Introduction
Cancer is a major cause of mortality worldwide and
in Taiwan (1). Liver cancer is the
second most frequent cause of cancer death, and colorectal cancer
is the third most frequent cause of cancer death in Taiwan
(2,3). Approximately 26.0 per 100,000
individuals succumb to liver cancer and 25.3 per 100,000
individuals succumb to colorectal cancer each year, according to
the Department of Health, Executive Yuan, Taiwan in 2010
(www.doh.gov.tw/CHT2006/DM/DM2_2.aspx?now_fod_list_no=12336&class_no=440&level_no=4).
Chemotherapy is one of the treatment options in liver and
colorectal cancer, but the anticancer effects of chemotherapeutic
agents are not fully satisfactory. Thus, the discovery of new
antiliver and anticolorectal cancer chemotherapeutic agents is
urgently required. The induction of cancer cell apoptosis has been
shown to be the major anticancer mechanism for chemotherapeutic
agents (4,5). Apoptosis has stimulated interest in
caspases as potential therapeutic targets of chemotherapeutic
agents (6,7).
Previously, we designed and synthesized a series of
carboxamide derivatives as novel anticancer agents (8). We found that many of these compounds
exhibited potent cytotoxicities against various human cancer cell
lines (8,9). ITR-284
[N-(2-Dimethylaminoethyl)-4,8-dihydrobenzo (1,2-b;4,5-b’)
dithio-phene-2- carboxamide phosphoric acid salt] (Fig. 1A) is one of the most potent agents.
The previous studies suggested that ITR-284 significantly inhibited
the proliferation of HL60 and WEHI-3 leukemia cells, with low
toxicity to normal cells (8,9). In
the current study, we investigated the antiproliferative effects
and apoptotic induction of ITR-284 on human hepatocellular cancer
cell lines (Hep G2, Hep 3B, SK-HEP-1 and J5) and colorectal cancer
cell lines (HT 29, COLO 205, HCT 116 and SW 620). We demonstrated
that ITR-284 has a greater growth inhibition effect than that of
other compounds in various cancer cells, with a half maximal
effective concentration (EC50) of 50 to 75 nM. We
explored the mechanism of apoptotic induction by ITR-284 in Hep 3B
and COLO 205 cells. Our results suggest that ITR-284 induced
apoptosis in Hep 3B and COLO 205 cells through caspase
cascade-mediated pathways. ITR-284 may be selected as the lead
compound of an antihepatocellular and colorectal cancer agent to
trigger cell apoptosis in the future.
Materials and methods
Chemicals and reagents
MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was
purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Fetal
bovine serum (FBS), L-glutamine, penicillin-streptomycin, cell
culture medium DMEM and trypsin-EDTA were obtained from Gibco/Life
Technologies (Carlsbad, CA, USA). Caspase-3 (Z-DEVE-FMK), -8
(Z-IETD-FMK) and -9 (Z-LEHD-FMK) inhibitors were dissolved in DMSO
and diluted in cell culture medium prior to use (R&D Systems,
Minneapolis, MN, USA).
Cell culture
The human hepatocellular cancer cell lines (Hep G2,
Hep 3B, SK-HEP-1 and J5) and human colorectal cancer cell lines (HT
29, COLO 205, HCT 116 and SW 620) were purchased from the Food
Industry Research and Development Institute (Hsinchu, Taiwan). All
cells were cultured with DMEM and plated into a 75-T flask with 2
mM L-glutamine and were adjusted to contain 10% FBS and 1%
penicillin-streptomycin (100 U/ml penicillin and 100 μg/ml
streptomycin). All cells were grown at 37°C in a humidified
atmosphere comprised of 95% air and 5% CO2.
Cell viability assay
Cell viability was assessed using the MTT assay as
described previously (10,11). Approximately 2×104
cells/well were plated onto 96-well plates and then were exposed to
ITR-284 (0, 20, 40, 60, 80 and 100 nM). DMSO (0.1%) in media served
as a vehicle control. Cell viability was also used to examine Hep
3B and COLO 205 cells following pretreatment with or without 10 μM
of caspase-3, -8 and -9 inhibitors for 1 h, followed by treatment
with 50 nM ITR-284 and 0.1% DMSO as a control. After a 48-h
incubation, 100 μl of MTT solution (0.5 mg/ml) was added to each
well, and the plate was incubated at 37°C. Approximately 100 μl of
0.04 M HCl/isopropanol was added and the absorbance at 570 nm was
measured for each well. The cell survival ratio was expressed as a
percentage of the control. All results were formed of three
independent experiments.
Cell morphological examination
A total of 2×105 cells/well of Hep 3B and
COLO 205 cells in 24-well plates were exposed to 50 nM ITR-284 for
48 h. The cell morphology was directly examined and images were
captured under a contrast-phase microscope (12).
Assays for caspase-3, -8 and -9
activities
The activities of caspase-3, -8 and -9 were
determined according to the manufacturer’s instructions (Caspase
colorimetric kits, R&D Systems). Hep 3B and COLO 205 cells were
inoculated into a 75-T flask at a density of 1×107.
After being treated with ITR-284 (50 nM) for 48 h, cells were
harvested and lysed in lysis buffer (50 μl) for 10 min. After
centrifugation, the supernatants containing 100 μg protein were
incubated with caspase-3, -8 and -9 substrate (Z-DEVE-pNA,
Z-IETD-pNA and Z-LEHD-pNA for caspase-3, -8 and -9, respectively)
in reaction buffer. Samples were incubated in a 96-well
flat-bottomed microplate at 37°C for 1 h. The levels of released
pNA were measured with an ELISA reader (Anthos Labtec Instruments
GmbH, Salzburg, Austria) at a wavelength of 405 nm (13,14).
Statistical analysis
The statistical results were expressed as the means
± SEM of triplicate samples, and the difference between groups was
analyzed using a two-tailed Student’s t-test. P<0.001 was
considered to indicate a statistically significant difference.
Results
ITR-284 inhibits cell growth in human
hepatocellular and colorectal cancer cells
Our previous study reported that ITR-284 is capable
of inhibiting cell growth of HL-60 and WEHI-3 leukemia cells
(8). In the present study, we
investigated the growth inhibition effect of ITR-284 on human
hepatocellular cancer cells (Hep G2, Hep 3B, SK-HEP-1 and J5) and
colorectal cancer cells (HT 29, COLO 205, HCT 116 and SW 620). The
anti-proliferative effects of ITR-284 on those cells were evaluated
by the MTT assay. As shown in Fig.
2, exposure to various concentrations of ITR-284 (0, 20, 40,
60, 80 and 100 nM) for 48 h resulted in dose-dependent decreases in
cell viability of Hep G2 (Fig.
2A), Hep 3B (Fig. 2B),
SK-HEP-1 (Fig. 2C) and J5 cells
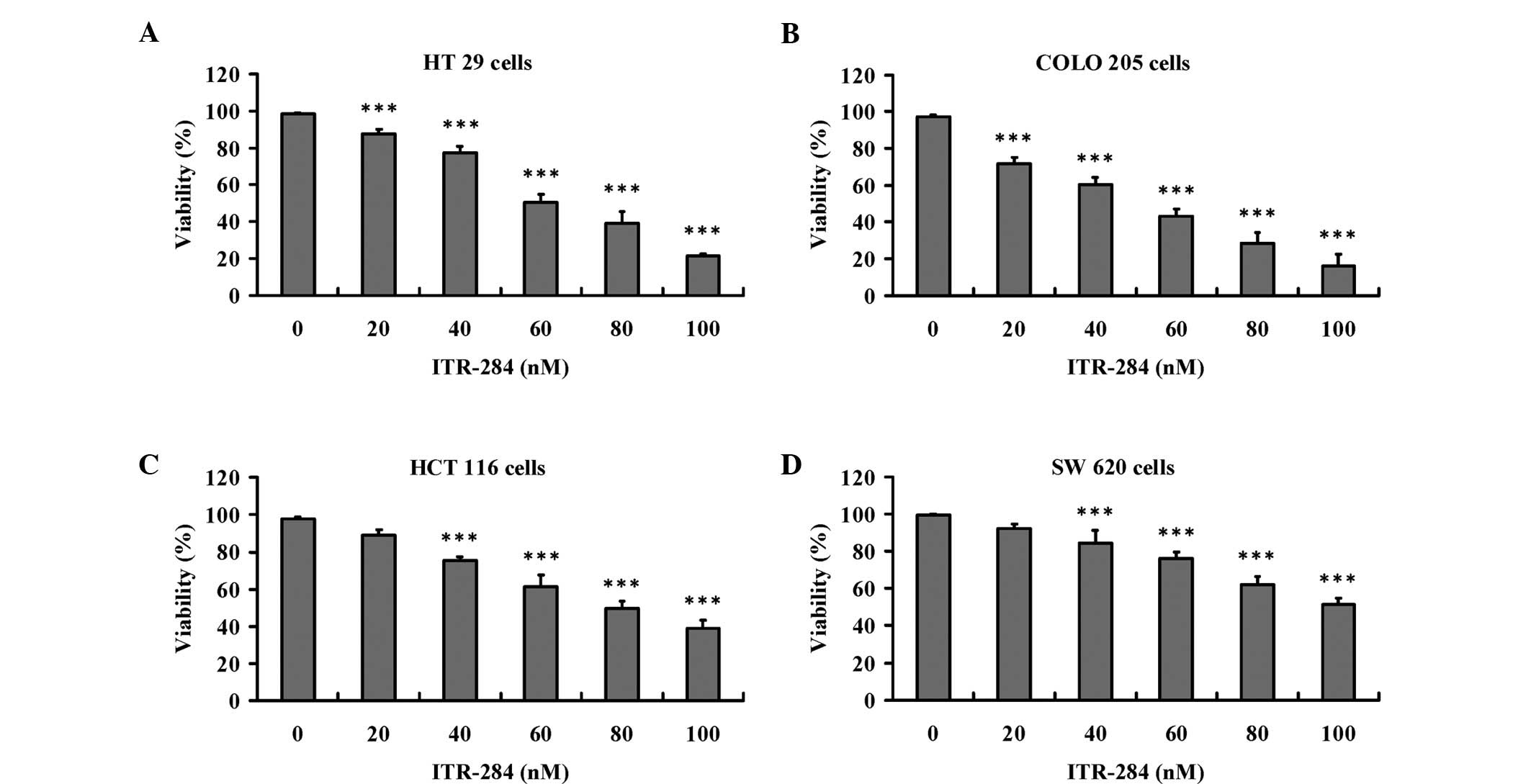
(Fig. 2D). In Fig. 3, we observed that ITR-284 (0, 20,
40, 60, 80 and 100 nM) also dose-dependently reduced cell viability
of HT 29 (Fig. 3A), COLO 205
(Fig. 3B), HCT 116 (Fig. 3C) and SW 620 cells (Fig. 3D). The results presented in
Table I show the EC50
values of ITR-284 in various cancer cell lines. Our results
demonstrated that the ITR-284 had highly selective effects on Hep
3B and COLO 205 cells in vitro.
 | Table IIn vitro cytotoxicity of
ITR-284. |
Table I
In vitro cytotoxicity of
ITR-284.
| Cell line | Cell type |
EC50a (nM) |
|---|
| Hep G2 | Human
hepatoblastoma | 86.39±4.18 |
| Hep 3B | Human hepatocellular
carcinoma | 51.23±2.98 |
| SK-HEP-1 | Human hepatocarcinoma
cells | 95.69±3.25 |
| J5 | Human hepatocellular
carcinoma | 106.25±4.40 |
| HT 29 | Human colorectal
adenocarcinoma | 76.58±6.25 |
| COLO 205 | Human colon
adenocarcinoma | 47.56±3.69 |
| HCT 116 | Human colorectal
carcinoma | 96.25±5.58 |
| SW 620 | Human colorectal
adenocarcinoma | 126.32±4.01 |
ITR-284 induces apoptosis in Hep 3B and
COLO 205 cells
ITR-284-induced reduction of cell viability may be
due to apoptosis. A 48-h exposure to 50 nM ITR-284 caused the Hep
3B cells (Fig. 4A) and COLO 205
cells (Fig. 4B) to round and
shrink morphologically. Treatment of Hep 3B and COLO 205 cells with
50 nM of ITR-284 also induced the translocation of
phosphatidylserine (PS) from the inner side of the plasma membrane
to the outer layer of the cell membrane by Annexin V analysis (data
not shown). Our results indicated that ITR-284 treatments provoked
apoptosis in human hepatocellular cancer Hep 3B and colorectal
cancer COLO 205 cells.
ITR-284-triggered apoptosis involves the
activation of caspase-3, -8 and -9
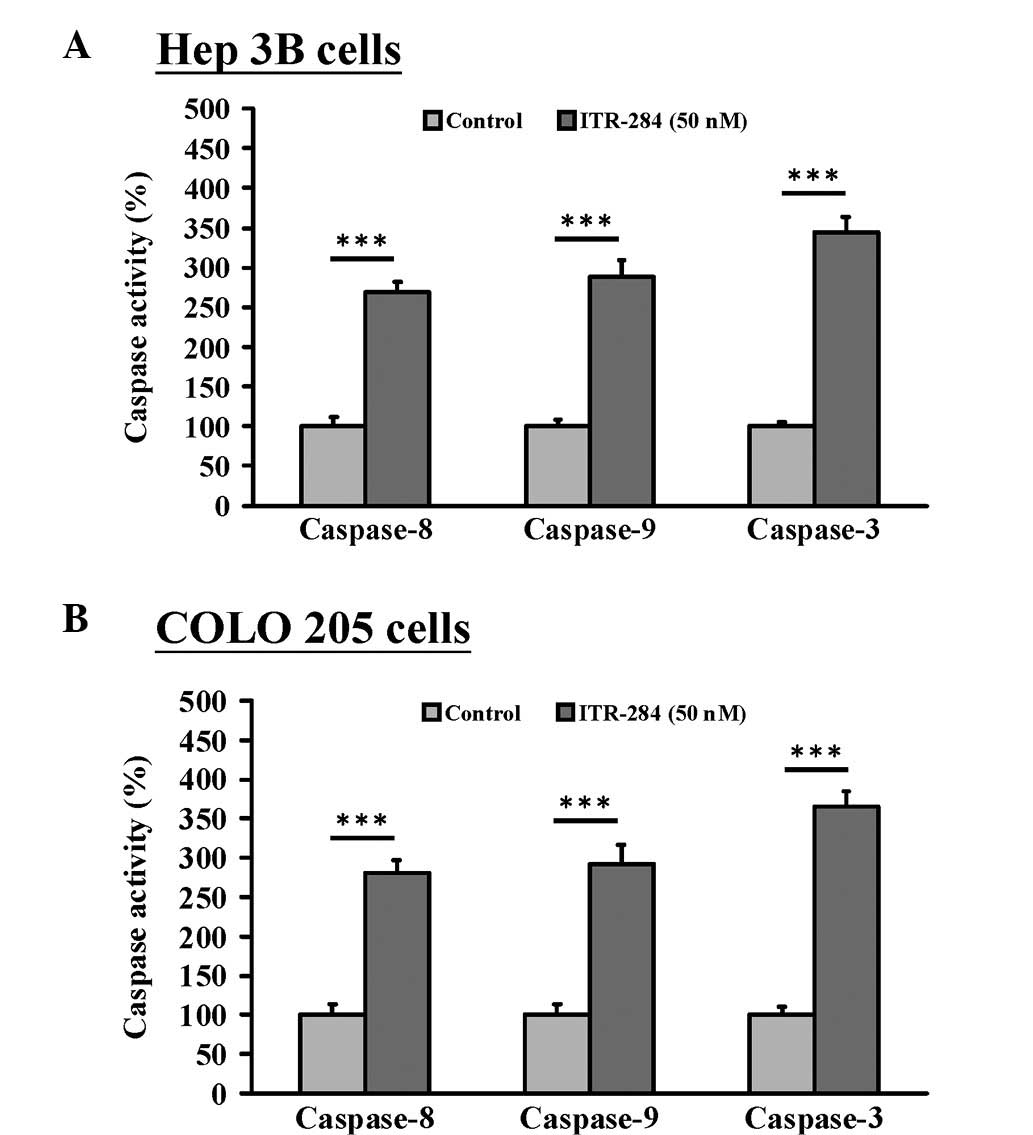
To determine whether caspases are majorly involved
in ITR-284-induced apoptotic cell death, the caspase-3, -8 and -9
activities were examined using the caspase colorimetric activity
assay. Our results demonstrated that caspase-3, -8 and -9
activities were all elevated following 48 h of exposure to 50 nM
ITR-284 in both Hep 3B (Fig. 5A)
and COLO 205 cells (Fig. 5B). We
suggested that ITR-284-induced apoptosis occurs through the
induction of caspase-3, -8 and -9 activities.
Effects of caspase-3, -8 and -9
inhibition on apoptosis in ITR-284-treated cells
The aforementioned results showed that
ITR-284-induced apoptosis occurs through the activation of
caspase-3, -8 and -9 activities. In the present study, Hep 3B and
COLO 205 cells were pre-treated with 10 μM caspase-3, -8 and -9
inhibitors for 1 h, and then exposed to 50 nM ITR-284.
Subsequently, cells were harvested for measuring the cell viability
by MTT assay. Pre-incubation with caspase-3, -8 and -9 specific
inhibitors significantly reduced ITR-284-induced viability in Hep
3B (Fig. 6A) and COLO 205 cells
(Fig. 6B). Our results suggest
that caspase-3, -8 and -9 activation may be involved in
ITR-284-induced apoptotic cell death.
Discussion
ITR-284 is a prospective anticancer compound and was
first described and synthesized in cooperation with the laboratory
of Dr. Yen-Fang Wen. An earlier study has verified that ITR-284
significantly inhibited the cell proliferation of human leukemia
cells (8). Furthermore, ITR-284
has much less cytotoxicity in normal peripheral blood mononuclear
cells (PBMCs) than in leukemia cells (8). The previous study has demonstrated
that ITR-284 (30 nM) substantially inhibits the growth of HL60 and
WEHI-3 leukemia cells in vitro. In a leukemia orthotopic
model, ITR-284 significantly prolonged the survival rate, prevented
body weight loss, inhibited spleen enlargement and reduced
infiltration of immature myeloblastic cells into splenic red pulp
in an in vivo experiment (8). However, combined treatment of ITR-284
with ATRA is more effective for differentiation therapy of
leukemia. Our data indicated that ITR-284 represents a promising
candidate as an anticancer drug with low toxicity to normal cells
(8). The purpose of this study was
to determine whether ITR-284 affects cell growth, and we
investigated cell death signaling pathways and induction of
apoptosis in human hepatocellular and colorectal cancer cells.
A number of studies have showed that the carboxamide
derivatives function via certain molecular mechanisms, including
the inhibition of topoisomerase activities and the induction of
apoptosis (15–17). In the present study, our results
demonstrate that ITR-284 treatment decreased the viability
(Figs. 2 and 3) of human hepatocellular cancer cells
(Hep G2, Hep 3B, SK-HEP-1 and J5) and colorectal cancer cells (HT
29, COLO 205, HCT 116 and SW 620). ITR-284 may cause cytotoxicity
by inducing cell death. Notably, the EC50 for 48-h
treatment of ITR-284 in hepatocellular and colorectal cancer cell
lines was different (Table I); one
of the reasons for the differences in sensitivities of different
cell lines may be the inherent different doubling time in various
cell lines, and another reason may be the differential gene
expression in various cell types. It is well known that Hep G2, J5
and SK-HEP-1 cell lines are p53-positive, but Hep 3B cells are
p53-negative. HT 29, COLO 205 and SW 620 lines have p53 mutation,
but the HCT 116 cell line has wild-type p53.
This is the first study to investigate the
anticancer effects of ITR-284 on human hepatocellular and
colorectal cancer cells, and the results suggest that ITR-284
induced apoptotic cell death and inhibited the growth of cancer
cells in a concentration-dependent manner. This observation is
similar to our earlier study addressing ITR-284, which showed that
ITR-284 initially affected the induction of apoptosis in HL60 and
WEHI-3 leukemia cell lines. As shown in Fig. 5, ITR-284 induced apoptosis through
the activation of caspases-3, -8 and -9 in Hep 3B and COLO 205
cells. These results suggest that the anticancer activity of
ITR-284 occurs through the induction of apoptotic cell death. Hep
3B and COLO 205 cells were pretreated with caspase-3, -8 and -9
inhibitors and then exposed to ITR-284, leading to increases in the
percentage of viable cells when compared with the ITR-284-treated
only cells (Fig. 6). Our data
indicated that these three caspases (-3, -8 and -9) were activated
following ITR-284 treatment. Thus, we proposed that ITR-284-induced
apoptosis may be carried out through the extrinsic and intrinsic
signaling pathways.
In conclusion, ITR-284 has growth inhibition effects
on human hepatocellular cancer cells (Hep G2, Hep 3B, SK-HEP-1 and
J5) and colorectal cancer cells (HT 29, COLO 205, HCT 116 and SW
620) by inducing cell apoptosis. Our study has clearly revealed
that the activation of caspase-3, -8 and -9 is the major
pharmacological action of ITR-284. Based on our results, ITR-284
has the potential to become one of the leading compounds for the
development of a novel antihepatocellular and colorectal cancer
agent in the future.
Acknowledgements
This study was supported by a research grant from
the National Science Council of the Republic of China awarded to Dr
Tian-Shung Wu and a grant from the China Medical University
(CMU-99-pharmacy-01 and CMU-99-pharmacy-02).
References
|
1
|
Zimonjic DB, Keck CL, Thorgeirsson SS and
Popescu NC: Novel recurrent genetic imbalances in human
hepatocellular carcinoma cell lines identified by comparative
genomic hybridization. Hepatology. 29:1208–1214. 1999. View Article : Google Scholar
|
|
2
|
Nowak AK, Chow PK and Findlay M: Systemic
therapy for advanced hepatocellular carcinoma: a review. Eur J
Cancer. 40:1474–1484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma CY, Ji WT, Chueh FS, Yang JS, Chen PY,
Yu CC and Chung JG: Butein inhibits the migration and invasion of
SK-HEP-1 human hepatocarcinoma cells through suppressing the ERK,
JNK, p38, and uPA signaling multiple pathways. J Agric Food Chem.
59:9032–9038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lin JJ, Huang WW, Tsuzuki M, Lee TH and Chung JG: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar
|
|
6
|
Kelloff GJ, Crowell JA, Steele VE, et al:
Progress in cancer chemoprevention: development of diet-derived
chemopreventive agents. J Nutr. 130:467S–471S. 2000.PubMed/NCBI
|
|
7
|
Lavrik IN, Golks A and Krammer PH:
Caspases: pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen YF, Lee KH, Huang PT, Chen MH, Shin
WC, Huang LJ, Hsu MH, Chen CJ and Kuo SC: Cell differentiation
enhancement by hydrophilic derivatives of
4,8-dihydrobenzo[1,2-b:5,4-b’]dithiophene-4,8-diones in HL-60
leukemia cells. Bioorg Med Chem Lett. 17:2908–2912. 2007.PubMed/NCBI
|
|
9
|
Wen YF, Yang JS, Kuo SC, Hwang CS, Chung
JG, Wu HC, Huang WW, Jhan JH, Lin CM and Chen HJ: Investigation of
anti-leukemia molecular mechanism of ITR-284, a carboxamide analog,
in leukemia cells and its effects in WEHI-3 leukemia mice. Biochem
Pharmacol. 79:389–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ip SW, Wei HC, Lin JP, Kuo HM, Liu KC, Hsu
SC, Yang JS, Mei-Dueyang, Chiu TH, Han SM and Chung JG: Bee venom
induced cell cycle arrest and apoptosis in human cervical
epidermoid carcinoma Ca Ski cells. Anticancer Res. 28:833–842.
2008.PubMed/NCBI
|
|
11
|
Lin ML, Chen SS, Lu YC, Liang RY, Ho YT,
Yang CY and Chung JG: Rhein induces apoptosis through induction of
endoplasmic reticulum stress and Ca2+-dependent
mitochondrial death pathway in human nasopharyngeal carcinoma
cells. Anticancer Res. 27:3313–3322. 2007.PubMed/NCBI
|
|
12
|
Yang JS, Chen GW, Hsia TC, Ho HC, Ho CC,
Lin MW, Lin SS, Yeh RD, Ip SW, Lu HF and Chung JG: Diallyl
disulfide induces apoptosis in human colon cancer cell line (COLO
205) through the induction of reactive oxygen species, endoplasmic
reticulum stress, caspases casade and mitochondrial-dependent
pathways. Food Chem Toxicol. 47:171–179. 2009. View Article : Google Scholar
|
|
13
|
Yang JS, Hour MJ, Kuo SC, Huang LJ and Lee
MR: Selective induction of G2/M arrest and apoptosis in HL-60 by a
potent anticancer agent, HMJ-38. Anticancer Res. 24:1769–1778.
2004.PubMed/NCBI
|
|
14
|
Packard BZ, Toptygin DD, Komoriya A and
Brand L: Profluorescent protease substrates: intramolecular dimers
described by the exciton model. Proc Natl Acad Sci USA.
93:11640–11645. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lukka PB, Kestell P, Paxton JW and Baguley
BC: Development and validation of a liquid chromatography-mass
spectrometry (LC-MS) assay for the determination of the anti-cancer
agent
N-[2-(dimethylamino)ethyl]-2,6-dimethyl-1-oxo-1,2-dihydrobenzo[b]-1,6-naph
thyridine-4-carboxamide (SN 28049). J Chromatogr B Analyt Technol
Biomed Life Sci. 875:368–372. 2008.PubMed/NCBI
|
|
16
|
Feng W, Satyanarayana M, Tsai YC, Liu AA,
Liu LF and LaVoie EJ: 11-Substituted
2,3-dimethoxy-8,9-methylenedioxybenzo[i]phenanthridine derivatives
as novel topoisomerase I-targeting agents. Bioorg Med Chem.
16:8598–8606. 2008.PubMed/NCBI
|
|
17
|
Creighton-Gutteridge M, Cardellina JH II,
Stephen AG, Rapisarda A, Uranchimeg B, Hite K, Denny WA, Shoemaker
RH and Melillo G: Cell type-specific, topoisomerase II-dependent
inhibition of hypoxia-inducible factor-1alpha protein accumulation
by NSC 644221. Clin Cancer Res. 13:1010–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|