Introduction
Mesenchymal stem cells (MSCs) are a type of adult
stem cell that have attracted significant attention due to their
unique properties. The first source of human MSCs was identified
and isolated from bone marrow (BM) (1). The BMMSCs showed capacity for
self-renewal and multipotent differentiation. The potential uses of
MSCs have been studied extensively in vitro and in animal
models (2–4). MSCs are hypothesized to have a high
potential for use in tissue engineering and regenerative medicine
(5,6). However, the availability of BMMSCs is
limited due to the location of the cells. BM aspiration, or biopsy,
is an invasive surgery, and the the differentiation capacity varies
relative to the age of the donor (7). Optional MSC sources have been
identified, including adipose tissue (8), umbilical cord blood (9) and peripheral blood (10) and Wharton’s jelly (WJ) (11). The most noteworthy source of MSCs
is the perinatally-derived stem cells i.e., stem cells from the
placenta (PL), amniotic fluid and umbilical cord (UC). These stem
cells have a number of advantages, including their ability to be
easily obtained, a low immunogenicity and lack of ethical concerns
(12). MSCs may be isolated and
cultured from the UC, PL and WJ (13).
Coronary heart disease is the third leading cause of
mortality worldwide. A number of risk factors have been revealed to
play a role in the pathogenesis of this disease, including smoking,
obesity, hypertension and hypercholesterolemia. The main mechanism
is the blockage of blood flow through the heart leading to
myocardial infarction (the death of cardiac muscle cells). Cell
therapy is one of a number of therapeutic methods developed to
improve the quality of life of patients suffering from heart
problems. Various cell types have been studied extensively to
determine the most effective outcome. Adult stem cells are one of
the candidates for cell therapy. MSCs are the best option due to
three important properties; multilineage differentiation, possible
autologous sources and their immunoregulatory role. The
transdifferentiation of MSCs has been studied extensively and it is
widely accepted that MSCs are multipotent. The role of MSC
differentiation into cardiomyocyte-like cells has been demonstrated
in animal models and in vitro studies (14–16).
The present study analyzed the cardiomyocyte differentiation
ability of MSCs from perinatal sources in comparison with
BMMSCs.
Materials and methods
Perinatal cell culture
The present study was approved by the Siriraj
Institution Review Board (SIRB). Human UCs and PLs were collected
from full-term deliveries and immersed in sterile
phosphate-buffered saline (PBS) supplemented with 100 U/ml
penicillin and 100 μg/ml streptomycin under sterile conditions. The
WJ, PLs and UCs were stored and processed separately. The samples
were washed twice using PBS prior to their preparation into
2–3-cm2 sections. Perinatal tissues were collected in 15
ml centrifugal tubes containing PBS and then washed twice under
centrifugation at 984 × g for 5 min. The pellets from the washing
procedure were digested using 0.5% trypsin-EDTA at 37°C for 30 min
and placed in 25-cm2 tissue culture flasks in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 μg/ml streptomycin. The tissues were incubated under 5%
CO2 until MSC outgrowth occurred from the explant. The
complete medium was changed twice a week. Subpassaging was
performed using 0.5% trypsin-EDTA to expand the quantity of cells
used in the experiment.
BMMSCs
BM aspirations were obtained from healthy donors
following written informed consent. The BM aspiration was collected
in 5,000 IU sodium heparin and diluted by PBS at a 1:1 ratio prior
to the isolation of mononuclear cells. Following the method of
Boyum (17), the cell suspension
was layered on Ficoll-Hypaque at a 1:1 ratio followed by
centrifugation at 984 × g for 30 min. Mononuclear cells were
harvested from the interphase layer into new centrifugal tubes for
2 cycles of washing. The cells were seeded in T25 flasks using DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin. The culture supernatant was removed 5–7 days later
and the adherent cells were maintained at 37°C with 5%
CO2. The BMMSCs from passages 2–5 were used in this
study.
MSC characterization
Criteria
The cells from the BM and perinatal sources were
characterized as MSCs based on the following criteria: plastic
adherence, positive and negative specific cell surface markers and
multi-potential differentiation (13).
MSC surface markers
The cell surface markers for the MSCs were examined
using a flow cytometer (FACSCalibur™, Becton-Dickinson,
Franklin Lakes, NJ, USA). The immunophenotype of the MSCs was
characterized using the following mouse monoclonal antibodies:
cluster of differentiation CD34-PE, CD45-FITC, CD73-PE (all
purchased from BD Pharmingen, San Diago, CA, USA), CD90-FITC,
CD105-FITC and CD106-PE (all purchased from AbD SeroTec, Raleigh,
NC, USA). The MSCs between passages 2 and 5 were harvested using
0.5% trypsin-EDTA. A 5×105 cell suspension (50 μl) was
incubated at 4°C for 30 min in the dark with mouse anti-human
antibodies. Following incubation, the cells were washed twice with
cold PBS and centrifuged at 984 × g for 5 min at 4°C. The cells
were then fixed with 300 μl 1% paraformaldehyde prior to analysis
by a flow cytometer (Becton-Dickinson).
Osteogenic and adipogenic
differentiation of MSCs
The MSCs from passages 2–5 were cultured in Advance
STEM Osteogenic Differentiation medium (OsteoDiff) for osteoblast
differentiation (Hyclone Laboratories, Inc., Logan, UT, USA) and
Advance STEM Adipogenic Differentiation medium (AdipoDiff; Hyclone
Laboratories) for adipocyte differentiation. The MSC cells were
seeded at a density of 1×105 cells/ml and maintained in
each differentiation medium. The media were changed twice a week.
The differentiated conditioned MSCs were maintained for 3 weeks, at
which time their osteogenic and adipogenic properties were
evaluated using immunocytochemical staining with alkaline
phosphatase and Oil Red O (Sigma-Aldrich, St. Louis, MO, USA).
In vitro treatment with
5-azacytidine
The MSCs obtained from the BM, UC, PL and WJ during
passages 2–5 were used in this experiment. The MSCs were seeded at
densities of 1×105 cells in DMEM supplemented with 10%
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin for 24 h. The
treatment group was treated with 10 μM 5-azacytidine
(Sigma-Aldrich) in complete medium for 24 h subsequent to which it
was changed into complete medium for 3 weeks. The morphology,
growth rate and viability were then observed.
RNA preparation and real-time
RT-PCR
RNA was collected from the control and
5-azacytidine-treated groups at weeks 1 and 3. The total RNA was
isolated from the cells using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and quantified by
spectrophotometry at 260 nm. The total RNA (2 μg) was used for
reverse transcription with the first-strand cDNA synthesis kit
(Invitrogen Life Technologies). Real-time PCR was performed for 40
cycles using the ABI 7500 real-time PCR system (Applied Biosystems,
Bedford, MA, USA) and analyses for cardiac- and myogenic-specific
genes were performed using the following primers: α-cardiac actin
(260 bp), sense 5′-TCTATGAGGGCTACGCTTTG-3′ and antisense
5′-GCCAATAGTGATGACTTGGC-3′; Troponin T (TnT; 225 bp), sense
5′-AGAGCGGAAAAGTGGGAAGA-3′ and antisense
5′-CTGGTTATCGTTGATCCTGT-3′; Nk×2.5 (136 bp), sense
5′-CTGCCGCCGCCAACAAC -3′ and antisense 5′-CGCGGGTCCCTTCCCTACCA-3′;
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 139 bp) sense
5′-GTCAACGGATTTGGTCGTATTG-3′ and antisense
5′-CATGGGTGGAATCATATTGGAA-3′. Each cycle consisted of denaturation
at 95°C for 10 sec, annealing at 60°C for 10 sec and extension at
72°C for 40 sec. The products were quantified by the comparative
Ct method. Samples were compared with the control group,
normalized against GAPDH and presented as fold change
increases.
Immunofluorescence staining
The MSCs from passages 2–5 were cultured on chamber
slides (Lab-Tek, Thermo Fisher Scientific, Rochester, NY, USA) at
1×105 cells/well with DMEM supplemented with 10% FBS,
100 U/ml penicillin and 100 μg/ml streptomycin. The 10 μM
5-azacytidine treatment and control groups from days 3 and 7 were
used for immunofluorescence analysis. The cells were washed twice
with PBS prior to fixation using 4% paraformaldehyde in PBS at 4°C
for 30 min. Non-specific binding was blocked using 4% bovine serum
albumin (BSA), followed by incubation with purified goat polyclonal
TnT and mouse monoclonal GATA4 primary antibodies (both Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The samples were then
washed twice with PBS and overlaid with rabbit anti-mouse FITC-
(Millipore, Billerica, MA, USA) and donkey anti-goat PE-conjugated
secondary antibodies (Santa Cruz Biotechnology, Inc.) for 30 min at
4°C in the dark. The samples were washed twice, followed by DAPI
counterstaining and mounting using ProLong Gold Antifade
(Invitrogen Life Technologies).
Statistical analysis
The data were analyzed using SPSS version 11.5
(SPSS, Inc., Chicago, IL). Data are expressed as the mean ± SEM.
The significance was analyzed using the Mann-Whitney U test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphology and characterization of
perinatal MSCs
The UCMSCs, PLMSCs and WJMSCs were observed to
exhibit different morphologies following tissue culture for 1 week.
There were limited morphological differences between the tissue
sources (Fig. 1A-C). The cells
continued to grow and reached confluence within week 2 of the
primary culture. Subpassages of MSCs grown in complete medium
revealed a spindle-shaped cell morphology (Fig. 1D-F). The MSCs from passages 3 and 4
were characterized for osteogenic-adipogenic differentiation
capacity and cell surface markers. Following culture of the MSCs in
adipogenic and osteogenic media for 3 weeks, cell-specific
cytochemical staining was performed. The UCMSCs, PLMSCs and WJMSCs
were positive for Oil Red O (Fig.
1G-I) and alkaline phosphatase staining (Fig. 1J-L). The MSCs were shown to be
positive for MSC surface markers CD73, CD90 and CD105 and negative
for hematopoietic cell surface markers CD34 and CD45 (Fig. 2).
 | Figure 1Morphology and characterization of
perinatal MSCs. Perinatal MSCs from the tissues were observed to be
spindle-shaped; (A) UCMSCs, (B) WJMSCs and (C) PLMSCs. Following
subpassaging of all MSC sources, the morphology was
fibroblast-like; (D) UCMSCs, (E) WJMSCs and (F) PLMSCs. Following
culturing of perinatal MSCs in adipogenic medium for 3 weeks, the
cells were stained with Oil Red O and small fat droplets were
revealed in the cytoplasm; (G) UCMSCs, (H) WJMSCs and (I) PLMSCs.
Alkaline phosphatase staining was used to characterize osteogenic
differentiation and all MSC sources showed positive staining; (J)
UCMSCs, (K) WJMSCs and (L) PLMSCs (magnification, ×100). MSCs,
mesenchymal stem cells; UC, umbilical cord; PL, placenta; WJ,
Wharton’s jelly. |
Effect of 5-azacytidine on the MSCs
The UCMSCs, WJMSCs and PLMSCs from passage 3 were
used at this stage of the study. The MSCs were treated with 10 μM
5-azacytidine for 24 h and maintained in complete medium for 3
weeks. The morphology, cell number and percent viability from week
1 were observed and compared against the control group. The
viability of all the MSC sources following treatment with
5-azacytidine was 94–100% in the UCMSCs; 84–100% in the WJMSCs and
93–100% in the PLMSCs. The morphology of the treated cells revealed
similarities with the control group in all MSC sources subsequent
to being cultured for 6 days (data not shown). The cell numbers in
the 5-azacytidine-treated group were lower than those of the
control group during the initial days in all the MSC sources.
Immunofluorescence study of cardiomyocyte
markers during MSC differentiation
The UCMSCs, WJMSCs, PLMSCs and BMMSCs from passages
3–5 were used at this stage of the study. The MSCs were treated
with 10 μM 5-azacytidine for 24 h and then changed into complete
medium for 7 days. The cardiac-specific markers, GATA4 and cardiac
TnT, were examined by immunofluorescence staining. The treated and
control MSCs revealed GATA4 expression in the nucleus, green in
Fig. 3E-H. The cardiac TnT in the
cytoplasm is presented in red in Fig.
3M-P. The expression of the two markers was higher in the
treated cells compared with the control.
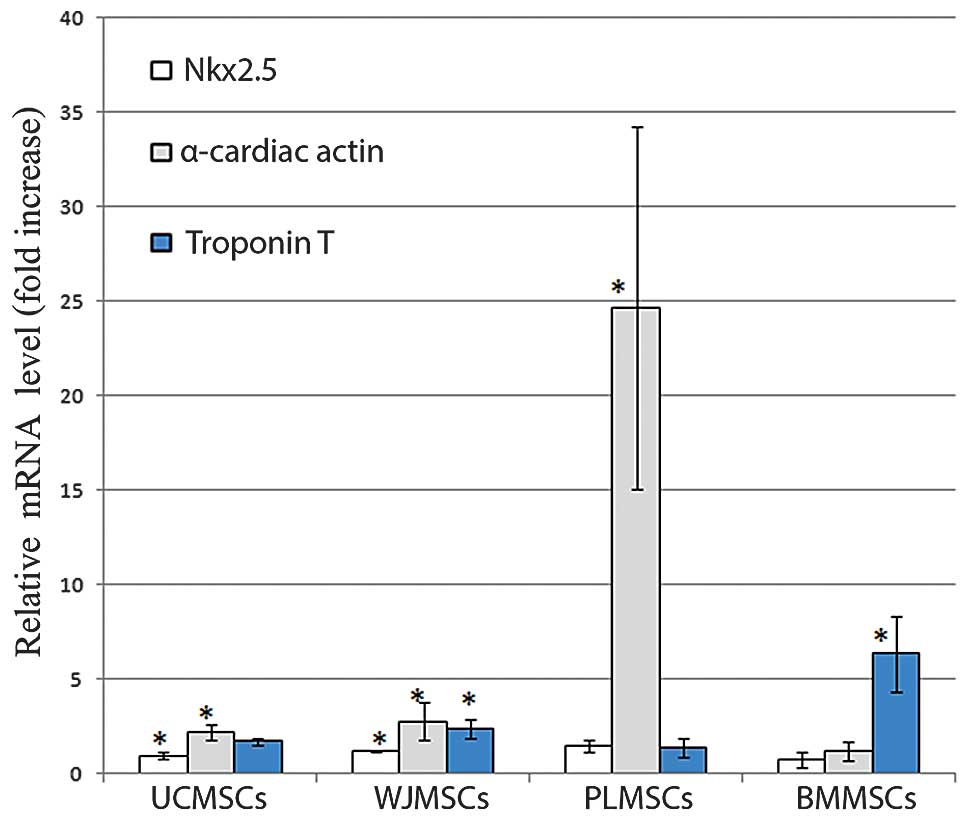
Expression of cardiomyocyte-specific
genes following treatment with 5-azacytidine
The mRNA expression of the cardiomyocyte specific
markers Nk×2.5, α-cardiac actin and cardiac TnT, was examined in
the BMMSCs, UCMSCs, PLMSCs and WJMSCs following treatment with 10
μM 5-azacytidine for 24 h and maintenance in culture for 1–3 weeks.
The quantitative analysis of the expression of the cardiac-specific
markers was performed by real-time RT-PCR and reported as a fold
increase of the control. The cardiac-specific markers showed a
higher expression level than that of the controls in the samples
treated with 5-azacytidine for 24 h and cultured for 1 week
(Fig. 4). The expression decreased
in samples maintained for 2 and 3 weeks (data not shown).
Discussion
BMMSCs have been studied extensively and a number of
promising medicinal applications have been reported. However, at
present, the clinical utilization of BMMSCs is limited due to the
invasiveness of the harvesting procedure (18). A new source of MSCs has been
identified that demonstrates a similar potential to BMMSCs
(8,9,11).
MSCs from perinatal tissue (amniotic membrane, UC and PL) were
identified as potential sources of MSCs due to their immunogenic
property, non-invasive sourcing and absence of ethical issues
(11). Moreover, the unlimited
differentiation potential of perinatal MSCs has been identified and
extensively studied (19,20).
The present study aimed to develop
cardiomyocyte-like cells from perinatal MSCs and compare them with
BMMSCs. The UC, WJ and PL were isolated and explants of each were
cultured for their residing MSCs. The MSCs were treated with the
hypomethylating agent, 5-azacytidine, and the cardiomyocyte
characteristics were evaluated. Expression of the cardiomyocyte
markers was analyzed by real-time RT-PCR and an increased
expression of Nk×2.5, TnT and α-cardiac actin was observed in the
5-azacytidine-treated MSCs compared with the controls. However, the
cardiomyocyte gene expression patterns varied depending on the
source of the MSCs. These observations may be due to variations in
the capacity and fate of each MSC source (21), which is determined by the
epigenetic state of the cells (22,23).
The effect of 5-azacytidine in the present study was
consistent with previous studies (18,24,25).
However, the mechanism of this hypomethylating agent yielded a
non-specific pattern and remains undefined. In addition, a
reduction in cardiac-specific marker expression was identified
following 5-azacytidine treatment for 2–3 weeks. The transient
expression of these markers may be a limitation of using
5-azacytidine as a differentiation initiation factor. The
combination of 5-azacytidine with other factors may be more
successful for improving the properties of
cardiomyocyte-differentiated MSCs.
The role of MSCs in the regeneration of damaged
tissue remains controversial. The mechanisms include the role of
MSCs in neoangiogenesis (26,27),
transdifferentiation (28),
anti-inflammatory responses (29)
and as a protective factor (30).
The success of transplantation may be due to various aspects that
play a concerted role in tissue regeneration. A clinical trial of
MSCs in the treatment of cardiac ischemia revealed a reduced
efficacy in the engraftment rate and functional capacity (31). The majority of studies concerning
BMMSCs have revealed that these cells are autologous and
allogeneic. The allogeneic origin of MSCs from other sources may be
optional and is particularly true of perinatal MSCs due to their
potential role in growth and neonatal development and their
availability. However, further studies are required to ensure the
proper use of these MSCs for stem cell transplantation.
Acknowledgements
The present study was supported by grants from the
Thailand Research Fund (no. RTA 488-0007) and the Commission on
Higher Education (no. CHERES-RG-49).
Abbreviations:
|
BM
|
bone marrow
|
|
BSA
|
bovine serum albumin
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
MSC
|
mesenchymal stem cell
|
|
PL
|
placenta
|
|
TnT
|
troponin T
|
|
UC
|
umbilical cord
|
|
WJ
|
Wharton’s jelly
|
References
|
1
|
Friedenstein AJ, Chailakhyan RK, Latsinik
NV, Panasyuk AF and Keiliss-Borok IV: Stromal cells responsible for
transferring the microenvironment of the hemopoietic tissues.
Cloning in vitro and retransplantation in vivo. Transplantation.
17:331–340. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Y, Chen L, Scott PG and Tredget EE:
Mesenchymal stem cells enhance wound healing through
differentiation and angiogenesis. Stem Cells. 25:2648–2659. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morigi M, Introna M, Imberti B, et al:
Human bone marrow mesenchymal stem cells accelerate recovery of
acute renal injury and prolong survival in mice. Stem Cells.
26:2075–2082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu X and Li H: Mesenchymal stem cells and
skin wound repair and regeneration: possibilities and questions.
Cell Tissue Res. 335:317–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richardson SM, Hoyland JA, Mobasheri R,
Csaki C, Shakibaei M and Mobasheri A: Mesenchymal stem cells in
regenerative medicine: opportunities and challenges for articular
cartilage and intervertebral disc tissue engineering. J Cell
Physiol. 222:23–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mueller SM and Glowacki J: Age-related
decline in the osteogenic potential of human bone marrow cells
cultured in three-dimensional collagen sponges. J Cell Biochem.
82:583–590. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bunnell BA, Flaat M, Gagliardi C, Patel B
and Ripoll C: Adipose-derived stem cells: isolation, expansion and
differentiation. Methods. 45:115–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multipotent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villaron EM, Almeida J, López-Holgado N,
et al: Mesenchymal stem cells are present in peripheral blood and
can engraft after allogeneic hematopoietic stem cell
transplantation. Haematologica. 89:1421–1427. 2004.PubMed/NCBI
|
|
11
|
Wang HS, Hung SC, Peng ST, et al:
Mesenchymal stem cells in the Wharton’s jelly of the human
umbilical cord. Stem Cells. 22:1330–1337. 2004.
|
|
12
|
Surbek D, Wagner A and Schoeberlein A:
Perinatal stem cell therapy. Perinatal Stem Cells. Cetrulo CL,
Cetrulo KJ and Cetrulo CL Jr: John Wiley & Sons; Hoboken, NJ:
pp. 51–60. 2009
|
|
13
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
14
|
Shim WS, Jiang S, Wong P, et al: Ex vivo
differentiation of human adult bone marrow stem cells into
cardiomyocyte-like cells. Biochem Biophys Res Commun. 324:481–488.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hattan N, Kawaguchi H, ando K, et al:
Purified cardiomyocytes from bone marrow mesenchymal stem cells
produce stable intracardiac grafts in mice. Cardiovasc Res.
65:334–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kruglyakov PV, Sokolova IB, Zin’kova NN,
et al: In vitro and in vivo differentiation of mesenchymal stem
cells in the cardiomyocyte direction. Bull Exp Biol Med.
142:503–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boyum A: Isolation of leucocytes from
human blood. A two-phase system for removal of red cells with
methylcellulose as erythrocyte-aggregating agent. Scand J Clin Lab
Invest Suppl. 97:9–29. 1968.PubMed/NCBI
|
|
18
|
Xu W, Zhang X, Qian H, et al: Mesenchymal
stem cells from adult human bone marrow differentiate into a
cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood).
229:623–631. 2004.PubMed/NCBI
|
|
19
|
Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee
JL and Chang YJ: Clonal amniotic fluid-derived stem cells express
characteristics of both mesenchymal and neural stem cells. Biol
Reprod. 74:545–551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu KH, Zhou B, Lu SH, et al: In vitro and
in vivo differentiation of human umbilical cord derived stem cells
into endothelial cells. J Cell Biochem. 100:608–616. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kadivar M, Khatami S, Mortazavi Y,
Shokrgozar MA, Taghikhani M and Soleimani M: In vitro
cardiomyogenic potential of human umbilical vein-derived
mesenchymal stem cells. Biochem Biophys Res Commun. 340:639–647.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benayahu D, Shefer G and Shur I: Insights
into the transcriptional and chromatin regulation of mesenchymal
stem cells in musculo-skeletal tissues. Ann Anat. 191:2–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collas P: Epigenetic states in stem cells.
Biochim Biophys Acta. 1790:900–905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin-Rendon E, Sweeney D, Lu F,
Girdlestone J, Navarrete C and Watt SM: 5-Azacytidine-treated human
mesenchymal stem/progenitor cells derived from umbilical cord, cord
blood and bone marrow do not generate cardiomyocytes in vitro at
high frequencies. Vox Sang. 95:137–148. 2008. View Article : Google Scholar
|
|
25
|
Zhang Y, Chu Y, Shen W and Dou Z: Effect
of 5-azacytidine induction duration on differentiation of human
first-trimester fetal mesenchymal stem cells towards
cardiomyocyte-like cells. Interact Cardiovasc Thorac Surg.
9:943–946. 2009. View Article : Google Scholar
|
|
26
|
Nagaya N, Kangawa K, Itoh T, et al:
Transplantation of mesenchymal stem cells improves cardiac function
in a rat model of dilated cardiomyopathy. Circulation.
112:1128–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silva GV, Litovsky S, Assad JA, et al:
Mesenchymal stem cells differentiate into an endothelial phenotype,
enhance vascular density, and improve heart function in a canine
chronic ischemia model. Circulation. 111:150–156. 2005. View Article : Google Scholar
|
|
28
|
Shake JG, Gruber PJ, Baumgartner WA, et
al: Mesenchymal stem cell implantation in a swine myocardial
infarct model: engraftment and functional effects. Ann Thorac Surg.
73:1919–1926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo J, Lin GS, Bao CY, Hu ZM and Hu MY:
Anti-inflammation role for mesenchymal stem cells transplantation
in myocardial infarction. Inflammation. 30:97–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Markel TA, Wang Y, Herrmann JL, et al:
VEGF is critical for stem cell-mediated cardioprotection and a
crucial paracrine factor for defining the age threshold in adult
and neonatal stem cell function. Am J Physiol Heart Circ Physiol.
295:H2308–H2314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Copland IB and Galipeau J: Death and
inflammation following somatic cell transplantation. Semin
Immunopathol. 33:535–550. 2011. View Article : Google Scholar : PubMed/NCBI
|