulation of smooth muscle cell proliferation. Am J Med.
115:87–92. 2003. View Article : Google Scholar
|
|
2
|
Carmody BJ, Arora S, Wakefield MC, Weber
M, Fox CJ and Sidawy AN: Progesterone inhibits human infragenicular
arterial smooth muscle cell proliferation induced by high glucose
and insulin concentrations. J Vasc Surg. 36:833–838. 2002.
View Article : Google Scholar
|
|
3
|
Cifarelli V, Luppi P, Tse HM, He J,
Piganelli J and Trucco M: Human proinsulin C-peptide reduces high
glucose-induced proliferation and NF-κB activation in vascular
smooth muscle cells. Atherosclerosis. 201:248–257. 2008.PubMed/NCBI
|
|
4
|
Isenović ER, Kedees MH, Tepavcević S,
Milosavljević T, Korićanac G, Trpković A and Marche P: Role of
PI3K/AKT, cPLA2 and ERK1/2 signaling pathways in insulin regulation
of vascular smooth muscle cells proliferation. Cardiovasc Hematol
Disord Drug Targets. 9:172–180. 2009.PubMed/NCBI
|
|
5
|
Gizard F, Nomiyama T, Zhao Y, et al: The
PPARalpha/p16INK4a pathway inhibits vascular smooth muscle cell
proliferation by repressing cell cycle-dependent telomerase
activation. Circ Res. 103:1155–1163. 2008. View Article : Google Scholar
|
|
6
|
Herbert KE, Mistry Y, Hastings R, Poolman
T, Niklason L and Williams B: Angiotensin II-mediated oxidative DNA
damage accelerates cellular senescence in cultured human vascular
smooth muscle cells via telomere-dependent and independent
pathways. Circ Res. 102:201–208. 2008. View Article : Google Scholar
|
|
7
|
Jacob T, Hingorani A and Ascher E:
Evidence for telomerase activation in VSMCs exposed to
hyperglycemic and hyperhomocysteinemic conditions. Angiology.
60:562–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Serrano AL and Andrés V: Telomeres and
cardiovascular disease: does size matter? Circ Res. 94:575–584.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gancarcíková M, Zemanová Z, Brezinová J,
Berková A, Vcelíková S, Smigová J and Michalová K: The role of
telomeres and telomerase complex in haematological neoplasia: the
length of telomeres as a marker of carcinogenesis and prognosis of
disease. Prague Med Rep. 111:91–105. 2010.PubMed/NCBI
|
|
10
|
Jones CH, Pepper C and Baird DM: Telomere
dysfunction and its role in haematological cancer. Br J Haematol.
156:573–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sprouse AA, Steding CE and Herbert BS:
Pharmaceutical regulation of telomerase and its clinical potential.
J Cell Mol Med. 16:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cesselli D, Beltrami AP, D’Aurizio F, et
al: Effects of age and heart failure on human cardiac stem cell
function. Am J Pathol. 179:349–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El Assar M, Angulo J, Vallejo S, Peiró C,
Sánchez-Ferrer CF and Rodríguez-Mañas L: Mechanisms involved in the
aging-induced vascular dysfunction. Front Physiol.
3:1322012.PubMed/NCBI
|
|
14
|
Fyhrquist F and Saijonmaa O: Telomere
length and cardiovascular aging. Ann Med. 44(Suppl 1): S138–S142.
2012. View Article : Google Scholar
|
|
15
|
Cao Y, Li H, Mu FT, Ebisui O, Funder JW
and Liu JP: Telomerase activation causes vascular smooth muscle
cell proliferation in genetic hypertension. FASEB J. 16:96–98.
2002.PubMed/NCBI
|
|
16
|
Coppey LJ, Davidson EP, Rinehart TW,
Gellett JS, Oltman CL, Lund DD and Yorek MA: ACE inhibitor or
angiotensin II receptor antagonist attenuates diabetic neuropathy
in streptozotocin-induced diabetic rats. Diabetes. 55:341–348.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HJ, Jin YX, Shen W, Neng J, Wu T, Li
YJ and Fu ZW: Low dose streptozotocin (STZ) combined with high
energy intake can effectively induce type 2 diabetes through
altering the related gene expression. Asia Pac J Clin Nutr.
16(Suppl 1): 412–417. 2007.PubMed/NCBI
|
|
18
|
Wang Y, Qiao L, Qiu J, Mi W, Han Y and
Zhong C: Establishing primary cultures of vascular smooth muscle
cells from the spiral modiolar artery. Int J Pediatr
Otorhinolaryngol. 76:1082–1086. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He X, Qiao Q, Ge N, et al:
Irradiation-induced telomerase activity and gastric cancer risk: a
case-control analysis in a Chinese Han population. BMC Cancer.
10:3122010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Li LS, Yang DL, Gong QH, Deng J
and Huang XN: Inhibitory effect of ginsenoside rg1 on vascular
smooth muscle cell proliferation induced by PDGF-BB is involved in
nitric oxide formation. Evid Based Complement Alternat Med.
2012:3143952012. View Article : Google Scholar
|
|
21
|
McMurray HF, Parrott DP and Bowyer DE: A
standardised method of culturing aortic explants, suitable for the
study of factors affecting the phenotypic modulation, migration and
proliferation of aortic smooth muscle cells. Atherosclerosis.
86:227–237. 1991. View Article : Google Scholar
|
|
22
|
Sun X, Han F, Yi J, Han L and Wang B:
Effect of aspirin on the expression of hepatocyte NF-kappaB and
serum TNF-alpha in streptozotocin-induced type 2 diabetic rats. J
Korean Med Sci. 26:765–770. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jacob T, Clouden N, Hingorani A and Ascher
E: The effect of cotinine on telomerase activity in human vascular
smooth muscle cells. J Cardiovasc Surg (Torino). 50:345–349.
2009.PubMed/NCBI
|
|
24
|
Belair CD, Yeager TR, Lopez PM and
Reznikoff CA: Telomerase activity: a biomarker of cell
proliferation, not malignant transformation. Proc Natl Acad Sci
USA. 94:13677–13682. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matthews C, Gorenne I, Scott S, et al:
Vascular smooth muscle cells undergo telomere-based senescence in
human atherosclerosis: effects of telomerase and oxidative stress.
Circ Res. 99:156–164. 2006. View Article : Google Scholar
|
|
26
|
Chen H, Li Y and Tollefsbol TO: Strategies
targeting telomerase inhibition. Mol Biotechnol. 41:194–199. 2009.
View Article : Google Scholar : PubMed/NCBI
|